Abstract
Vestibular-evoked myogenic potentials (VEMPs) are short latency manifestations of vestibulo-ocular and vestibulocollic reflexes that originate from the utricle and saccule. Although cervical and ocular VEMPs have mostly been applied to peripheral vestibular disorders, the characteristics and the diagnostic values of VEMPs have been expanded to assess the function of the central otolithic pathways. In the central nervous system, the cervical VEMPs (cVEMPs) are mediated by the vestibular nuclei and uncrossed medial vestibulospinal tract descending in the lower brainstem and spinal cord. In contrast, the ocular VEMPs (oVEMPs) reflect the function of the vestibular nuclei and the crossed vestibulo-ocular reflex (VOR) pathways, mostly contained in the medial longitudinal fasciculus (MLF). Therefore, lesions involving the vestibular nuclei can present abnormalities of both cVEMPs and oVEMPs. The medullary lesions involving the descending MLF or the spinal accessory nucleus impair cVEMPs. In contrast, the lesions involving the MLF, the crossed ventral tegmental tract, oculomotor nuclei and the interstitial nucleus of Cajal can impair oVEMPs. Patients with unilateral cerebellar infarctions may show abnormal VEMPs especially when they have the ocular tilt reaction. Delayed responses of VEMPs are characteristic of multiple sclerosis (MS). Reduced VEMP responses can be observed in patients with vestibular migraine. VEMPs are useful in evaluating central as well as peripheral otolithic function that are not readily defined by conventional vestibular function tests, and can aid in detecting and localizing central lesions, especially silent brainstem lesions such as tiny infarctions or MS plaques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vestibular-evoked myogenic potentials (VEMPs) are now widely used to assess the function of otolithic pathways [1–3]. Cervical VEMPs (cVEMPs) are a manifestation of the vestibulocollic reflex (VCR) and involve measuring the electromyographic (EMG) activity from the tonically activated sternocleidomastoid (SCM) muscles in response to saccular stimulation [2]. VEMPs can also be recorded from the extraocular muscles using the surface electrodes placed over the inferior oblique and inferior rectus muscles, and is termed ocular VEMPs (oVEMPs) [4]. In contrary to the cVEMPs which are an uncrossed inhibitory vestibulospinal response, the oVEMPs represent a crossed excitatory vestibulo-ocular reflex (VOR) (Fig. 1) [5–7]. The VOR is mediated by the vestibular end organ receptors, the neurons in the vestibular nuclei, and the ocular motoneurons. Pathways carrying these signals mostly run in the MLF in the upper medulla and pons, but also in other ascending otolithic pathways such as the central ventral tegmental tract, ascending tract of Deiters and the ipsilateral vestibulo-thalamic tract from the vestibular nuclei to the upper brainstem and thalamus [5, 8].
Schematic illustration of the anatomic pathways involved in the generation of ocular and cervical vestibular-evoked myogenic potentials (VEMPs). Cervical VEMPs are the result of inhibitory postsynaptic potentials on the ipsilateral SCM motoneurons and are mediated by the descending medial vestibulospinal tract (VST) within the medial longitudinal fasciculus (MLF). And, the MLF from the upper medulla to the midbrain is the main pathway of the crossed otolith-ocular reflex responsible for ocular VEMPs. The utricular projections for the vestibulo-ocular function are directed more to the superior and medial nuclei while the saccular projections for the vestibulospinal function are more to the spinal and the lateral vestibular nuclei [12]. The mossy fiber arising from the superior and medial vestibular nuclei mediates projections from the utricle and saccule to the ipsilateral uvula and nodulus [12]. Regarding cVEMPs, saccular stimulation induces inhibitory potentials in the ipsilateral SCM while utricular stimulation evokes ipsilateral inhibitory and contralateral excitatory potentials [16]. However, the utricular contribution to the ipsilateral cVEMP responses has been assumed to be negligible (thin line). For oVEMPs, stimulation of the utricular nerve gives rise to strong activation of the ipsilateral superior oblique and contralateral inferior oblique muscles [14]. Saccular nerve stimulation, in contrast, produces no activation in the majority of extraocular motor neurons. CN III oculomotor nucleus, CN XI accessory nerve, F flocculus, MLF medial longitudinal fasciculus, MVST medial VST, Inf. VIII inferior division of the vestibular nerve, Sup. VIII superior division of the vestibular nerve, VN vestibular nuclei, S saccular macula, SCM sternocleidomastoids, U utricular macula or uvular in cerebellum, V vermis. (Blue line excitatory, red line inhibitory)
VEMPs have generally been regarded the tests for evaluating the function of the otolithic end organs and their afferents. Thus, those have mostly been applied to the disorders involving the peripheral vestibular system. However, VEMPs also can assess the function of central vestibulospinal and vestibulo-ocular pathways that include the vestibular fascicle and nuclei, the medial vestibulospinal tract (VST), and the medial longitudinal fasciculus (MLF). In addition, the cerebellum may affect VEMP responses by participating in the modulation of the otolithic signals. Therefore, due to possible damage to the vestibular fascicles, vestibular nuclei and their efferents, and cerebellum that are all involved in relaying and processing of the vestibular signals, central vestibular lesions may impair the VEMP responses along the descending (cVEMPs) and ascending (oVEMPs) tracts in the brainstem. Since the vestibulocollic (vestibulospinal) and VOR pathways diverge beyond the nerve root entry zone and the vestibular nuclei, both cVEMPs and oVEMPs would provide valuable information in localizing the central lesions when combined. Thus, a central lesion causing abnormal responses for both cervical and ocular VEMPs is likely to be localized to the vestibular nerve root entry zone or the vestibular nuclei [9, 10]. The cortical representation of saccular stimulation using cVEMPs involves the multisensory cortical network within both hemispheres including the posterior insular cortex, the middle and superior temporal gyri, and the inferior parietal cortex [11, 12].
