Abstract
Weight loss is increasingly considered as a negative prognostic marker in amyotrophic lateral sclerosis (ALS). Despite the critical importance of nutritional issues in ALS, and the common use of percutaneous endoscopic gastrostomy (PEG), there is a general lack of knowledge on peri-interventional treatment, optimal parameters of enteral nutrition, its timing during disease progression and its potential disease-modifying effects in ALS patients. Here we report the results of a multi-center prospective study of percutaneous endoscopic gastrostomy (PEG) in ALS. In this observational clinical trial, 89 ALS patients were prospectively enrolled over a 3-year period and longitudinal data were collected over 18 months. PEG was a safe procedure even in patients with low forced vital capacity, and prophylactic single-shot antibiosis as well as slow increase of caloric nutrition via PEG was beneficial to avoid complications. No signs of refeeding syndrome were observed. High-caloric intake (>1,500 kcal/d) via PEG in patients that lived at least 12 months after PEG insertion was correlated with prolonged survival. Additional oral food intake was not associated with a worse prognosis. Our results suggest that peri-interventional PEG management should include prophylactic single-shot antibiosis, slow increase of caloric intake, and long-term high-caloric nutrition. Although our results indicate that PEG might be more beneficial when applied early, we believe that it can also be performed safely in patients with far advanced disease. Because of its explorative and observational character, most of our results have to be confirmed by a randomized interventional trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is commonly associated with rapid weight loss as the result of multiple causes including dysphagia, but also increased metabolism [1, 2]. Metabolic alterations in ALS patients precede motor symptoms as shown by two long-term prospective studies [3, 4], but their underlying mechanisms remain obscure. Importantly, multiple retrospective studies have observed positive correlations between survival and either pre-morbid body mass index (BMI) [5], smaller weight loss at disease onset [6], or circulating lipids [7, 8]. Consistently, animal studies support the notion that high-caloric intake and weight gain in ALS patients could increase survival [9].
Nutritional management of ALS patients generally includes enteral nutrition, through percutaneous endoscopic gastrostomy (PEG) or radiologically inserted gastrostomy (RIG). There are several studies suggesting that PEG might be subject to more frequent complications than RIG [10, 11], but this point remains controversial. Current guidelines suggest proposing enteral nutrition to patients on an individual basis, mostly in case of prominent bulbar symptoms and/or loss of more than ten percent of initial body weight [12]. PEG is currently considered to be at higher risk in patients with low forced vital capacity [13], although this notion was based mostly on one retrospective analysis of two clinical trials [14]. In general, definitive evidence with respect to timing of enteral nutrition during disease progression is lacking. Another critical unresolved question is whether enteral nutrition should be performed with an isocaloric diet or by increasing caloric intake (hypercaloric diet). So far, there has been only one recent clinical trial which observed increased survival of ALS patients under PEG with hypercaloric diet as compared with isocaloric diet [15]. Moreover, little is known about the best peri-procedural treatment of ALS patients, including antibiotic treatment, patterns of adapting patients to PEG nutrition, and whether or not small amounts of additional oral food intake are acceptable. Thus, there appears to be a general lack of knowledge on enteral nutrition, its timing during disease progression, its proper execution, and its potential disease-modifying effects despite its very common use in ALS patients.
To further clarify these questions, we generated a prospective multi-center register of PEG in ALS patients. The aim of this study was to prospectively analyze factors associated with outcome after PEG insertion. Since this is a purely observational study without a specific hypothesis or a control group, all results have to be regarded as descriptive. However, we believe that we discovered new aspects which will help to design future interventional trials which will directly affect the treatment of ALS patients with PEG.
Methods
Study design and participants
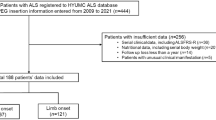
This study reports the results of a multi-center study for ALS patients with PEG, initiated by the German MND network which comprises specialized ALS centers from Germany and one center (St. Gallen) from Switzerland (Table 1). Because of the explorative character, the complexity of the research issue, and since there is no generally accepted standard of care, the study was designed as a register to collect as many data as possible and to analyze the current practice rather than providing a specific hypothesis. For this purpose we allowed every participating center to conduct its own established peri-interventional setups and guidelines and carefully recorded center-specific aspects. Over a time period of 3 years (October 2010 until October 2013) we subsequently included 89 patients in the register. The differences between the centers regarding the number of included patients are mainly explained by varying catchment areas and levels of specialization and therefore unequal total numbers of patients. The study was conducted in concordance with the STROBE checklist for cohort studies [16].
