Abstract
Background and objectives
Pompe disease is a rare metabolic disorder due to lysosomal alpha-glucosidase (GAA) deficiency. It is considered as a multi-systemic disease since, although glycogen accumulation is largely prominent in heart, skeletal and respiratory muscles, other organs can also be affected. As regards the vascular system, few reports have documented cerebrovascular malformations in Pompe patients. The aim of this study was to define the presence and type of intracranial arterial abnormalities in a cohort of late onset Pompe disease (LOPD) patients.
Methods
We have studied 21 LOPD patients with cerebral CT angiography (CTA), using maximum intensity projection and volume rendering technique for 3D-image reconstruction.
Results
We found intracranial arterial abnormalities in 13/21 patients (62 %), of whom: 2/21 patients (9.5 %) showed an unruptured intracranial aneurysm (respectively 2 and 4 mm), 10/21 (47 %) had a vertebrobasilar dolichoectasia (VBD) and 1/21 a basilar artery fenestration. Signs of lacunar encephalopathy (insular, capsular and frontal subcortical lesions) were detected in 13/21 patients (62 %) and this correlated with the presence of respiratory impairment (p = 0.017).
Conclusions
These findings differ from what has been previously observed in healthy, aged-matched populations and confirm that cerebral arteries abnormalities, mainly involving the posterior circle, are not so rare in LOPD patients and are often accompanied by a lacunar encephalopathy that might represent a hypoxic-ischemic origin. A CTA or an MRA is recommended, in LOPD patients, for early detection of cerebrovascular malformations as they could lead to life-threatening events such as sub-arachnoid haemorrhage or brainstem compression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pompe disease (glycogen storage disease type II - OMIM # 232300) is a rare, inherited, metabolic disorder caused by lysosomal α-glucosidase (GAA) (EC number 3.2.1.20) deficiency, which leads to glycogen accumulation across different tissues, mainly heart, skeletal and respiratory muscles.
Depending on age of onset, two clinical forms are known: infantile onset Pompe disease (IOPD) (onset <1 year of age) and late pnset Pompe disease (LOPD). LOPD phenotype is extremely heterogeneous, ranging from cases having a presymptomatic hyperCKemia to others with proximal/axial muscle weakness and/or respiratory impairment. The disease course is usually slow but progressive often causing, overtime, loss of ambulation and dependency to mechanical ventilation.
In 2006, enzyme replacement therapy (ERT) with alglucosidase alfa became available, changing, in several cases, the natural course of IOPD (i.e. the oldest IOPD survivor is now 16 years old) (Kanters et al 2014), and improving/stabilizing motor and respiratory performances in at least 2/3 of LOPD cases (Toscano and Schoser 2013).
In the last few years, several reports highlighted the systemic nature of Pompe disease, demonstrating glycogen accumulation and tissue damage in many organs and systems: central and peripheral nervous system (Montagnese et al 2015; DeRuisseau et al 2009), heart (Hobson-Webb et al 2012), lung (Gaeta et al 2013), bone (Bertoldo et al 2015), gastrointestinal tract (Karabul et al 2014), hearing system (Musumeci et al 2012) and bladder (Remiche et al 2012). The involvement of the cerebrovascular system has been documented, mostly, in sporadic cases presenting aneurysms, vertebrobasilar dolichoectasia (VBD) or dilative arteriopathy (Table 1). In some of these cases, aneurysm rupture caused a subarachnoid haemorrhage (SAH) with a fatal outcome; in others, intracerebral haemorrhage (ICH), aneurysm thrombosis or brainstem compressions were reported (Table 1).
Autopsy studies documented the presence of glycogen-filled vacuoles within the smooth muscle cells in the tunica media of cerebral arterioles and arteries, along with numerous small aneurysmal dilatations (Kretzschmar et al 1990; Hobson-Webb et al 2012). In a German cohort of LOPD patients, the authors identified a subset of cases that presented with cardiac and cerebrovascular alterations and proposed the term “cardio-cerebrovascular pattern” to underscore the frequency of such findings (Schüller et al 2012). The above mentioned autoptic and clinical data suggest that the cerebrovascular system involvement should be investigated further in LOPD.