In this review, we present an overview of basic peripheral physiology and cervical and ocular VEMP abnormalities in central vestibular disorders, and suggest the localizing and diagnostic value of VEMP testing in various central vestibular disorders.
Neural connections of the utricle and saccule
The semicircular canals sense angular acceleration of the head, whereas the otolith organs sense its linear acceleration. The otolith organ consists of two receptors: the saccular and utricular maculae, which are sensitive to vertical and horizontal linear acceleration, respectively. Both the utricular and saccular afferents reach the target neurons in the vestibular nuclei of the brainstem [12, 13]. Even though these otolithic projections are superimposed on each other, the saccular projections are mainly toward the lateral, particularly the spinal (inferior) vestibular nucleus and the superior vestibular nucleus while the utricular nerve projects toward the medial, the superior vestibular nucleus, and rostral portion of the spinal vestibular nucleus (Fig. 1). These arrangements are consistent with the saccular projection that plays a role in the VCR mediated by the saccular macule, inferior vestibular nerve, the medial VST from the medial, inferior, and lateral vestibular nuclei, and finally the motor neurons of the accessory spinal nuclei reaching the neck muscles. In contrast, the utricle contributes to the VOR that is transmitted through the MLF and other tracts in the dorsomedial brainstem. In monkeys, the principal cerebellar projection from the saccule is to the uvula with a less dense projection to the nodulus. In contrast, a strong projection from the utricle to the cerebellum is to the nodulus and weak projections are to the flocculus, paraflocculus, and uvula [12].
Selective stimulation of the utricular nerve in cats evokes excitatory postsynaptic potentials (EPSPs) at the ipsilateral lateral rectus motoneurons and small ocular counter-rotations [14, 15]. Utricular nerve stimulation also induces excitatory and inhibitory responses in the contralateral superior oblique motoneurons and the ipsilateral inferior oblique motoneurons which play a role in eye rotation during head tilt [15]. In cats, utricular stimulation evokes longer latency inhibitory postsynaptic potentials (IPSPs) in the contralateral extensor and flexor motoneurons [16] and in the SCM motoneurons, predominantly on the ipsilateral side [17]. In contrast, stimulation of the saccular nerve in cats induces no or only slight responses from the extraocular motoneurons in spite of the existence of a vertical VOR [18, 19]. Instead, the inhibitory disynaptic connections between the saccule and ipsilateral SCM motoneurons are strong enough to explain the underlying neural mechanism of cVEMPs in cats [20]. Therefore, these two sensory organs have rather different neural projections. There are strong projections of the utricular afferents to the oculomotor system and of the saccular projections to the cervical spinal neurons (Fig. 1).
Brainstem representation
cVEMPs
The topology of ischemic lesions for impaired cVEMPs was revealed in 29 patients with brainstem infarcts (Table 1) [11]. Using probabilistic lesion maps for unilaterally abnormal (12/29, 41.4 %) and normal cVEMPs (10/29, 34.5 %), the study showed that the lesions causing abnormal cVEMPs were mostly located in the areas of the vestibular nuclei and spinal accessory nerve in the lateral medulla oblongata. In the pons, the lesions were also frequently found in the anterolateral parts of the pyramidal tract fibers, and in the tegmental area of the pons, including the vestibular nuclei in a few patients. It is not surprising that lesions involving the vestibular nuclei at the level of the lateral lower pons or the spinal accessory nerve in the lateral medulla oblongata can impair cVEMPs (Fig. 2). However, more rostral brainstem lesions up to the mesencephalon may also impair cVEMPs [13, 21]. This suggests descending modulatory pathways for cVEMPs in the brainstem.