ALS patients with an indication for PEG as evaluated by the participating centers were included. Apart from PEG indication, inclusion criteria were the diagnosis of possible, probable laboratory-supported, probable, or definite ALS by El Escorial criteria [17], and provision of informed consent by the participant or an authorized caregiver. There were no exclusion criteria.
After obtaining informed consent the patients were included in the register by generating a computer-encoded pseudonym number. For input and storage of data we used an internet-based, password protected platform provided by 2mt software GmbH, Ulm. The software included an automatic plausibility check; additionally, all data were monitored in Ulm. In case of ambiguities, queries were sent using the same abovementioned platform.
Procedures and outcomes
Baseline investigation (V0) was conducted one to 5 days prior to PEG and included individual data, medical history, medication, medical indicators for PEG, body weight, BMI, ALS functional rating scale revised (ALS-FRS-r) [18], forced vital capacity (FVC), laboratory data (hemoglobin, leukocytes, thrombocytes, sodium, potassium, calcium, magnesium, phosphate, creatinine, urea, CK, glucose, ALT, AST, GGT, cholesterol, CRP), and arterial blood analysis (pH, pO2, pCO2, pHCO3–, BE).
All investigations were repeated one to 3 days after PEG when patients were still in hospital (post-PEG investigation, V1). Additionally, PEG specific data (use of antibiotics before and after PEG, anesthesia, and schemes of PEG nutrition), adverse events, and complications were recorded.
Patients were seen every 3 months (V2, V3, up to V7) in the outpatient clinics. In addition to the data above, patients were asked about their PEG usage (daily caloric intake, additional oral feeding, and frequency of PEG nutrition). In case of patient’s death the date and circumstances were recorded. To minimize the risk of selection bias, we performed a post-study survey to assure that all PEG patients who suffered from severe adverse events had been entered into our register.
A web-based electronic data system was used to record all study data in a standardized manner.
Statistical analysis
Statistical analysis included the effect of age, sex, ALS-FRS-r, BMI, FVC, and daily caloric intake on survival using Kaplan–Meier product-limit method and generalized Wilcoxon test. As thresholds for group distribution of quantitative variables we either used commonly accepted values or, if not applicable, we built groups of approximate equal numbers. Patients lost to follow-up or still alive at the end of the study were censored. We tried to complete missing electronic data by contacting the corresponding center. If the request was not successful, missing data were accordingly labeled in the statistics. To control for confounding variables we performed multivariate analysis. For comparison of mean values we used T Test. To compare the distribution of independent samples non-parametric tests (Mann–Whitney U and Kruskal–Wallis) were performed. The level of significance was set at p = 0.05. For statistical analyses SPSS Statistics 21 (IBM) was used.
Role of the funding source
The sponsors had no role in study design, data collection, data analysis, data interpretation, or writing of the report. JD had access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Recruitment and follow-up
Over a time period of 3 years (November 2010 until November 2013) we included 89 patients (43 male, 46 female, age 63.1 ± 10.0 years) from nine centers in the register. The number of recruited patients ranged from one (Bochum, Wiesbaden) to 17 (Hannover) with a mean of 9.9 ± 5.6. Table 1 shows distribution of patients per center, number of patients per visit, and patient’s characteristics. Reasons for missing longitudinal data included death, loss to follow-up, and termination of study. Follow-up time was up to a maximum of 18 months (V7); average follow-up time was 4.49 ± 4.74 months. In total 311 study visits were conducted (3.49 ± 1.55 per patient).
Peri-procedural practice
Indication criteria for PEG as communicated by the according centers were severe dysphagia with risk of aspiration (N = 31, 35.6 %), loss of body weight (N = 23, 26.4 %), or both (N = 33, 37.9 %).