The aim of this study was to assess the presence and type of cerebrovascular abnormalities in a cohort of LOPD patients by systematic application of cerebral computed tomography angiography (CTA) and CT-scan.
Materials and methods
We studied 21 LOPD patients; diagnostic criteria were: age at onset >1 year, GAA residual activity below 30 % of controls (assayed in skeletal muscle) (Lim et al 2014) and/or identification of pathogenic mutations with GAA sequence analysis. In patients with only one detected pathogenic mutation (patients n°1–4 and 21, Table 2), research for deletions has been performed by MLPA analysis with negative results. The respiratory impairment was assessed by pulmonary function tests measuring FVC in upright and supine position (calculating ΔFVC), maximum inspiratory (MIP) and expiratory pressures (MEP).
All patients underwent a non-contrast brain CT scan and a CTA with a 64 row, multi-slice CT equipment (Somatom Sensation 64; Siemens Medical Systems, Forchheim, Germany). Acquisition and reconstruction parameters were: rotation time 0.5 s, pitch 1, 100 kVp, 200 effective milliamps, slice thickness 0.75 mm; reconstruction interval 0.40 mm. An intravenous antecubital administration of an 85–100 mL bolus of non-ionic iodinated contrast material (Xenetix 350 mg I/mL; Guerbet, Aulnay-sous-Bois, France) was performed at a 4 mL/s flow rate. The imaging start delay was determined by a bolus tracking facility and a threshold fixed at 80 Hounsfield units. The covered area extended from the first cervical vertebra to the superior aspect of the frontal sinuses. Post-processing of the image data was performed on a Leonardo workstation (InSpace 3D software, Siemens); maximum intensity projection (MIP) and volume rendering (VR) reconstruction were obtained.
Two experienced neuroradiologists independently looked for:
-
a)
Presence of aneurysms with specific consideration to their size and location.
-
b)
Evidence of vertebrobasilar dolichoectasia (VBD) according to Smoker’s criteria; evaluating the diameter (>4.5 mm), the height of bifurcation and the laterality of the basilar artery as surrogate measures for tortuosity and elongation (Gutierrez et al 2014);
-
c)
Other anatomical variants
In addition, a non-contrast brain CT scan was used to evaluate parenchymal alterations, involving both grey and white matter.
Statistical analysis
Statistical analysis was conducted using SAS (SAS version [9.2] of the SAS System (Copyright © 2002–2008 by SAS Institute Inc., Cary, NC, USA). Descriptive analysis included mean ± standard deviation (SD) or median and interquartile range (IQR), as appropriate, for continuous variables. Frequencies were calculated for categorical variables. For normally distributed continuous variables unpaired Student’s t-tests were used to assess differences between groups, while for non-normally distributed continuous variable the Mann–Whitney U tests were used. χ2 or Fisher’s exact test were used to compare frequencies. All hypothesis tests conducted were 2-tailed. A p-value < 0.05 was considered significant.
Results
This cohort of patients included 12 males and nine females of Caucasian origin. The mean age was 50 ± 14.97 years, ranging from 16 to 76 years. The most relevant clinical features, as well as cardiovascular risk factors, are summarized in Table 2.
The median disease duration was 13 years (range 2–32); 13/21 (62 %) patients had respiratory impairment (seven patients used NIV), seven patients (33 %) had obstructive sleep apnoea syndromes (OSAS) (Table 2).
None of the patients complained of central nervous system symptoms or signs except for six patients who suffered from morning headache, likely related to respiratory impairment and/or OSAS.
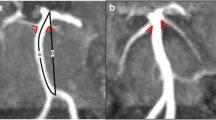
CTA revealed a saccular aneurysm in 2/21 patients (9,5 %); one with a diameter of 4 mm (Fig. 1a and b) and a second of 2 mm (Fig. 1c and d), both located at the M1 tract of the right middle cerebral artery (MCA). A basilar artery fenestration was detected in one patient (Fig. 2c-d)
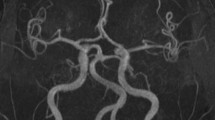
The frequency of VBD in this cohort was 47 % (10/21 pts): a basilar artery enlargement in 2/21 pts (9.5 %) and an abnormal height of bifurcation or a lateral displacement of the basilar artery (BA) in 8/21 pts (38 %).