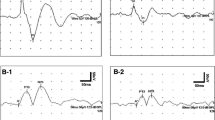
Cervical and ocular vestibular-evoked myogenic potentials (VEMPs) from representative cases of brainstem infarctions at different anatomical locations. a A patient with acute midbrain infarction does not generate ocular VEMPs during contralesional ear stimulation, in combination with a contraversive ocular tilt reaction and tilt of the subjective visual vertical. b A patient with isolated unilateral internuclear ophthalmoplegia (INO) shows abnormal ocular VEMPs induced by air-conducted tone bursts during stimulation of the contralesional ear but sparing of cervical VEMPs. c A patient with medial medullary infarction (MMI) shows abnormal cervical VEMPs in the lesion side probably due to disruption of the medial vestibulospinal tract (VST) descending within the medial longitudinal fasciculus (MLF). d A patient with isolated vestibular nuclear infarction in the right side shows decreased cervical VEMPs and absent response of ocular VEMPs during tone burst stimulation of the ipsilesional ear (right ear). e A patient with acute infarction involving the lateral medulla shows decreased amplitude of cervical VEMPs during stimulation of the ipsilesional ear while the oVEMPs were symmetric. The side of VEMPs is indicated according to the ear stimulated
In another study, cVEMPs were abnormal in all lower brainstem lesions with decreased or absent wave formations or increased latencies [22]. In contrast, in patients with upper brainstem infarction, cVEMPs were normal while auditory brainstem reflexes were abnormal. The authors concluded that testing of cVEMPs is useful for identifying lower brainstem lesions.
oVEMPs
More than a half (27/52, 52 %) of the patients with acute brainstem lesions showed abnormal oVEMPs induced by air-conducted tone burst sounds (ACS) (Table 1) [5]. Four of the five patients with acute midbrain lesions (80 %) also showed abnormal oVEMPs during contralesional ear stimulation, mostly in combination with a contraversive ocular tilt reaction (OTR) and contraversive tilt of the subjective visual vertical (Fig. 2). Of the 28 patients with acute pontine lesions, 16 (57.1 %) showed abnormal oVEMPs. Responses were also abnormal in 47 % of the patients with medullary strokes. The majority of patients with abnormal oVEMPs to ACS had lesions in the dorsomedial brainstem that contains the MLF, the crossed ventral tegmental tract, and the interstitial nucleus of Cajal.
Lateral medullary infarction (Wallenberg syndrome)
Due to damage of the vestibular fascicles and vestibular nuclei that are involved in relaying and central processing of the peripheral vestibular inputs, lateral medullary lesions may cause abnormal VEMPs. One study showed that cVEMPs were abnormal in 43 % (9/21) of patients with lateral medullary infarction (LMI) (Table 1) [23]. The cVEMP abnormalities included decreased amplitude or delayed responses in the ipsilesional (n = 5), contralesional (n = 2), or on both sides (n = 2) (Fig. 2) [23]. However, only one patient (4.7 %) exhibited a canal paresis. In contrast to the cVEMPs, caloric tests evaluate the ascending horizontal VOR originating from the horizontal semicircular canal [20]. Therefore, in LMI, topographical correlations could be made that caloric paresis is more likely linked to rostrally located infarctions while absent or delayed cVEMPs may indicate caudally located ones [24]. The contralesional or bilateral abnormalities of cVEMPs in patients with unilateral LMI were ascribed to disruption of commissural modulation between the vestibular nuclei [23]. Of the 19 patients with medullary lesions, patients with lateral medullary lesions (n = 14) showed abnormal oVEMPs less frequently than those with medial medullary lesions (4/14, 28.5 % vs. 5/5, 100 %) [5].
Recently, two patients with isolated vestibular nuclear infarction showed decreased or absent responses of both cervical and ocular VEMPs during tone burst stimulation of the ipsilesional ear [10]. They also exhibited spontaneous torsional-horizontal nystagmus that beat away from the lesion side, gaze-evoked nystagmus, ipsilesional canal paresis, and decreased head-impulse VOR gains for the horizontal and posterior semicircular canals on both sides, but more for the ipsilesional ones [10]. Thus, the lesions involving the vestibular nuclei may present features of both peripheral and central vestibulopathies including abnormal cVEMPs and oVEMPs during ipsilesional ear stimulation (Fig. 2).
Medial medullary infarction (MMI)
In about a half of the patients with MMI showed abnormal cVEMPs in the lesion side, and has been explained by disruption of the medial VST descending within the MLF (Fig. 2) [25]. The abnormal cVEMPs in patients with infarctions involving the medullary tegmentum support that cVEMPs are mediated by the medial VST descending within the MLF. Patients with abnormal cVEMPs frequently showed abnormal ocular motor findings, indicating that the lesions involved the medullary tegmentum in those patients.
