Abstract
The objective of the study was to better characterize the clinical phenotype associated with the A8344G “MERRF” mutation of mitochondrial DNA. Fifteen mutated patients were extensively investigated. The frequency of main clinical features was: exercise intolerance and/or muscle weakness 67 %, respiratory involvement 67 %, lactic acidosis 67 %, cardiac abnormalities 53 %, peripheral neuropathy 47 %, myoclonus 40 %, epilepsy 40 %, ataxia 13 %. A restrictive respiratory insufficiency requiring ventilatory support was observed in about half of our patients. One patient developed a severe and rapidly progressive cardiomyopathy requiring cardioverter-defibrillator implantation. Five patients died of overwhelming, intractable lactic acidosis. Serial muscle MRIs identified a consistent pattern of muscle involvement and progression. Cardiac MRI showed non-ischemic late gadolinium enhancement in the left ventricle inferolateral part as early sign of myocardial involvement. Brain spectroscopy demonstrated increased peak of choline and reduction of N-acetylaspartate. Lactate was never detected in brain areas, while it could be documented in ventricles. We confirm that muscle involvement is the most frequent clinical feature associated with A8443G mutation. In contrast with previous reports, however, about half of our patients did not develop signs of CNS involvement even in later stages of the disease. The difference may be related to the infrequent investigation of A8344G mutation in ‘pure’ mitochondrial myo-cardiomyopathy, representing a bias and a possible cause of syndrome’s underestimation. Our study highlights the importance of lactic acidosis and respiratory muscle insufficiency as critical prognostic factors. Muscle and cardiac MRI and brain spectroscopy may be useful tools in diagnosis and follow-up of MERRF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myoclonic epilepsy with ragged red fibers (MERRF) is a multisystem disorder characterized by a variable combination of myoclonus, epilepsy, ataxia, muscle weakness and dementia. Hearing loss, peripheral neuropathy, exercise intolerance, cardiomyopathy and multiple lipomas are also observed. The mitochondrial DNA (mtDNA) MT-TK encoding tRNALys is the most commonly mutated gene and the A-to-G transition at nucleotide 8344 (m.8344A>G) is present in over 80 % of affected individuals [1]. We report detailed clinical, laboratory, imaging, morphological and biochemical features of 15 consecutive Italian patients carrying the A8344G MERRF mutation, all of them followed in our University Hospital.
Patients and methods
Fifteen patients (10 females, 5 males) harboring the A8344G mutation from seven unrelated Italian families were investigated. The following assessments were performed: extensive clinical examination, laboratory tests including creatine kinases (CK), venous blood lactic acid at rest and after cycloergometer exercise and troponin HS serum levels; electromyography (EMG) and nerve conduction studies (NCS), electroencephalography (EEG), dynamic EEG monitoring (24 and 48 h), basal 12-lead electrocardiography (EKG) and 24-h Holter monitoring, echocardiography, pulmonary function test and nocturnal polysomnography (PSG).
Imaging
All patients were fully cooperative and had given written consent prior to the investigations. Magnetic resonance (MR) exams were performed at 1.5 Tesla. Neuroimaging studies were performed using brain computer tomography (CT) or Magnetic Resonance Imaging (MRI) and Magnetic Resonance Spectroscopy study (MRS). Brain MRI included diffusion weighted imaging (DWI) acquisitions. Spectra were recorded with the standard quadrature headcoil following a diagnostic MRI exam. Voxels were either centered in basal ganglia, ventricles, stroke-like lesions visible on T2-weighted and DWI images or in the normal-appearing gray matter and parenchyma.
Muscle MRI protocol included fast spin echo (FSE) and Fast Short Tau Inversion Recovery (F-STIR) sequences. The degree of atrophy and fatty transformation was best assessed on T1 weighted images. Transverse sections of lower limbs and pelvic girdle muscles were obtained.
Cardiac magnetic resonance (CMR) included both functional and late gadolinium enhancement (LGE) imagines.
Muscle biopsy and genetic analysis
Muscle biopsy and blood samples for genetic analysis were collected for diagnostic purposes after written informed consent. Cryosections were analyzed using standard procedures [2] and respiratory chain enzymes activities in muscle were measured as previously described [3]. Molecular study was conducted in muscle and/blood and/or fibroblasts by Restriction Fragment Length Polymorphism (RFLP) analysis [4]. To quantitate the relative percentage of mutation in tissues 50 pmol of [γ-32P]ATP end-labeled backward primer was added to each PCR reaction before the last cycle. Densitometric analysis of the X-ray film was done using a Biorad Image Analyzer [5].