Information about antibiotic treatment was present for 77 patients. Antibiotic prophylaxis (single-shot) before PEG was given to 61 patients (79.2 %), 16 patients (20.8 %) had no antibiotic treatment. Antibiotic substances included ceftriaxone (N = 33, 42.9 %), cefuroxime (N = 17, 22.1 %), metronidazole (N = 5, 6.5 %), amoxicillin (N = 3, 3.9 %), tazobactam (N = 1, 1.1 %), levofloxacin (N = 1, 1.1 %), and ampicillin (N = 1, 1.1 %). Local anesthesia was used for 65 patients (73.0 %), 20 patients (22.5 %) received general anesthesia; no information was given for 4 patients (4.5 %).
The time of first application of food via PEG was 1 day (N = 56, 74.7 %), 2 days (N = 16, 21.3 %), or 3 days (N = 3, 4.0 %) after PEG insertion; no information was given for 14 patients (14.7 %). Caloric intake was increased every 2 days (N = 54, 70.1 %), 3 days (N = 20, 26.0 %), or 4 days (N = 3, 3.9 %), and the increase of caloric intake ranged from 37.5 kcal/d to 450 kcal/d with a mean of 143.5 ± 76.9 kcal/d. No information was given for 12 patients (13.5 %).
Adverse events and complications
Peri-interventional mortality was 1/89 (1.1 %). This patient had severe aspiration pneumonia prior to PEG and died from respiratory failure 8 days after PEG. Procedure-related mortality was 0 %. Minor adverse events and complications were present in 82/89 patients (92.1 %, Table 2). One more severe complication (abscess with need of surgical revision) was reported in 1/89 patients (1.1 %). During further longitudinal observation, no PEG-associated mortality (0 %) was reported. Minor adverse events were present in 24/32 patients (75.0 %, Table 2). There were reports of more severe adverse events in 4/32 patients (12.5 %), including one abscess with need of surgical revision, one severe peritonitis, and two keloids with the need of multiple minor interventions.
Laboratory changes
PEG procedure was associated with a number of laboratory alterations (Table 3). Significants were decrease of hemoglobin (p < 0.0001), thrombocytes (p = 0.002), urea (p = 0.003), albumin (p = 0.004), and CK (0.002) as well as increase of leucocytes (p = 0.032) and CRP (p < 0.0001). Blood gas analysis showed a significant decrease of pO2 (p = 0.017).
Antibiotic Treatment
Patients who received a prophylactic single-shot antibiosis with cefuroxime (1.53 ± 2.67 mg/l) or ceftriaxone (11.34 ± 22.98 mg/l; p = 0.002; Fig. 1) had significantly lower CRP values than patients without antibiosis (16.31 ± 36.45; p = 0.001). On a clinical level, both patients with peritonitis received no prophylactic antibiosis; the patient with abscess received ceftriaxone.
Caloric intake
Patients who underwent a slower increase rate of caloric intake (≤200 kcal/d) reported significantly less feeling of fullness (N = 10/28; 35.7 %) than patients who received a higher increase rate of caloric intake (>200 kcal/d; N = 15/17, 88.2 %; p = 0.001). There were no clinical or laboratory signs of refeeding syndrome; electrolytes including potassium, calcium, magnesium, and phosphate remained stable before and after PEG (Table 3).
Body weight
Difference of body weight after 3 months was 0 ± 3.8 kg and after 6 months −1.3 ± 5.6 kg overall. There was no significant difference in body weight means before, 3 months after, and 6 months after PEG, indicating a stabilization of body weight in most patients (Fig. 2). After 3 months, differences in body weight ranged from −12 to +6 kg, and after 6 months from −15 to +15 kg.
PEG usage
After three months, patients reported that they used the PEG once a day (N = 3, 8.1 %), twice a day (N = 7, 18.9 %), three times a day (N = 12, 32.4 %), four times a day (N = 6; 16.2 %), five times a day (N = 3, 8.1 %), six times a day (N = 3, 8.1 %), or never (N = 3, 8.1 %). Additional intake of water via PEG ranged from 0 to 2.3 liters with a mean of 953 ± 515 ml. 12 patients (37.5 %) reported that they did not have any additional oral intake of food, 12 patients (37.5 %) had minor additional oral food intake for taste purposes, and eight patients (25 %) had predominant or exclusive oral food intake.