Of the 19 patients with medullary lesions, all five with upper medial medullary lesions showed abnormal oVEMPs in response to ACS [5]. Three of them exhibited absent oVEMP formation only during contralesional ear stimulation while the other two had absent formation during stimulation of the ear on the lesion side or the ear on either side. These findings indicate a possible decussation of the otolithic fibers for oVEMPs in the upper medulla.
Internuclear ophthalmoplegia (INO)
One study evaluated 13 patients with internuclear ophthalmoplegia (INO) due to multiple sclerosis (MS) to determine whether oVEMPs are mediated by the MLF (Table 1) [26]. The study found that oVEMPs induced by ACS were more frequently abnormal (69 %) than cVEMPs (8 %). The oVEMPs in these patients were usually absent (13/26 ears) or delayed (5/26 ears) in 18 of the 26 ears (69.2 %). Another study found abnormal oVEMPs induced by ACS in 8 (67 %) of the 12 patients with isolated unilateral INO due to brainstem infarction [21]. In contrast, cVEMPs were abnormal in only 3 patients (25 %) (Fig. 2). The occasional abnormality of cVEMPs in these patients suggests a modulatory pathway for the inhibitory sacculocollic reflex descending in the MLF [21]. A recent study also showed absent oVEMPs in response to ACS during contralesional ear stimulation in all 7 patients with unilateral INO and no wave formation on both sides in another patient with bilateral INO [5]. Two patients with one-and-a-half syndrome exhibited absent or delayed responses of oVEMPs only during contralesional ear stimulation. All these findings support that oVEMPs are mediated by the fibers ascending in the MLF.
Cerebellar representation
The cerebellum has reciprocal connections with the vestibular nuclei and plays a major role in balance control [27]. The saccular nerve has a strong projection to the uvula and nodulus, and the utricular nerve to the nodulus, uvula, flocculus/paraflocculus, and bilateral fastigial nuclei [12, 28]. In monkeys, the cerebellar vermis and flocculus have direct and indirect projections to the vestibular nuclei [29]. In general, the flocculus is important for control of the angular VOR, while the nodulus and uvula are mainly involved in the control of otolith-related reflexes [30]. Therefore, in addition to conventional neurotologic tests, evaluation of VEMPs can provide valuable information for understanding the pathomechanism of cerebellar disorders.
cVEMPs
Cerebellar lesions in the non-AICA (anterior inferior cerebellar artery) territories have generally been known to spare cVEMP responses (Table 1) [31, 32]. In a previous study, the mean p13 latencies and peak-to-peak amplitudes did not differ between the patients with cerebellar stroke and controls [32]. There were no differences in the latency or amplitude between the ipsi- and contralesional sides either. However, in AICA territory infarctions, the abnormal cVEMP responses can be explained by concomitant inner ear involvements [33]. The cVEMPs induced by click sounds were recorded in 16 patients with unilateral AICA territory infarction, and 50 % of them exhibited abnormal responses including absent or decreased amplitude on the side of infarction [33]. Patients with abnormal cVEMPs more frequently had canal paresis or sensorineural hearing loss compare to those with normal cVEMPs. This suggests that abnormal cVEMPs might result from ischemia to the peripheral vestibular system from the root entry zone of the eight nerves to the inner ear [33]. Twenty-seven patients with acute unilateral cerebellar infarctions were subjected to cVEMPs in response to ACS and oVEMPs induced by bone-conducted vibration [34]. In this study, the patients with the OTR showed abnormal VEMPs more frequently than those without (11/15 vs. 3/12, respectively). This study also revealed frequent abnormalities of cervical (11/27, 41 %) and ocular (9/27, 33 %) VEMPs in patients with unilateral cerebellar infarction. However, the absence of correlation in the directionality between the OTR and VEMP abnormalities suggests either deactivation or disinhibition as the cerebellar role in otolithic modulation [34].
In patients with cerebellar ataxia and bilaterally impaired head-impulse tests, cVEMPs were abnormal in 71 % (22/31), reduced amplitude in 18 and absent responses in 7, either bilaterally (n = 13) or unilaterally (n = 9) [35]. In this report, voxel-based morphometry revealed a cerebellar atrophy in both the vermis and cerebellar hemispheres, especially in the flocculi on both sides, which could have contributed to abnormal vestibulocollic responses [35]. Studies of the patients with spinocerebellar degeneration including olivo-ponto-cerebellar atrophy (OPCA, n = 10), cortical cerebellar atrophy (CCA, n = 3), and Machado–Joseph disease (MJD, n = 3) showed that patients with OPCA and CCA had well-preserved cVEMPs while two of the three patients with MJD had abnormal results [36]. Some cerebellar patients may show dissociation between canal and otolith dysfunction [37].