Results
Clinical features
Clinical features, laboratory data and neurophysiological studies are reported in Table 1. Morphological, molecular and biochemical findings are described in Table 2. All patients had normal early development. Age of onset varied from 6 to 56 years (mean age 25). Six patients (40 %) had facial weakness, four (27 %) showed extra-ocular muscle involvement with bilateral eyelid ptosis (three patients) and ophthalmoparesis (one patient). Ten patients (67 %) complained muscle fatigue after mild effort and severe exercise intolerance. Eight patients (53 %) showed muscle weakness with predominant involvement of axial muscles, girdle and proximal limb muscles.
Ten patients (67 %) had high blood lactate levels at rest or an abnormal lactate increase after cycloergometer test. Nine patients (60 %) showed high CK levels (twice to six times normal). EMG was myopathic in 10 patients (67 %), and ENG detected a peripheral axonal sensory motor neuropathy in 7 (47 %). Ten patients (67 %) had restrictive respiratory impairment associated in 7 with sleep-disordered breathing. Seven patients (47 %) had severe respiratory insufficiency requiring nocturnal ventilatory support or tracheostomy and mechanical ventilation. Cardiac abnormalities were present in 8 patients (53 %). Two patients complained dyspnoea (#7,11) and 2 palpitations (#5,11), no one referred chest pain or syncope. Basal troponin HS was assessed in four patients (#7,11,12,13) and was abnormal in all (3–6 times normal), but serial evaluation did not show a rise and fall pattern characteristic of acute coronary syndrome. Six patients (40 %) had cardiac dysrhythmias as sinus tachycardia (#6), supraventricular tachycardia (#2), non-sustained ventricular tachycardia (#11), ventricular extrasystoles (#12), right bundle branch block (#4,12) and Wolff–Parkinson–White syndrome (#7). Echocardiography was abnormal in 7 patients (47 %) showing left ventricular systolic dysfunction (#11), hypertrophic cardiomyopathy (#13), left ventricular hypertrophy (#3,12), septal hypertrophy (#4) and regional wall abnormalities (#3,6,7). Within 2 years, patient 11 developed significant dilatation and dysfunction of the left ventricle with global hypokinesis and severe systolic dysfunction (EF 33 %), despite appropriate therapy with ACE inhibitors, beta-blockers, furosemide and spironolactone. Cardiac MRI confirmed the global hypocinesia of the left ventricle with EF 28 % and detected a depressed global systolic dysfunction also of the right ventricle with EF 38 %. Coronary TC angiography was normal. He was implanted with implantable cardioverter-defibrillator (ICD) at the age of 60 years.
Myoclonus was detected in 6 (40 %) and generalized epileptic seizures were present in 6 (40 %) of our patients; myoclonus and epilepsy were both present in 4 patients. Critical events usually consisted of tonic–clonic seizures (#2,3,6,13,15) and more rarely of absences (#3). EEG abnormalities as generalized spike and wave discharges with background slowing or focal epileptiform discharges were seen in 11 patients (73 %), regardless of the presence of seizures. Photo-paroxysmal response was observed in 2 (13 %). Two patients (13 %) showed cerebellar ataxia. A progressive cognitive decline was observed in 3 (20 %). Patient 1 and 4 developed severe cognitive deterioration with predominant memory disturbances. Psychomotor regression was the presenting symptom in patient 15 with Leigh phenotype. Psychiatric involvement characterized by severe depressive disorder was observed in two patients (13 %). Two patients (13 %) complained migraine. A sensorineural hearing loss was found in 6 patients (40 %).
Liver impairment was observed in 2 patients and it was characterized by recurrent episodes of liver failure concomitantly with marked increase in blood lactate. Liver biopsy was performed in both these patients and showed steatosis (#4), centrilobular cholestasis, mild inflammatory activity and portal fibrosis (#13). Endocrine dysfunction was observed in 2 patients (13 %) and was represented by hyperthyroidism and hypogonadism, respectively. Three patients had lipomas (20 %).
