Abstract
Although the global benefits of gastrostomy have been proven in amyotrophic lateral sclerosis (ALS), the impact on biological parameters has not been explored yet. The aim of this preliminary work was to evaluate the modification of biological parameters in patients with ALS undergoing gastrostomy. We retrospectively collected clinical and biological data from 44 patients having undergone gastrostomy at three time points (T0, T1 and T2: before, at the time of and after gastrostomy). We examined the relationship between the biological parameters and disease progression. Variations of the concentrations of total cholesterol significantly differed before (T1–T0) vs those after gastrostomy (T2–T1; P=0.0044). The variations of total cholesterol and low-density lipoprotein cholesterol concentrations after gastrostomy were negatively associated with survival (P=0.0002). This study showed for the first time that patients with ALS fed quite exclusively by gastrostomy had decreased blood cholesterol after gastrostomy. We suggest that a restoration of normal lipid metabolism should be planned in patients with ALS.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal condition, and Riluzole remains the single drug approved for ALS. Accurate management of alteration of nutritional status could positively modify the evolution of ALS, as denutrition has been proved to be a major prognostic factor.1 Numerous causes can induce weight loss and denutrition through hypermetabolism, swallowing disturbances and psychological and cognitive troubles. The recourse of percutaneous endoscopic gastrostomy (gastrostomy) is discussed for patients with ALS in the presence of swallowing troubles and/or alteration of nutritional parameters in order to supplement oral feeding. Despite the recognition of the global benefit for patients with ALS undergoing gastrostomy being reported,2, 3 its impact on the quality of life and on survival remains a subject of debate, and its long-term influence on metabolic parameters has not been confirmed. The aim of this preliminary work was to evaluate the modification of biological parameters in patients undergoing gastrostomy and its effect on survival.
Patients and methods
Study design and participants
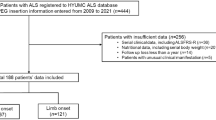
Data from our referral centre were collected from 44 patients who were having a definite or a probable ALS4 and were undergoing gastrostomy. Biological and clinical data were collected at the following three time points: before gastrostomy (T0), at time of intervention (T1) and after gastrostomy (T2). Data from a control cohort of 225 patients with ALS who did not undergo gastrostomy were collected at two time points (t0 and t2) in order to have an overview of standard evolution of biological parameters (control group). Hypolipemic therapeutics were noted.
Clinical variables
Data on site of onset, age of onset and gender were collected. The survival post gastrostomy was evaluated from the time of gastrostomy to death or tracheostomy. The Revised ALS Functional Rating Scales (ALSFRS-R) and body mass index were noted at T0, T1 and T2. Body composition was assessed at T0 and T2 as previously reported.5
Biological data
Numerous blood parameters (including albumin, creatinine, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol and triglycerides) were collected at T0, T1, T2, t0 and t2 for patients with gastrostomy and for controls, respectively. The Ethics Committee of Tours Hospital agreed that signed consent from participants was not required, as these blood tests were part of a normal follow-up procedure in patients with ALS.
Statistical analysis
The evolution of each variable before gastrostomy (T1 vs T0) and after gastrostomy (T2 vs T1) was expressed as percentages of the parameters’ variations, weighted by the time frame between the two time points. Available parameters were compared by paired Student’s t-test or a Wilcoxon paired t-test. The evolution of parameters between t0 and t2 in patients without gastrostomy was considered as standard evolution of biological parameters in the ALS population. Therefore, the parameters that underwent significant modification after gastrostomy were compared to those observed in the control population during the same period (T0–T2, t0–t2). We also performed a multivariate analysis including the relevant biological parameters and the parameters that significantly differed between patients with and without gastrostomy. Disease evolution analysis was performed with either the Kaplan–Meier method or the Cox proportional hazard model. To perform the Kaplan–Meier curve, we calculated the median of the relevant parameter to constitute two groups (inferior and superior to the median). The differences between these results were deemed significant after Benjamini–Hochberg adjustment (level of significance: q). Values were given with a confidence interval of 95%, using the computing environment R (R version 3.3.1, the R Foundation for Statistical Computing Platform).