oVEMPs
It remains unclear whether the cerebellum has any influence on the oVEMP responses. However, damage to the cerebellum has been implicated as a cause of skew deviation and OTR which have been attributed to asymmetric modulation of the vestibular projections from the receptors of the utricle [38]. The primary utricular afferents have strong direct projections to the vestibular nuclei, the cerebellar nodulus, and ventral uvula and weaker projections to the anterior vermis, the fastigial nuclei, and the flocculus and ventral paraflocculus [12]. Lesions of the nodulus or uvula may affect the otolith-ocular reflexes and oVEMPs [30]. A study found absent oVEMPs in patients with cerebellar lesions extending into the brainstem, but normal responses in patients with isolated cerebellar lesions [39]. Abnormal VEMPs in isolated cerebellar lesions suggest a ‘modulatory role’ of the cerebellum on the otolithic function, or some cases of technically abnormal VEMPs, or perhaps the lesions larger than they appear on imaging.
Multiple sclerosis
Patients with MS frequently report dizziness and disequilibrium related to vestibular dysfunction in the course of their illness [40]. Although they do not always show brainstem or cerebellar lesions on imaging studies, the vertigo and imbalance mostly arise from structural or functional involvement of the vestibular system. Patients with lesions involving the VOR and vestibulospinal reflex (VSR) pathways may show various degrees of functional impairment. Therefore, investigations of cervical and ocular VEMPs are useful in assessing the VOR and VSR pathways in MS patients.
cVEMPs
Three patients with definite MS and MRI lesions involving the VST showed a prolonged latency of cVEMPs (Table 2) [41]. This could be attributed to demyelination of either the primary afferent axons at the root entry zone or the secondary VST axons rather than to the lesions involving the vestibular nucleus neurons. Another study of 40 MS patients found abnormal cVEMPs in 28 patients (70 %) [42]. In 24 (85.7 %) of them, the cVEMPs were delayed on one or both sides, and were not generated at all on one side in the remaining 4 patients. These results were concordant with the clinical findings of brainstem involvement in 55 % and with the abnormal MRI findings in 65 % of the cases [42]. In contrast to most previous studies with MS patients that showed increased latencies of cVEMPs, one retrospective study involving 70 MS patients described abnormal cVEMPs in 31 % of them, but increased latencies only in 11.8 %, and reduced peak-to-peak amplitude or absent responses in 19.1 % [43]. The absent cVEMPs might have been caused by axonal damage or severe loss of the myelin sheaths.
A previous study attempted to determine whether VEMPs are useful in detecting “silent” demyelinating lesions involving the brainstem in MS patients [44]. The study found that only those with a history or symptoms/signs of brainstem dysfunction showed abnormal cVEMPs while those without did not show differences in the VEMP findings compared to normal controls [44]. The abnormalities were mostly delayed responses (p < 0.001) rather than reduced amplitudes. Overall, a number of studies showed abnormal cVEMPs in 31–70 % of patients with definite MS [42–46]. Delayed responses were the most frequent abnormality but showed little correlation with the radiological findings in most studies [42–46].
oVEMPs
Regarding oVEMPs, prolonged latency was found in 30 % and absent response in 40 % of patients on at least one side in a study of 30 MS patients [47]. The mean latencies of oVEMPs and cVEMPs were significantly increased in MS patients, and also correlated with the expanded disability status scale (EDSS) [47]. Another study of 62 MS patients found a higher prevalence of abnormal oVEMPs than abnormal cVEMPs (45.2 vs. 17.7 %, p < 0.01) [48]. In this study, the abnormalities were mostly increased latencies rather than reduced amplitudes. Although correlations with clinical or MRI evidence of brainstem involvements were not significant, both ocular (p < 0.05) and cervical VEMP latencies (p < 0.01) were significantly correlated with EDSS. These results appear to indicate the possibility of asymptomatic or pre-radiological involvement of the vestibulocollic or VOR pathways in MS patients. Another study found a higher frequency of abnormalities in oVEMP (69 %) than in cVEMPs (8 %) in patients with INO mostly due to MS [26]. These findings may be ascribed to the lesions mostly located in the upper brainstem.
Increased latency of VEMPs, however, is not specific for MS, and does not help distinguishing MS from other etiologies because prolongation of the latency has been reported in other pathologies affecting the brainstem, such as strokes and tumors [49].
Vestibular migraine
Patients with acute vestibular migraine (VM) may show clinical signs suggestive of central as well as peripheral vestibular dysfunction [50].
cVEMPs
One study showed that cVEMP amplitudes were reduced in 43 (68 %) of the 63 patients with VM while the latencies were within normal range (Table 2) [51]. In this study, the reduced cVEMP amplitudes in the presence of normal latencies suggest hypoperfusion-induced ischemia or serotonergic-induced extravasation of the inner ear affecting the saccule. They also compared the findings of cVEMPs induced by ACS between the patients with VM and Meniere disease (MD) to determine electrophysiological differences between the two disorders (Table 2) [52]. Similar prevalence of reduced cVEMP amplitude was found between the patients with VM (68 %, 43/63) and MD (69 %, 11/16), but the latencies were within normal range [52]. These results suggest that the saccule or its afferent pathway may be affected in both disorders, and probably due to labyrinthine pathogenesis.