CTA resulted normal in nine patients (43 %); posterior circle anatomical variants, such as dominant vertebral artery or foetal origin of the posterior cerebral artery, were present in four cases.
Signs of lacunar encephalopathy (insular, capsular and frontal subcortical white matter lesions) were detected in 13/21 pts (62 %); two of whom, aged 64 and 36 years old, showed respectively an old parietal and temporal silent ischemic stroke (Table 2). In the remaining 8/21 pts (38 %), brain CT was normal.
In order to evaluate if the presence of VBD correlated with disease duration, enzymatic activity, cardiovascular risk factors, vascular encephalopathy, BMI or age, we compared the demographic and clinical characteristics of the two groups of patients with or without VBD; no statistically significant difference was found between the two groups. The presence of encephalopathy was associated with respiratory impairment (p = 0.017).
Discussion
This study aimed to systematically investigate cerebrovascular malformations in LOPD patients. For such purpose, first tier imaging modalities are CTA or MRA. In this study, CTA was chosen considering the following advantages over MRA. The 3D-time-of-flight (3D-TOF) is the preferred MRA technique for the detection of intracranial aneurysms. It provides high-quality images without contrast administration but is prone to many artefacts, caused by turbulent blood flow. Furthermore, MRA is not accurate in depicting small-sized aneurysms (≤3 mm) located near to the skull base or in defining the morphology of aneurysms (a key indicator of a rupture) and finally in reliably identifying lesions with turbulent intraluminal flow (Kapsalaki et al 2012). For the diagnosis of VBD, a substantial comparability between CT/CTA and MRI/TOF-MRA has been demonstrated (Förster et al 2014).
On the other hand, CTA has many advantages related to its worldwide availability and low cost, optimal spatial and contrast resolution and a rapid acquisition time (about 2 min). In particular shorter acquisition time, in comparison to the longer acquisition time required by MRA, makes CTA a better choice for Pompe patients who typically have respiratory impairment and cannot lie supine long (in our cohort, eight patients).
CTA disadvantages are related to the use of iodinated contrast material, potentially causing allergy or nephrotoxicity, and to ionizing radiation exposure. None of our patients manifested side effects during or immediately after the study.
Intracranial saccular aneurysms are bulging, weak areas in the arterial walls; they are usually asymptomatic but may rupture causing a stroke. Findings from analysis of 68 prevalence studies showed an overall prevalence of 3.2 % in a population without comorbidity and a mean age of 50 years (Vlak et al 2011). Nevertheless, the reported aneurysm prevalence for the general population widely varies among different studies according to evaluation methods (1,5T MRA, 3T MRI, CTA) and ethnic differences. The occurrence of intracranial aneurysms is associated with several inherited disorders such as, autosomal dominant polycystic kidney disease (ADPKD), Ehlers-Danlos syndrome type IV, Marfan’s syndrome and neurofibromatosis type I (Brown and Broderick 2014). In Pompe disease, few case reports described patients having a cerebral aneurysm, sometimes complicated by SAH and only one recent study systematically evaluated the cerebral arteries of ten LOPD patients, not finding any aneurysm (Hensel et al 2015).
In our cohort of LOPD patients, we have identified an isolated unruptured intracranial aneurysm (UIA) in 9.5 % of patients, significantly higher than the 3.2 % reported for the general population (Vlak et al 2011). However, given the relatively small number of patients studied, this aspect needs further confirmation on larger LOPD cohorts.
Aneurysm pathophysiology is regulated by complex interactions between anatomical (vascular anatomy, aneurysm size, shape, location), structural (arterial walls) and hemodynamic factors (flow patterns, wall shear stress) that play a role in aneurysm formation, growth and rupture. Autoptic studies on LOPD patients highlighted the presence of PAS-positive vacuoles within smooth muscle cells of the arterial walls media, together with numerous small aneurysmal dilatations (Kretzschmar et al 1990; Hobson-Webb et al 2012); these data suggest that glycogen accumulation alters arterial walls architecture and may contribute to aneurysms formation.