Survival
Overall survival from time of PEG insertion was 18.95 ± 1.64 months. Less overall weight loss (<5 kg; p = 0.025), and higher cholesterol serum levels (>220 mg/dl; p = 0.03) at time of PEG intervention were associated with significantly better survival; BMI, ALS-FRS-r, FVC, age, and sex as well as serum laboratory values had no significant impact on survival (Fig. 3). Overall we found no difference in survival (p > 0.05) for patients who received high-caloric nutrition (≥1,500 kcal/d) compared to patients with lower calorie intake (<1,500 kcal/d). Within a subgroup of patients who survived at least 12 months after PEG, survival was significantly prolonged (23.6 ± 3.8 vs. 21.1 ± 2.2 months) in the high-caloric group (p = 0.042; Fig. 4). Additional oral food intake had no impact on survival (Fig. 4).
Predictive factors for survival. a Patients who lost <5 kg of body weight during the course of disease had a significantly prolonged survival of 6.2 months after PEG compared to patients who lost ≥5 kg of body weight (21.5 ± 2.1 vs. 15.3 ± 2.4 months; p = 0.025). b Patients with cholesterol ≥220 mg/dl had a significantly prolonged survival of 9.24 months after PEG compared to patients with cholesterol <220 mg/dl (23.2 ± 3.5 vs 13.9 ± 2.6 months; p = 0.03). c FVC had no impact on survival (FVC > 50 %: N = 40, FVC < 50 %: N = 35)
Nutrition over PEG and survival. a Patients with a survival of at least 12 months and high-caloric nutrition (≥1,500 kcal/d, N = 6) had a significantly prolonged survival of 2.5 months (23.6 ± 3.8 vs. 21.1 ± 2.2 months; p = 0.042) compared to patients with lower caloric intake (<1,500 kcal/d, N = 12). b Additional oral food intake had no impact on survival
Discussion
Strengths and limitations of the study
Use of enteral nutrition in ALS is increasing, yet knowledge on the timing or the desirable characteristics of its composition, as well as its consequences for disease progression remain based on retrospective studies which are subject to bias and confounders. The major strength of the current study is that it represents the first large prospective study of PEG use in ALS patients. The main limitations are due to the design as a register study which implies the absence of predefined hypotheses and control groups. Also, a bias based on center-specific conditions cannot be excluded. Therefore results of this study have to be regarded as descriptive and must be proven by randomized placebo-controlled interventional trials. However, this design was chosen to analyze the current practice, to identify possible targets for future research, and to help with the planning of PEG-related interventional studies.
Overall safety of PEG
Our results showed that PEG was a very safe procedure in our collective, and devoid of both short-term and long-term complications. Only one patient died during the peri-operative period, and death was probably unrelated with PEG but due to pre-existing advanced disease and aspiration pneumonia. Serious adverse events were rare.
In our study, PEG led to decreased hemoglobin, decreased thrombocytes along increased leukocytes and CRP immediately after tube implantation. Our interpretation of this finding is that these changes are due to bleeding during or immediately after surgery, and to low grade inflammatory processes related with surgery or tube insertion. However, those laboratory changes were rarely associated with clinical symptoms. Still, levels of CRP should be carefully monitored in the peri-operative period since high (>10 mg/l) levels of CRP were shown to be a risk factor for early mortality in a prospective study [19].
Risk of PEG in patients with low vital capacity
Surprisingly, we observed that worse respiratory status was not associated with shorter survival after PEG which somewhat contradicts existing literature which previously recommended that PEG should be avoided in patients with FVC below 50 %. This notion is based on the retrospective analysis of BDNF and CNTF trials showing peri-operative death in patients with low FVC [14]. However, more recently several retrospective studies did not observe an influence of FVC on survival after PEG [20–22]. Our study for the first time provides prospective evidence that FVC does not influence peri-procedural survival of patients after PEG, and that PEG can be safely performed even in far advanced stages of disease. Although we found a significant reduction of pO2 post PEG, this did not seem to influence the clinical outcome. We believe that the increasing use of NIV in general as well as the possibility to use NIV during PEG insertion might contribute to the better prognosis of patients with low FVC. This view is also supported by recent retrospective studies [20, 23–25]. However, since we did not record the exact numbers of peri-interventional use of NIV, this relationship still has to be confirmed by a controlled prospective study.