Compared to ordinary migraineurs and healthy controls, VM patients frequently show absence of cVEMP responses (16/37, 44 % in VM, vs. 8/32, 25 % in the migraineurs vs. 3/30, 3.3 % in the controls) even though the sound intensity thresholds and latencies of cVEMPs were similar among the groups [53] (Table 2).
oVEMPs
Even though both VM and MD groups showed significantly reduced cVEMP and oVEMP amplitudes in recent studies [52, 54], there were some differences in the result of VEMPs in response to different stimulus modalities [54, 55]. During stimulation with click-evoked sounds, the amplitudes of both cVEMPs and oVEMPs were significantly reduced in both VM and MD groups compared with controls [54]. In contrast, the VM group did not show a difference in oVEMPs induced by tone burst sounds while patients with MD showed a significant reduction in oVEMP amplitudes. However, when stimulated with bone-conducted vibration (BCV) using a tendon hammer or a Mini-Shaker at the midline forehead, the amplitude of oVEMPs did not show any differences between the VM and MD groups [54]. Another study did not show any difference in the amplitudes or symmetry of oVEMPs among the controls, VM and MD either [55]. These results suggest that, compared to MD, VM may show fewer abnormalities of both cVEMPs and oVEMPs in response to click sounds and cVEMPs induced by minitaps. Therefore, although VM and MD behave similarly on most of the VEMP tests, further studies adopting different stimuli are required to resolve this issue in larger number of patients.
Cortical representation
The significant overlap of utricular and saccular afferents in the vestibular nuclei suggests a convergence of these inputs for functional integration of the vestibular reflexes [12]. The cortical representation of the semicircular canals or the entire vestibular nerve has been demonstrated using caloric activation and galvanic stimulation [56, 57].
The ascending cortical projections of the otoliths were demonstrated with cVEMPs induced by ACS using fMRI [58, 59]. These studies found a significant activation in the multisensory cortical vestibular network within both hemispheres, including the posterior insular cortex, the middle and superior temporal gyri, and the inferior parietal cortex [58]. The activation was bilateral with a predilection for the right hemisphere in right-handers and with a predominant ipsilateral projection. These patterns of cortical activation are very similar to those with galvanic and caloric stimulations [56, 57, 60]. This indicates that the semicircular canal and otolith inputs may converge at the brainstem level, e.g., within the vestibular nuclei, and reach the vestibular cortical areas as integrated information.
However, we should interpret the VEMP results with caution since: (1) Much of the data are from studies using ACS to stimulate the vestibular receptors. Several control studies on healthy individuals have shown the high false positive abnormal rate of VEMPs in response to ACS, whereas they do have clear responses to BCV [61]. (2) Most studies do not have an age-matched control group, and the VEMP amplitudes were not normalized. (3) Most studies involved measuring VEMPs in a small group of non-randomly selected patients with a specific diagnosis (e.g., MS) or lesion site (e.g., brainstem), which make it impossible to extrapolate the sensitivity and specificity of particular VEMP abnormalities for a specific diagnosis. (4) Definitions of the abnormalities regarding the latency and amplitude are not clear. (5) Lack of standardization of VEMP techniques and analysis makes it difficult to compare the findings among the studies. To improve consistency of the recordings within and among the laboratories, standardized minimum requirements and guidelines are required for proper recording and interpretation of VEMPs [62].
In conclusion, cervical and ocular VEMPs appear to be effective in evaluating the function of central vestibular pathways, especially in the patients with brainstem or cerebellar lesions. Cervical VEMPs provide a valuable tool for investigating the integrity of the vestibulo (sacculo)-spinal pathways [11], while oVEMPs reflect the function of the vestibulo (utriculo)-ocular pathways (Fig. 1) [5]. Combined evaluation of ocular and cervical VEMPs permits assessment of both ascending and descending vestibular pathways in the brainstem and should be considered an important neurophysiological tool for investigating the central vestibulopathies. These evoked potentials may be even useful in detecting central vestibulopathies that are not readily defined by conventional vestibular function tests. However, we still await well-designed studies with standardization of the measurements and analyses, and with age-matched normal controls.