Incidentally discovered, intact, aneurysms could be either observed or electively treated, depending on their size and on patients’ conditions. In the absence of specific guidelines for aneurysm management in patients with LOPD, we would suggest adopting the general guidelines for UIAs surgical treatment (Komotar et al 2008); none of our patients met the criteria for surgical intervention. If surgery is not indicated, a neuroradiological follow up, performed every year for the first 2 years and, later, if the aneurysm is stable, every 2–5 years, is suggested (Wiebers 2006).
In one patient, we found a fenestration of the basilar artery (BA); this is a rare anatomical variant resulting, during embryogenesis, from an incomplete fusion of the paired longitudinal neural arteries forming the BA. These fenestrations may evolve into saccular aneurysm, due to hemodynamic stress and absence of the media (Sogawa et al 2013). While this embryogenic pathophysiology seems not related to Pompe disease, it is possible that glycogen accumulation could make the arterial walls further vulnerable. Therefore, finding a fenestration could be more dangerous in patients with LOPD than in the general population indicating an importance of close follow-up.
VBD is characterized by expansion, elongation and tortuosity of the vertebrobasilar arteries that may become symptomatic due to brainstem compression, obstructive hydrocephalus and/or haemorrhage. Its prevalence in the general population widely varies depending upon the diagnostic methods used for its detection and the population studied, increasing with age and cardiovascular risk factors. Angiography and autoptic results suggest an overall incidence between 0.05 % (Yuan et al 2014) and 3.7 % (Kumral et al 2005). Gutierrez et al found among a population of 718 subjects, studied by MRA, at least one dolichoectatic artery in 19 % of them (Gutierrez et al 2014); considering that their mean age (71.6 ± 8 years) was notably older in comparison to our patients (50 ± 14.97 years), the higher frequency of VBD detected in our LOPD cohort (47 %) is even more significant.
The association between VBD and congenital diseases such as sickle cell anaemia, Marfan or Ehlers Danlos syndrome, autosomal recessive polycystic kidney (ARPKD), Fabry disease has been previously reported (Yuan et al 2014). Since the first description of VBD in Pompe disease, that date back to a single case report published in 2005 (Anneser et al 2005), several other studies reported analogous observations (Laforêt et al 2008, 2013; Sacconi et al 2010) (Table 1). Hensel et al, in their study on ten LOPD patients, described three cases of dilative arteriopathy, two of whom had VBD, and documented that the diameter of dilated cerebral arteries increased with disease duration. The authors suggested that the increased pCO2, sometimes present in LOPD patients with respiratory impairment, might play a role in causing vasodilation (Hensel et al 2015). In our group of patients, no correlations were found between the occurrence of dilative arteriopathy and disease duration or with respiratory impairment.
VBD detection is also relevant as it is considered an independent risk factor for ischemic lesions. Tanaka et al have shown that basilar artery dilation is a predictor of future cerebrovascular events (Tanaka et al 2013) and a prospective study on 156 patients with VBD (average follow-up 11.7 years) recorded in 37.8 % of patients an ischemic stroke, in 19.9 % cranial nerve or brainstem compression, in 13.5 % a cerebral haemorrhage, and 1.3 % a hydrocephalus (Passero and Rossi 2008).
In this LOPD group, there were no significant differences as regards risk factors in patients with or without VBD, but the presence of VBD should lead to a recommendation of a better control of cardiovascular risk factors, considering the increased risk for cerebrovascular events.
For these reasons, we also evaluated the brain parenchyma of our patients and detected that 13/21 (62 %) presented signs of lacunar encephalopathy, ranging from mild forms with few subcortical lesions to severe patterns with multiple subcortical and white matter lesions or, even, displaying previously unrecognized ischemic strokes.
In this study, the presence of a lacunar encephalopathy did not correlate with age, cardiovascular risk factors, disease duration and VBD but we found a significant association (p = 0.017) with respiratory impairment.