Five patients died because of an overwhelming lactic acidosis. In patients 1, 4 and 7, lactic acidosis acutely worsened a pre-existing cardio-respiratory failure and they died, respectively, after a 5 (#4) and a 15 (#1,7) years history of disease. Patient 6, affected by photo-paroxysmal episodes, exercise intolerance and severe lactic acidosis since the age of 13 years, died at 16 years of metabolic failure and epileptic status. Patient 13 was a 41-year-old woman with subacute onset of ataxic syndrome, severe depressive disorder, myoclonic jerks, generalized epileptic seizures, persistent lactic acidosis and recurrent episodes of liver failure. She also had one isolated stroke-like episode (SLE) and one episode of severe pulmonary artery hypertension (PAH, 50 mmHg). PAH was concomitant with an acute rise of the lactic acid to 12 mEq/l (v.n. 0.5–22), in absence of overt heart or pulmonary disease, and resolved with the lowering of lactic acidemia. The patient died of cardio-metabolic failure 2 years after the onset of the disease.
Muscle MRI features
One or serial muscle MRI findings obtained in 5 patients (# 2,7,11,12,13) with different severity of muscle weakness are reported in Fig. 1. Early changes were found at pelvic and thigh level followed by lower legs. At pelvic level, gluteus maximus was more commonly involved followed by gluteus medius and minimus (Fig. 1a, b, e, f). At thigh level, the posterior compartment was more commonly involved, with early affection of biceps femoris and adductor magnus (Fig. 1a–g) and relative sparing of semitendinosus, sartorius and gracilis even in more severely affected patients (Fig. 1b, e, g). In the anterior compartment, vastus intermedius and lateralis were more commonly affected (Fig. 1a, b, e–g). In lower legs, there was a prominent involvement of posterior compartment with predominant impairment of soleus (Fig. 1a, b, e, g). Muscle involvement was generally symmetric and characterized by progressive atrophy and fatty infiltration. In patients 7 and 11, STIR images demonstrated diffuse increase signal intensity in affected muscles as for oedema (Fig. 1h). Serial MRI studies were performed in patient 11 (Fig. 1a, b) and 12 (Fig. 1c, d) and showed a rapid progression of atrophy and fat infiltration of glutei and posterior thigh muscles (Fig. 1b, d) and then of vastus lateralis and intermedius in the anterior compartment of the thigh (Fig. 1b).
Cardiac MRI and histological features
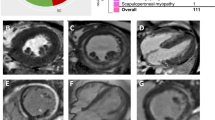
Three patients (#6,11,12) underwent cardiac MRI that showed in all, LGE in the basal inferolateral part of left ventricle with subepicardic distribution pattern and extensive intramural involvement both following a non-coronary distribution (Fig. 2a). Coronary TC angiography excluded an ischemic heart disease. Two patients (#4,11) underwent heart biopsy that showed the presence of focal accumulation of strongly SDH-reactive and COX-negative mitochondria (Fig. 2c, d) compared to control (Fig. 2b).
a cardiac MRI: LGE images show contrast enhancement of the interventricular septal and lateral walls (arrows). Heart biopsy SDH-stain: left ventricle endomyocardial biopsy in patient 11 (c and d at higher magnification) shows focal accumulation of strongly SDH-positive fibers compared to control (b)
Brain MRI features
Neuroimaging showed diffuse cerebral and cerebellar atrophy in 3 patients (#1,3,13) and thin corpus callosum in one patient (#3). White matter focal abnormalities were observed in patient 11. Bilateral necrosis in brainstem, basal ganglia and subthalamic nuclei was detected in patient 15. MRI in patient 13 showed acute lesions characterized by high-intensity signal areas on T2-weighted and DWI sequences that predominantly involved gray and white matter of parietal and occipital lobes bilaterally without vascular pattern distribution (Fig. 3a). An abnormal high-intensity area was also observed in the right thalami. Spectroscopy revealed decreased N-acetylaspartate (NAA) and increased Choline/Cr ratio in basal ganglia and occipital regions, but no lactate signal (Fig. 3b). One month later, cerebral lesions had completely disappeared and spectroscopy showed partial recovery of neurochemical abnormalities (Fig. 3d), whereas a diffuse cerebral atrophy became evident (Fig. 3c). MRS was performed in 4 additional patients (#2,7,11,12), no lactate signal was noted in basal ganglia and/or brain tissue at any stage of the disease (Fig. 3e); while a relatively increase of choline peak and a variable reduction of NAA was a constant feature in all. Spectroscopy was also obtained in cerebral ventricles in 2 patients (#2,11) and a small peak of lactic acid was instead observed in both (Fig. 3f).