Timing of PEG
Regarding the question of optimal PEG timing, our study has limited significance due to the missing of a control group. However, we found that weight loss before PEG, and even more cholesterol levels, were predictors of survival after PEG. If weight loss before PEG was less than 5 kg, survival after PEG was increased by 3 months. This may indicate that PEG should be performed as early as possible. However, since weight loss is known to be an independent prognostic factor in ALS in general, a controlled interventional trial has to answer the question if this finding is also true in a PEG-specific context. Interestingly, we observed that rather than initial BMI, it is the extent of weight loss that predicts survival in PEG patients. This is consistent with previous results in the general ALS population [6].
Use of single-shot antibiosis before PEG
Importantly, CRP levels were significantly lower in patients receiving single-shot antibiosis without any clinical signs of relevant inflammation. This was true for both cefuroxime and ceftriaxone. Although it has already been known that prophylactic antibiosis before PEG reduces the risk of peristomal infection in general [26], this insight is new for the subgroup of ALS patients and specific antibiotics.
Risk of refeeding syndrome
A potential major concern when initiating enteral nutrition in ALS patients is the occurrence of refeeding syndrome, that develops usually after fasting and might lead to severe neurological complications. It is characterized by drops in electrolytes, especially phosphate and magnesium [27]. Indeed, severe weight loss, as experienced by ALS patients, is one of the major criteria for detection of individuals at risk for refeeding syndrome [28]. Here we observed that PEG did not modify electrolytes that are affected during refeeding syndrome, in particular phosphate, magnesium, sodium, and potassium. At the same time no clinical signs of refeeding syndrome were found in individual patients, even in patients who received high-caloric intake shortly after PEG insertion. This shows that the risk of refeeding syndrome after PEG might have been overestimated in ALS patients. This result is very important considering the increasing evidence of beneficial effects of high-caloric nutrition in ALS patients with PEG (see below).
High-caloric nutrition in PEG patients
A recent clinical trial has observed a positive effect of hypercaloric diet in a small number of ALS patients under PEG [15]. There were no deaths within the 5 months observational period in the high carbohydrate group, and this was significant compared to the control group receiving an isocaloric diet. The study, although extremely promising suffered from the major limitation of a small number of patients. Although we were not able to apply individually adjusted amounts of calories due to the study design, we tried to confirm the abovementioned result in our large collective by identifying and comparing patients with higher caloric diets (>1,500 kcal/day) and lower caloric diets (<1,500 kcal/day). Strikingly, although we did not find a significant difference for the overall group, we were able to prove a benefit for the subgroup of patients who survived at least 12 months after PEG. Interestingly, Wills and colleagues included only patients that “have already tolerated enteral nutrition” [15], and included patients which were already under PEG for a median time of about 100 days (AM Wills, personal communication). Thus, our study is complementary to the findings of Wills and colleagues. Drawing conclusions from both studies there is growing evidence that hypercaloric enteral nutrition may be protective in ALS patients with PEG provided that these patients tolerate enteral nutrition well. However, since the development of weight gain varied greatly between patients during the further course of the disease, we believe that the exact individual amount of calories has to be adjusted for each patient accordingly.
Additional oral feeding
Interestingly, patients who performed additional oral feeding did not have a worse prognosis, suggesting that a sufficient caloric intake might even outweigh the higher risk of aspiration pneumonia.
Schemes of increasing caloric intake after PEG
Most adverse events noted during the study were minor ones, and the most prominent of these events was feeling of fullness. Importantly, this appeared much less frequent in patients who received slower increase of caloric intake after PEG (≤200 kcal/d). Therefore we suggest that caloric intake after PEG should be increased carefully, although in the end a high-caloric intake as described above should be accomplished.
Summary
We believe that the following insights arise from these results:
-
1.
Low vital capacity should probably not be regarded as a contraindication for PEG since it was not associated with a higher risk of complications in our study.
-
2.
Earlier PEG insertion might improve survival in ALS since greater weight loss before PEG was an independent negative prognostic factor in our study.
-
3.
Single-shot antibiosis should probably be recommended for ALS patients receiving PEG. Future interventional studies should investigate and compare specific antibiotics in a controlled manner in this regard.