It is well known that LOPD patients develop respiratory impairment due to diaphragmatic weakness, inspiratory and upper airways muscles involvement, that also contribute to OSAS occurrence (Mellies and Lofaso 2009). Nocturnal hypercapnia and hypoventilation are the most common alterations, followed by daytime hypoventilation and chronic respiratory failure. OSAS is considered a risk factor for stroke, inducing hypertension, reduced cerebral blood flow and impaired tissue oxygenation (Pizza et al 2010). We hypothesize that the recurrent presence of a lacunar encephalopathy, detected in our cohort, may be attributed to an inadequate cerebral oxygenation, but glycogen accumulation in cerebral arteries may also alter their anti-thrombotic properties leading us to hypothesize a hypoxic-ischemic pathogenic mechanism.
The absence of a control group should be considered among the limitations of our study and comparisons with the general population could be made only according to previously published studies. A control group could not be collected since, prospectively, CTA (considering its potential complications) could not ethically be proposed to healthy subjects.
Conclusions
Cerebrovascular involvement is yet an underestimated manifestation of LOPD. Our study, in addition to previous reports, provides evidence that cerebral arteriopathy has to be considered part of the multisystem LOPD phenotype. The clinical importance of these results relies on the potential risks of cerebrovascular malformations in causing life-threatening events. CTA or MRA should be recommended in all LOPD patients to early detect potentially treatable cerebrovascular malformations. A lacunar encephalopathy seems a recurrent finding and may be caused by a hypoxic-ischemic mechanism. Further studies on a greater number of patients, along with follow-up data, are needed to better assess the prevalence and natural history of the cerebrovascular involvement in LOPD.
References
Anneser JM, Pongratz DE, Podskarbi T, Shin YS, Schoser BG (2005) Mutations in the acid alpha-glucosidase gene (M. Pompe) in a patient with an unusual phenotype. Neurology 64:368–70
Bertoldo F, Zappini F, Brigo M et al (2015) Prevalence of asymptomatic vertebral fractures in late-onset Pompe disease. J Clin Endocrinol Metab 100:401–6
Brown RD, Broderick JP (2014) Unrupted intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol 13:393–404
DeRuisseau LR, Fuller DD, Qiu K et al (2009) Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci U S A 106:9419–24
Förster A, Ssozi J, Al-Zghloul M, Brockmann MA, Kerl HU, Groden C (2014) A comparison of CT/CT angiography and MRI/MR angiography for imaging of vertebrobasilar dolichoectasia. Clin Neuroradiol 24(4):347–53
Gaeta M, Barca E, Ruggeri P et al (2013) A late-onset Pompe disease (LOPD): correlations between respiratory muscles CT and MRI features and pulmonary function. Mol Genet Metab 110:290–6
Gutierrez J, Bagci A, Gardener H et al (2014) Dolichoectasia diagnostic methods in a multi-ethnic, stroke-free cohort: results from the Northern Manhattan Study. J Neuroimaging 24:226–31
Hensel O, Hanisch F, Stock K et al (2015) Morphology and function of cerebral arteries in adults with pompe. Dis JIMD Rep 20:27–33
Hobson-Webb LD, Proia AD, Thurberg BL et al (2012) Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab 106:462–9
Kanters TA, Hoogenboom-Plug I, Rutten-Van Mölken MP et al (2014) Cost-effectiveness of enzyme replacement therapy with alglucosidase alfa in classic-infantile patients with Pompe disease. Orphanet J Rare Dis 9:75
Kapsalaki EZ, Rountas CD, Fountas KN (2012) The role of 3 tesla MRA in the detection of intracranial aneurysms. Int J Vasc Med 2012:792–834
Karabul N, Skudlarek A, Berndt J, Kornblum C, Kley RA, Wenninger S, Tiling N, Mengel E, Plöckinger U, Vorgerd M, Deschauer M, Schoser B, Hanisch F (2014) Urge incontinence and gastrointestinal symptoms in adult patients with pompe disease: a cross-sectionalsurvey. JIMD Rep 17:53–61