References
Colebatch JG, Halmagyi GM (1992) Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology 42(8):1635–1636
Colebatch JG, Halmagyi GM, Skuse NF (1994) Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 57(2):190–197
Curthoys IS (2010) A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol 121(2):132–144
Todd NP et al (2007) Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol 118(2):381–390
Oh SY et al (2013) Ocular vestibular evoked myogenic potentials induced by air-conducted sound in patients with acute brainstem lesions. Clin Neurophysiol 124(4):770–778
Oh SY et al (2013) Cervical and ocular vestibular-evoked myogenic potentials in vestibular neuritis: comparison between air- and bone-conducted stimulation. J Neurol 260(8):2102–2109
Iwasaki S et al (2007) Head taps evoke a crossed vestibulo-ocular reflex. Neurology 68(15):1227–1229
Buttner-Ennever JA (1992) Patterns of connectivity in the vestibular nuclei. Ann NY Acad Sci 656:363–378
Rosengren SM et al (2007) Delayed vestibular evoked responses to the eyes and neck in a patient with an isolated brainstem lesion. Clin Neurophysiol 118(9):2112–2116
Kim HJ et al (2014) Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol 261(1):121–129
Heide G et al (2010) Brainstem representation of vestibular evoked myogenic potentials. Clin Neurophysiol 121(7):1102–1108
Newlands SD et al (2003) Central projections of the saccular and utricular nerves in macaques. J Comp Neurol 466(1):31–47
Nyberg-Hansen R (1964) Origin and termination of fibers from the vestibular nuclei descending in the medial longitudinal fasciculus. An experimental study with silver impregnation methods in the cat. J Comp Neurol 122:355–367
Uchino Y et al (1994) Monosynaptic and disynaptic connections in the utriculo-ocular reflex arc of the cat. J Neurophysiol 71(3):950–958
Uchino Y et al (1996) Utriculoocular reflex arc of the cat. J Neurophysiol 76(3):1896–1903
Uchino Y, Kushiro K (2011) Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci Res 71(4):315–327
Kushiro K et al (1999) Saccular and utricular inputs to sternocleidomastoid motoneurons of decerebrate cats. Exp Brain Res 126(3):410–416
Isu N et al (2000) Sacculo-ocular reflex connectivity in cats. Exp Brain Res 131(3):262–268
Goto F et al (2004) Eye movements evoked by selective saccular nerve stimulation in cats. Auris Nasus Larynx 31(3):220–225
Uchino Y et al (1997) Sacculocollic reflex arcs in cats. J Neurophysiol 77(6):3003–3012
Kim HJ, Lee JH, Kim JS (2014) Ocular vestibular evoked myogenic potentials to head tap and cervical vestibular evoked myogenic potentials to air-conducted sounds in isolated internuclear ophthalmoplegia. Clin Neurophysiol 125(5):1042–1047
Itoh A et al (2001) Clinical study of vestibular-evoked myogenic potentials and auditory brainstem responses in patients with brainstem lesions. Acta Otolaryngol Suppl 545:116–119
Kim S, Kim HJ, Kim JS (2011) Impaired sacculocollic reflex in lateral medullary infarction. Front Neurol 2:8
Tseng CL, Young YH (2010) Topographical correlations of lateral medullary infarction with caloric- and vestibular-evoked myogenic potential results. Eur Arch Otorhinolaryngol 267(2):191–195
Kim S, Lee HS, Kim JS (2010) Medial vestibulospinal tract lesions impair sacculo-collic reflexes. J Neurol 257(5):825–832
Rosengren SM, Colebatch JG (2011) Ocular vestibular evoked myogenic potentials are abnormal in internuclear ophthalmoplegia. Clin Neurophysiol 122(6):1264–1267
Highstein SM, Holstein GR (2006) The anatomy of the vestibular nuclei. Prog Brain Res 151:157–203
Newlands SD et al (2002) Central projections of the utricular nerve in the gerbil. J Comp Neurol 452(1):11–23
Ito M (1982) Cerebellar control of the vestibulo-ocular reflex–around the flocculus hypothesis. Annu Rev Neurosci 5:275–296
Angelaki DE, Hess BJ (1995) Lesion of the nodulus and ventral uvula abolish steady-state off-vertical axis otolith response. J Neurophysiol 73(4):1716–1720
Welgampola MS, Colebatch JG (2005) Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology 64(10):1682–1688
Pollak L, Kushnir M, Stryjer R (2006) Diagnostic value of vestibular evoked myogenic potentials in cerebellar and lower-brainstem strokes. Neurophysiol Clin 36(4):227–233
Ahn BH et al (2011) Abnormal cervical vestibular-evoked myogenic potential in anterior inferior cerebellar artery territory infarction: frequency, pattern, and a determinant. J Neurol Sci 307(1–2):114–119
Choi SY et al (2014) Impaired modulation of the otolithic function in acute unilateral cerebellar infarction. Cerebellum 13(3):362–371
Kirchner H et al (2011) Clinical, electrophysiological, and MRI findings in patients with cerebellar ataxia and a bilaterally pathological head-impulse test. Ann NY Acad Sci 1233:127–138