Results
Patients
Main characteristics of patients with gastrostomy (n=44) are listed in Supplementary Table 1. The mean values (s.e.) for the duration between T1/T0, T2/T1 and t0/t2 were 9.0±6.9 (min 3.9–max 13.3) months, 6.1±3.1 (min 3.1–max 13.8 months) and 11.02±3.8 (min 3.2–max 20.0) months, respectively. The time period from the first symptoms to the first (T0, t0) and last (T2, t2) time point was not different (P=0.9 and 0.1, respectively). The evaluation of body composition did not differ before and after gastrostomy. No patient was under hypolipemic treatment during the time of data collection. All the characteristics of patients without gastrostomy were similar as those of patients with gastrostomy except for the following criteria: weight loss, body mass index and the site of onset at diagnosis (Table 1).
Biological evolution after gastrostomy
Results are summarised in Table 1.
Variations in the serum concentrations of total cholesterol (P=0.0044) significantly differed (n=31, q=0.0059) before (T1–T0) vs those after gastrostomy (T2–T1). We observed no significant variation for LDL cholesterol (P=0.004, q=0.0029), LDL/HDL ratio (P=0.017) and albumin (P=0.04, Supplementary Figure S1) for the same time points. The variation in serum concentration of total cholesterol was the only parameter that significantly differed in patients with gastrostomy compared to that in controls (for a comparison (T0–T2) vs (t0–t2) as well as (T2–T1) vs (t0–t2), P<0.0005). According to the difference in several parameters at diagnosis between both the groups, we performed a multivariate analysis including the site at onset, body mass index and weight loss. We confirmed the significant decrease of total cholesterol after gastrostomy (56 patients with gastrostomy and 28 without gastrostomy, P=0.0043).
Disease progression after gastrostomy
Body mass index decreased before but also after gastrostomy (n=35, P=0.0004 and n=25, P=0.017, respectively, Table 1). ALSFRS-R score significantly decreased before gastrostomy (n=36, P<0.0001) but not after gastrostomy (n=17). The variation of total cholesterol concentration after gastrostomy (T2–T1) was negatively associated with survival (n=31, with groups based on the median at −1.758, P=0.0002; Figure 1) as well as for LDL cholesterol (n=31, P=0.0002). Even if the modification of LDL/HDL ratio between (T1–T0) and (T1–T2) was not significant after correction for multiple test (P=0.017), we observed a relation between high LDL/HDL ratio and increase in survival (P=0.022, not shown).