Komotar RJ, Mocco J, Solomon RA (2008) Guidelines for the surgical treatment of unruptured intracranial aneurysms: the first annual J. Lawrence pool memorial research symposium—controversies in the management of cerebral aneurysms. Neurosurgery 62:183–93, discussion 193–4
Kretzschmar HA, Wagner H, Hübner G et al (1990) Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J Neurol Sci 98:169–83
Kumral E, Kisabay A, Ataç C et al (2005) The mechanism of ischemic stroke in patients with dolichoectatic basilar artery. Eur J Neurol 12:437–44
Laforêt P, Petiot P, Nicolino M et al (2008) Dilative arteriopathy and basilar artery dolichoectasia complicating late-onset Pompe disease. Neurology 70(22):2063–6
Laforêt P, Laloui K, Granger B, The French Pompe registry et al (2013) Baseline characteristics of a cohort of 126 patients with adult Pompe disease. Rev Neurol (Paris) 169(8–9):595–602
Lim JA, Li L, Raben N (2014) Pompe disease: from pathophysiology to therapy and back again. Front Aging Neurosci 6:177
Mellies U, Lofaso F (2009) Pompe disease: a neuromuscular disease with respiratory muscle involvement. Respir Med 103:477–84
Montagnese F, Barca E, Musumeci O et al (2015) Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): unusual features and response to treatment. J Neurol 262:968–78
Musumeci O, Catalano N, Barca E et al (2012) Auditory system involvement in late onset Pompe disease: a study of 20 Italian patients. Mol Genet Metab 107:480–4
Passero SG, Rossi S (2008) Natural history of vertebrobasilar dolichoectasia. Neurology 70:66–72
Pizza F, Biallas M, Wolf M et al (2010) Nocturnal cerebral hemodynamics in snorers and in patients with obstructive sleep apnea: a near-infrared spectroscopy study. Sleep 33:205–10
Remiche G, Herbaut AG, Ronchi D et al (2012) Incontinence in late-onset Pompe disease: an underdiagnosed treatable condition. Eur Neurol 68:75–8
Sacconi S, Bocquet JD, Chanalet S, Tanant V, Salviati L (2010) Desnuelle C abnormalities of cerebral arteries are frequent in patients with late-onset Pompe disease. J Neurol 257:1730–3
Schüller A, Wenninger S, Strigl-Pill N et al (2012) Toward deconstructing the phenotype of late-onset Pompe disease. Am J Med Genet C Semin Med Genet 160C:80–8
Sogawa K, Kikuchi Y, O’uchi T et al (2013) Fenestrations of the basilar artery demonstrated on magnetic resonance angiograms: an analysis of 212 cases. Interv Neuroradiol 19:461–5
Tanaka M, Sakaguchi M, Miwa K et al (2013) Basilar artery diameter is an independent predictor of incident cardiovascular events. Arterioscler Thromb Vasc Biol 33:2240–4
Toscano A, Schoser B (2013) Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J Neurol 260:951–9
Vlak MH, Algra A, Branderburg R et al (2011) Prevalence of unrupted intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 10:595–7
Wiebers DO (2006) Unruptured intracranial aneurysms: natural history and clinical management. Update on the international study of unruptured intracranial aneurysms. Neuroimaging Clin N Am 16:383–390
Yuan YJ, Xu K, Yu J (2014) Research progress on vertebrobasilar dolichoectasia. Int J Med Sci 11:1039–1048
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Antonio Toscano received from Genzyme-Sanofi grants and reimbursements for teaching courses and participation to meetings of Global Advisory Board for Pompe Disease. Federica Montagnese, Francesca Granata, Olimpia Musumeci, Carmelo Rodolico, Stefania Mondello, Emanuele Barca, Maria Cucinotta, Anna Ciranni and Marcello Longo declare that they have no conflict of interests.
Informed consent
All followed procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Details of the contributions of individual authors
All the authors have contributed to the present work either in its conception and design or in the analysis and interpretation of the data.
Additional information
Communicated by: Greg Enns
Rights and permissions
About this article
Cite this article
Montagnese, F., Granata, F., Musumeci, O. et al. Intracranial arterial abnormalities in patients with late onset Pompe disease (LOPD). J Inherit Metab Dis 39, 391–398 (2016). https://doi.org/10.1007/s10545-015-9913-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-015-9913-x