Takegoshi H, Murofushi T (2000) Vestibular evoked myogenic potentials in patients with spinocerebellar degeneration. Acta Otolaryngol 120(7):821–824
Marti S et al (2008) Dissociation between canal- and otolithfunction in cerebellar atrophy. J Neurol 255(5):769–771
Rabinovitch HE, Sharpe JA, Sylvester TO (1977) The ocular tilt reaction. A paroxysmal dyskinesia associated with elliptical nystagmus. Arch Ophthalmol 95(8):1395–1398
Su CH, Young YH (2011) Differentiating cerebellar and brainstem lesions with ocular vestibular-evoked myogenic potential test. Eur Arch Otorhinolaryngol 268(6):923–930
Williams NP, Roland PS, Yellin W (1997) Vestibular evaluation in patients with early multiple sclerosis. Am J Otol 18(1):93–100
Shimizu K et al (2000) Vestibular evoked myogenic potentials in multiple sclerosis. J Neurol Neurosurg Psychiatry 69(2):276–277
Alpini D et al (2004) Vestibular evoked myogenic potentials in multiple sclerosis: clinical and imaging correlations. Mult Scler 10(3):316–321
Versino M et al (2002) Vestibular evoked myogenic potentials in multiple sclerosis patients. Clin Neurophysiol 113(9):1464–1469
Bandini F et al (2004) The diagnostic value of vestibular evoked myogenic potentials in multiple sclerosis. J Neurol 251(5):617–621
Eleftheriadou A et al (2009) The diagnostic value of earlier and later components of vestibular evoked myogenic potentials (VEMP) in multiple sclerosis. J Vestib Res 19(1–2):59–66
Colebatch JG (2012) Vestibular evoked myogenic potentials in multiple sclerosis. Clin Neurophysiol 123(9):1693–1694
Gabelic T et al (2013) Ocular and cervical vestibular evoked myogenic potentials in patients with multiple sclerosis. J Clin Neurophysiol 30(1):86–91
Gazioglu S, Boz C (2012) Ocular and cervical vestibular evoked myogenic potentials in multiple sclerosis patients. Clin Neurophysiol 123(9):1872–1879
Murofushi T et al (2001) Diagnostic value of prolonged latencies in the vestibular evoked myogenic potential. Arch Otolaryngol Head Neck Surg 127(9):1069–1072
von Brevern M et al (2005) Acute migrainous vertigo: clinical and oculographic findings. Brain 128(Pt 2):365–374
Baier B, Stieber N, Dieterich M (2009) Vestibular-evoked myogenic potentials in vestibular migraine. J Neurol 256(9):1447–1454
Baier B, Dieterich M (2009) Vestibular-evoked myogenic potentials in “vestibular migraine” and Meniere’s disease: a sign of an electrophysiological link? Ann NY Acad Sci 1164:324–327
Boldingh MI et al (2011) Vestibular sensitivity in vestibular migraine: VEMPs and motion sickness susceptibility. Cephalalgia 31(11):1211–1219
Zuniga MG et al (2012) Can vestibular-evoked myogenic potentials help differentiate Meniere disease from vestibular migraine? Otolaryngol Head Neck Surg 146(5):788–796
Taylor RL et al (2012) Vestibular evoked myogenic potentials to sound and vibration: characteristics in vestibular migraine that enable separation from Meniere’s disease. Cephalalgia 32(3):213–225
Stephan T et al (2005) Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage 26(3):721–732
Fasold O et al (2002) Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage 17(3):1384–1393
Schlindwein P et al (2008) Cortical representation of saccular vestibular stimulation: VEMPs in fMRI. Neuroimage 39(1):19–31
Bremmer F et al (2001) Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29(1):287–296
Bense S et al (2001) Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol 85(2):886–899
Cheng PW et al (2009) Acoustic, mechanical and galvanic stimulation modes elicit ocular vestibular-evoked myogenic potentials. Clin Neurophysiol 120(10):1841–1844
Papathanasiou ES et al (2014) International guidelines for the clinical application of cervical vestibular evoked myogenic potentials: an expert consensus report. Clin Neurophysiol 125(4):658–666
Acknowledgments
This work was supported by research funds of Chonbuk National University in 2015 and by Research Institute of Clinical Medicine of Chonbuk National University-Chonbuk National University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Oh is supported by research funds of Chonbuk National University in 2014 and by Research Institute of Clinical Medicine of Chonbuk National University- Chonbuk National University Hospital. HJ Kim reports no disclosures. JS Kim serves as an Associate Editor of Frontiers in Neuro-otology and on the editorial boards of the Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, Journal of Vestibular Research, and Journal of Neurology.
Rights and permissions
About this article
Cite this article
Oh, SY., Kim, HJ. & Kim, JS. Vestibular-evoked myogenic potentials in central vestibular disorders. J Neurol 263, 210–220 (2016). https://doi.org/10.1007/s00415-015-7860-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7860-y