Discussion
This study showed that patients with ALS fed by gastrostomy had a decrease in blood cholesterol after gastrostomy conversely to what was observed during the period preceding the intervention. This contrasted with a stable value of lipidic parameters over evolution in patients without gastrostomy (that is, control group). Even if both populations have some differences in clinical characteristics at baseline, the biological parameters are similar at baseline. A recent study showed that the prevalence of hypercholesterolaemia decreased over 18 months but with no impact on survival.6 However, the recruited population included patients with and without gastrostomy and therefore we cannot compare their data with ours. Our findings showed a clear decrease in cholesterol and LDL cholesterol after gastrostomy and a trend to a decrease of LDL/HDL ratio (P=0.017, not significant after correction for multiple tests). We noted an association between all these parameters and disease progression, including the effect of LDL/HDL ratio (P=0.022, not detailed). These observations are consistent with previous published data in favour of a longer survival, where the LDL/HDL ratio >2.9.7 Although the association between lipid profile and survival remains a matter of debate,6, 8 the benefit of hypercaloric diet seems admitted. A double-blind, placebo-controlled, randomised phase 2 clinical trial revealed that patients receiving high-fat hypercaloric diet had similar adverse events, tolerability and disease progression as patients with high-carbohydrate hypercaloric diet (HC/HC) had.3 But they also described that hypercaloric enteral nutrition is safe and tolerable in ALS, thus supporting the study of nutritional interventions at earlier stages of the disease. Dorst et al.2 also showed an increased survival in ALS patients (n=89) who lived at least 12 months after endoscopic gastrostomy insertion with high caloric intake (1500 kcal per day). Another observational and retrospective study9 reported clinical data and serum albumin, transferrin and total cholesterol at the time of endoscopic gastrostomy (T0) and after 3 months (T3) in 37 ALS patients. They did not observe any difference in biological data after gastrostomy (only at T3) but underlined the safety and effectiveness of this technique for enteral feeding. To date, no studies evaluated this evolution of cholesterol concentration several months after gastrostomy in ALS patients, and our observation supported the necessity of rigorously analysing the content of gastrostomy diet. Surprisingly, none of the gastrostomy nutritional supply prescribed contained cholesterol, and the potential supplementary nutrition of patients with ALS may have included vitamins, proteins, carbohydrates but not cholesterol. Thus, we suggest that the decrease of cholesterol concentrations was probably more due to a modified nutrition rather than a modification of energetic metabolism. As the impact of lipid is still controversial in ALS, we think that the type of nutrition, especially a supplementation in cholesterol in addition to gastrostomy or a restoration of normal lipid metabolism, should be investigated further by an interventional study in a larger cohort of patients with ALS. Regular biological examinations, as well as clinical evaluation, may be used to suggest an adaptation of feeding, both quantitatively and qualitatively.
References
Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73: 1227–1233.
Dorst J, Dupuis L, Petri S, Kollewe K, Abdulla S, Wolf J et al. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: a prospective observational study. J Neurol 2015; 262: 849–858.
Wills AM, Hubbard J, Macklin EA, Glass J, Tandan R, Simpson EP et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2014; 383: 2065–2072.
Brooks BR, Miller RG, Swash M, Munsat TL . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 293–299.
Desport JC, Preux PM, Bouteloup-Demange C, Clavelou P, Beaufrere B, Bonnet C et al. Validation of bioelectrical impedance analysis in patients with amyotrophic lateral sclerosis. Am J Clin Nutr 2003; 77: 1179–1185.
Rafiq MK, Lee E, Bradburn M, McDermott CJ, Shaw PJ . Effect of lipid profile on prognosis in the patients with amyotrophic lateral sclerosis: insights from the olesoxime clinical trial. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16: 478–484.
Dupuis L, Corcia P, Fergani A, Gonzalez De Aguilar JL, Bonnefont-Rousselot D, Bittar R et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 2008; 70: 1004–1009.
Huang R, Guo X, Chen X, Zheng Z, Wei Q, Cao B et al. The serum lipid profiles of amyotrophic lateral sclerosis patients: a study from south-west China and a meta-analysis. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16: 359–365.
Nunes G, Santos CA, Grunho M, Fonseca J . Enteral feeding through endoscopic gastrostomy in amyotrophic lateral sclerosis patients. Nutr Hosp 2016; 33: 561.
Acknowledgements
Author contributions
HB, PC and PCou were involved in the conceptualisation and design of the study. OLT, SM and SB were involved in the acquisition of the data. Statistical analysis was conducted by HB and FP. HB wrote the first version of the manuscript. HB, PC, PCou, CRA, PV and VM were involved in the interpretation of data for the work and revised the manuscript critically for important intellectual content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Rights and permissions
About this article
Cite this article
Blasco, H., Patin, F., Molinier, S. et al. A decrease in blood cholesterol after gastrostomy could impact survival in ALS. Eur J Clin Nutr 71, 1133–1135 (2017). https://doi.org/10.1038/ejcn.2017.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2017.54
- Springer Nature Limited