Brain MRI showing acute bilateral cerebral lesions predominantly involving the parieto-occipital lobes (a, c) and neurochemical abnormalities on MRS (b, d) in patient 13. In all examined patients, spectroscopy showed no lactate signal in basal ganglia and/or brain tissue (e), whereas a small lactate peak was detected in cerebral ventricles (f)
Morphological, biochemical and molecular features
Histological, biochemical and molecular features are summarized in Table 2. SDH staining showed that all patients, except patient 15 with Leigh phenotype, had RRFs, ranging from 10 to 55 %. RRFs were absent in patient 4 at first muscle biopsy, at age of 28 years, becoming evident in a second biopsy performed 4 years later. Nine patients had variable proportion of fibers with reduced COX staining (ranging from 0 to 40 %); COX-negative fibers were both RRFs and non-RRFs. Diffuse COX deficiency on histochemical assay was observed in Leigh patient. Six patients (40 %) showed SSVs. Biochemistry revealed a variable and partial defect of activities of respiratory chain complexes I, III and IV, but COX was the most severely affected (COX deficiency in 9/15 patients when normalized for citrate synthase). Percentage of mutate genomes in muscle was quite homogeneous ranging from 75 to 88 %, except in patients 8 (50 % of mutant mtDNA) and 9 (10 % of mutant mtDNA) that were, respectively, non-symptomatic and mild-symptomatic. Interestingly in patient 4, who underwent two biopsies, mutational load in muscle remained unchanged; whereas, morphological features varied markedly (see above). Mutation load in blood was determined in 13 patients and varied from non-detectable (patient 9) to 80 %, it was lower than in muscle (Table 2) and did not correlated with “muscle phenotype” nor with clinical CNS involvement. Sural nerve biopsy was performed in two patients (#3,4) with peripheral neuropathy showing axonal loss.
Discussion
We report detailed clinical features, laboratory data, imaging, morphological, biochemical and molecular findings in 15 consecutive Italian patients harboring the A8344G mutation.
In agreement with previous reports [6, 7], also in our cohort exercise intolerance and/or muscle weakness were the most common features, observed in 80 % of patients. Muscle weakness predominantly involved girdle and proximal limb muscles and could be preceded by muscle fatigue even by years. Facial and extra-ocular muscles involvement was also frequent and it was encountered in about half of the patients. Other main clinical features were, in order of frequency, respiratory involvement (67 %), lactic acidosis (67 %), cardiac involvement (47 %) and peripheral neuropathy (47 %).
The frequent impairment of respiratory muscles was the most relevant aspect. A severe restrictive respiratory insufficiency requiring ventilatory support was in fact observed in about half of our patients. Severe respiratory involvement is rarely reported in MERRF [8], maybe due to the fact that it is not systematically investigated.
In our series, both respiratory failure and lactic acidosis were the factors that mostly influenced the prognosis. Highly abnormal lactate levels were observed in 60 % of our patients and five died of metabolic failure and overwhelming lactic acidosis. Two of them (#6,13) showed a rapidly fatal disease course that is seldom reported in MERRF [9], especially in adult onset form known to be generally slowly progressive [1, 10, 11]. In patient 13, a severe PAH was observed concomitantly with an acute increase in blood lactic acid. PAH has been described in a few children with mitochondrial diseases [12] as a new manifestation of mitochondrial disorders. However, some old reports correlated acute PAH both with idiopathic lactic acidosis in humans [13] and with progressive acidemia in dogs experimentally induced by an intravenous infusion of lactic acid [14]. Indeed, it is possible that lactic acidosis, triggering pulmonary artery vasoconstriction, may cause PAH in mitochondrial disorders. This is further supported by the fact that in our patient, PAH completely recovered with the lowering of lactic acid level.