-
4.
The risk of refeeding syndrome is not relevant today in the context of ALS and PEG.
-
5.
Our study provides confirmatory evidence in a high number of patients that high-caloric nutrition is beneficial in ALS patients with PEG.
-
6.
Additional application of small amounts of oral feeding does not seem to worsen the prognosis of ALS patients with PEG. Of course, this has to be confirmed and investigated in the context of different stages of dysphagia and the use of fiber-endoscopic diagnostic methods.
-
7.
Although a high-caloric nutrition should be achieved in the end (see 5), a slow increase of calories (<+200 kcal/day) following PEG insertion results in a better tolerance of PEG feeding for the patient.
All these different points might directly influence the treatment of ALS patients with PEG in the near future, and should be investigated in future interventional trials.
References
Dupuis L, Pradat PF, Ludolph AC, Loeffler JP (2011) Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol 10(1):75–82. doi:10.1016/s1474-4422(10)70224-6
Korner S, Hendricks M, Kollewe K, Zapf A, Dengler R, Silani V, Petri S (2013) Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol 13:84. doi:10.1186/1471-2377-13-84
O’Reilly EJ, Wang H, Weisskopf MG, Fitzgerald KC, Falcone G, McCullough ML, Thun M, Park Y, Kolonel LN, Ascherio A (2013) Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Later Scler Frontotemporal Degener 14(3):205–211. doi:10.3109/21678421.2012.735240
Gallo V, Wark PA, Jenab M, Pearce N, Brayne C, Vermeulen R, Andersen PM, Hallmans G, Kyrozis A, Vanacore N, Vahdaninia M, Grote V, Kaaks R, Mattiello A, Bueno-de-Mesquita HB, Peeters PH, Travis RC, Petersson J, Hansson O, Arriola L, Jimenez-Martin JM, Tjonneland A, Halkjaer J, Agnoli C, Sacerdote C, Bonet C, Trichopoulou A, Gavrila D, Overvad K, Weiderpass E, Palli D, Quiros JR, Tumino R, Khaw KT, Wareham N, Barricante-Gurrea A, Fedirko V, Ferrari P, Clavel-Chapelon F, Boutron-Ruault MC, Boeing H, Vigl M, Middleton L, Riboli E, Vineis P (2013) Prediagnostic body fat and risk of death from amyotrophic lateral sclerosis: the EPIC cohort. Neurology 80(9):829–838. doi:10.1212/WNL.0b013e3182840689
Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM (2011) Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 44(1):20–24. doi:10.1002/mus.22114
Marin B, Desport JC, Kajeu P, Jesus P, Nicolaud B, Nicol M, Preux PM, Couratier P (2011) Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 82(6):628–634. doi:10.1136/jnnp.2010.211474
Dorst J, Kuhnlein P, Hendrich C, Kassubek J, Sperfeld AD, Ludolph AC (2011) Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol 258(4):613–617. doi:10.1007/s00415-010-5805-z
Dupuis L, Corcia P, Fergani A, Gonzalez De Aguilar JL, Bonnefont-Rousselot D, Bittar R, Seilhean D, Hauw JJ, Lacomblez L, Loeffler JP, Meininger V (2008) Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70(13):1004–1009. doi:10.1212/01.wnl.0000285080.70324.27
Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP (2004) Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA 101(30):11159–11164. doi:10.1073/pnas.0402026101
Allen JA, Chen R, Ajroud-Driss S, Sufit RL, Heller S, Siddique T, Wolfe L (2013) Gastrostomy tube placement by endoscopy versus radiologic methods in patients with ALS: a retrospective study of complications and outcome. Amyotroph Later Sclero Frontotemporal Degener 14(4):308–314. doi:10.3109/21678421.2012.751613
Blondet A, Lebigot J, Nicolas G, Boursier J, Person B, Laccoureye L, Aube C (2010) Radiologic versus endoscopic placement of percutaneous gastrostomy in amyotrophic lateral sclerosis: multivariate analysis of tolerance, efficacy, and survival. J Vasc Interv Radiol 21(4):527–533. doi:10.1016/j.jvir.2009.11.022
Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, Hardiman O, Kollewe K, Morrison KE, Petri S, Pradat PF, Silani V, Tomik B, Wasner M, Weber M (2012) EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)–revised report of an EFNS task force. Eur J Neurol Off J Eur Fed Neurol Soc 19(3):360–375. doi:10.1111/j.1468-1331.2011.03501.x
Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, Kalra S, Katz JS, Mitsumoto H, Rosenfeld J, Shoesmith C, Strong MJ, Woolley SC (2009) Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 73(15):1218–1226. doi:10.1212/WNL.0b013e3181bc0141
Kasarskis EJ, Scarlata D, Hill R, Fuller C, Stambler N, Cedarbaum JM (1999) A retrospective study of percutaneous endoscopic gastrostomy in ALS patients during the BDNF and CNTF trials. J Neurol Sci 169(1–2):118–125
Wills AM, Hubbard J, Macklin EA, Glass J, Tandan R, Simpson EP, Brooks B, Gelinas D, Mitsumoto H, Mozaffar T, Hanes GP, Ladha SS, Heiman-Patterson T, Katz J, Lou JS, Mahoney K, Grasso D, Lawson R, Yu H, Cudkowicz M (2014) Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. doi:10.1016/s0140-6736(14)60222-1
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457. doi:10.1016/s0140-6736(07)61602-x
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Motor Neuron Disord Off Publ World Fed Neurol Res Group Motor Neuron Diseases 1(5):293–299
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169(1–2):13–21
Blomberg J, Lagergren P, Martin L, Mattsson F, Lagergren J (2011) Albumin and C-reactive protein levels predict short-term mortality after percutaneous endoscopic gastrostomy in a prospective cohort study. Gastrointest Endosc 73(1):29–36. doi:10.1016/j.gie.2010.09.012
Gregory S, Siderowf A, Golaszewski AL, McCluskey L (2002) Gastrostomy insertion in ALS patients with low vital capacity: respiratory support and survival. Neurology 58(3):485–487
Spataro R, Ficano L, Piccoli F, La Bella V (2011) Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: effect on survival. J Neurol Sci 304(1–2):44–48. doi:10.1016/j.jns.2011.02.016
Sarfaty M, Nefussy B, Gross D, Shapira Y, Vaisman N, Drory VE (2013) Outcome of percutaneous endoscopic gastrostomy insertion in patients with amyotrophic lateral sclerosis in relation to respiratory dysfunction. Amyotroph Later Scler Frontotemporal Degener 14(7–8):528–532. doi:10.3109/21678421.2013.812659
Boitano LJ, Jordan T, Benditt JO (2001) Noninvasive ventilation allows gastrostomy tube placement in patients with advanced ALS. Neurology 56(3):413–414
Czell D, Bauer M, Binek J, Schoch OD, Weber M (2013) Outcomes of percutaneous endoscopic gastrostomy tube insertion in respiratory impaired amyotrophic lateral sclerosis patients under noninvasive ventilation. Respir Care 58(5):838–844. doi:10.4187/respcare.02024
Sancho J, Servera E, Chiner E, Banuls P, Gomez-Merino E, Sancho-Chust JN, Marin J (2010) Noninvasive respiratory muscle aids during PEG placement in ALS patients with severe ventilatory impairment. J Neurol Sci 297(1–2):55–59. doi:10.1016/j.jns.2010.06.022
Lipp A, Lusardi G (2013) Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane database Syst Rev 11:CD005571. doi:10.1002/14651858.CD005571.pub3
Solomon SM, Kirby DF (1990) The refeeding syndrome: a review. J Parenter Enter Nutr 14(1):90–97
Rio A, Whelan K, Goff L, Reidlinger DP, Smeeton N (2013) Occurrence of refeeding syndrome in adults started on artificial nutrition support: prospective cohort study. BMJ Open. doi:10.1136/bmjopen-2012-002173
Acknowledgments
The study was supported by the German MND Network, the German Department of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) and Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany.
Conflicts of interest
FH reports personal fees from Biomarin, personal fees from genzyme, outside the submitted work. All other authors declare that they have no conflict of interest.
Ethical standard
The study was approved by the ethics committees of all participating centers and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorst, J., Dupuis, L., Petri, S. et al. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: a prospective observational study. J Neurol 262, 849–858 (2015). https://doi.org/10.1007/s00415-015-7646-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7646-2