Cardiac abnormalities, as minor conduction system defects and left ventricular hypertrophy and/or impaired function, were observed in about half of our patients with characteristics and percentage of incidence in agreement to that previously reported [15]. In one patient, cardiomyopathy had a severe and rapidly progressive course leading, within 2 years, to severe dilatation and global dysfunction with hypokinesis of the left ventricle requiring ICD to prevent sudden death. Cardiac involvement in MERRF syndrome is not rare and both hypertrophic and dilated cardiomyopathy are reported [16]. A progressive impairment of left ventricle function leading to severe dysfunction and death due to heart failure has been previously described [15] and our report further confirms the importance of a careful cardiac monitoring at diagnosis and during the follow-up of A8344G MERRF patients.
Peripheral neuropathy was detected in about 50 % of our patients, a percentage significantly higher than previously reported [6, 7].
Ataxia was observed in only 13 % against the 25–50 % reported in the literature [6, 7, 17].
Myoclonus and generalized epilepsy, the core features of the syndrome [1, 17], were both detected in 40 % of our cases, and only less than 30 % presented the ‘typical’ MERRF phenotype. Interestingly, however, paroxysmal EEG abnormalities, even in absence of seizures, were common in most of the patients with myopathy and/or exercise intolerance.
Deafness had the same incidence than previously described [6].
Muscle MRI showed a characteristic pattern of progression. Atrophy and fat infiltration predominantly involved glutei, with early affection of gluteus maximus, and posterior thigh muscles, with prominent impairment of biceps femoris and adductor magnus. At level of the leg, the posterior compartment was more commonly involved with a prevalent impairment of soleus. MRI findings correlated with clinically observed muscle deficits. Muscle weakness, after first manifestation, rapidly deteriorated and the progression was also documented by serial MRI.
Cardiac MRI showed a diffuse intramural LGE in inferolateral segments of the left ventricle of all investigated patients, indicating fibrosis, but coronary TC angiography was normal. Moreover, the presence of ‘ragged red fibers’ in the heart tissue ruled out an inflammatory disease and confirmed the mitochondrial origin of the cardiomyopathy. This characteristic pattern of non-ischemic LGE may represent an early sign even before evidence of heart impairment by echocardiography and it is similar to that recently reported in CPEO and KSS patients [18].
The neuroradiological features usually reported in MERRF as spongiform degeneration of cerebral white matter, calcifications in cerebellum and/or basal ganglia [19, 20], prominent cerebellar atrophy [21, 22] and bilateral putaminal necrosis [23], are also common in other mitochondrial diseases. Moreover, the A8344G mutation has been reported as an uncommon cause of Leigh disease [24–26]. We observed diffuse cortical and sub-cortical atrophy without disproportional cerebellar involvement, even in patients who presented an ataxic syndrome. Focal white matter abnormalities and bilateral and symmetric lesion of brainstem, subthalamic nuclei and basal ganglia were also present. SLEs typically seen in MELAS [27] are uncommon in MERRF harboring the A8344G mutation [28, 29]. Our patient 13, concomitant with sudden neurological deterioration, showed acute bilateral cerebral lesions predominantly involving the parieto-occipital lobes. In contrast with what is observed in MELAS, in this patient, however, spectroscopy showed no lactate peak, but reduction of NAA, a neuronal/axonal marker, and increased Cho/Cr ratio both in the basal ganglia and in the affected occipital regions. Neurochemical abnormalities partially recovered when clinical course improved, thus, representing a possible marker of disease activity and of acute central nervous system dysfunction. The same pattern with increased of choline-containing compounds, variable reduction of NAA, and absence of lactate peak was also found in four more patients. Similarly, previous MRS reports in symptomatic MERRF patients described absent lactate signal, increased Cho/Cr ratio and decreased NAA/Cr ratio [22, 30] in the basal ganglia [22]. This aspect is to a certain extent puzzling, but in agreement with what reported by histopathological studies of neuronal loss mainly in sub-cortical structures, including the nucleus dentatus [31]. This “characteristic” pattern may, thus, help to distinguish MERRF from other mitochondrial disorders as MELAS. However, in 2/2 patients, a small peak of lactic acid was found in the cerebrospinal fluid when the spectra were obtained in the ventricles as in MELAS [32].
Concerning morphological features, all patients had RRFs in muscle except the child with Leigh phenotype as already described [23]. In patient 4, despite the high mutational load, RRFs were absent in the first muscle biopsy becoming evident only in a second one 4 years later, supporting what previously stated [33] that the absence of RRFs does not allow to exclude a diagnosis of MERRF. The percentage of mutated genomes did not change in the two samples, as already observed [10]. A partial COX deficiency was detected in 60 % of the patients, confirming a preferential involvement of COX among respiratory chain enzymes [1, 7, 34]. The degree of biochemical and morphological abnormalities and the percentage of mutant genomes on muscle did not correlate with the severity of muscle involvement as assessed both by clinical examination and MRI. Mutation load in blood was always lower than in muscle confirming that muscle is the most reliable tissue to detect and quantify the A8344G mutation [6]. Again, the amount of mutated mtDNA did not correlate with either muscle or CNS phenotype. The variability of percentage and distribution of mutated genomes in different tissues of the same individual may, however, in part explain, as for other mitochondrial disorders, the absence of clinical manifestations of CNS involvement in some patients.
In conclusion, we report that “myo-cardiomyopathy” represents the most common clinical presentation associated with the A8443G mutation and most often patients do not develop epilepsy, myoclonus or ataxia even later in life. It is interesting to note that most of our myopathic patients had clear EEG abnormalities thus representing a diagnostic hint for the search of A8344G mutation.
Although our study population is relatively small, the difference with previous reports [1, 6, 17] may be related to the fact that the A8344G mutation is mainly investigated when classic clinical criteria are present. This represents a bias and a possible cause of underestimation of the syndrome. Indeed most of A8344G MERRF series were identified from retrospective database search and review of published data [6, 7]. Instead, the patients described in this report were all systematically followed in the same center and in the same setting.
Our study highlights the importance of lactic acidosis and respiratory muscle insufficiency as the main determinant factors of mortality and morbidity in MERRF patients and the need to carefully monitor respiratory and heart function.
We also characterized the pattern of distribution and progression of muscle involvement and cardiac impairment. Finally, our report points out the role of muscle and cardiac MRI and brain spectroscopy as useful tools in diagnosis and follow-up of 8344-MERRF patients.
References
DiMauro S, Hirano M, Kaufmann P et al (2002) Clinical features and genetics of myoclonic epilepsy with ragged red fibers. Adv Neurol 89:217–229
Sciacco M, Bonilla E (1996) Cytochemistry and immunocytochemistry of mitochondrial tissue sections. Methods Enzymol 264:509–521
DiMauro S, Servidei S, Zeviani M et al (1987) Cytochrome oxidase deficiency in Leigh syndrome. Ann Neurol 22(4):498–506
Zeviani M, Gellera C, Antozzi C et al (1991) Maternally inherited myopathy and cardiomyopathy: association with mutation in mitochondrial DNA tRNALeu(UUR). Lancet 338(8760):143–147
Silvestri G, Servidei S, Rana M et al (1996) A Novel Mitochondrial DNA point mutation in the tRNA(Ile) gene is associated with progressive external ophthalmoplegia. Biochem Biophys Res Commun 20(2):221–225
Chinnery PF, Howell N, Lightowlers RN et al (1997) Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain 120(10):1713–1721
Mancuso M, Orsucci D, Angelini C et al (2013) Phenotypic heterogeneity of the 8344A>G mtDNA “MERRF” mutation. Neurology 80(22):2049–2054
Wiedemann FR, Bartels C, Kirches E, Mawrin C, Wallesch CW (2008) Unusual presentations of patients with the mitochondrial MERRF mutation A8344G. Clin Neurol Neurosurg 110(8):859–863
Sanger TD, Jain KD (1996) MERRF syndrome with overwhelming lactic acidosis. Pediatr Neurol 14(1):57–61
Silvestri G, Ciafaloni E, Santorelli FM et al (1993) Clinical features associated with the A → G transition at nucleotide 8344 of mtDNA (“MERRF mutation”). Neurology 43(6):1200–1206
Fukuhara N (1991) MERRF: a clinicopathological study. Relationships between myoclonus epilepsies and mitochondrial myopathies. Rev Neurol (Paris) 147(6–7):476–479
Finsterer J, Zarrouk Mahjoub S (2013) Pulmonary hypertension in mitochondrial disorders. Brain Dev 35(5):466. doi:10.1016/j.braindev.2012.07.015
Sproule BJ, Phillipson EA, Couves CM, Brownlee RT (1966) Acute pulmonary hypertension in idiopathic lactic acidosis. Can Med Assoc J 94(3):141–143
Teplinsky K, O’Toole M, Olman M, Walley KR, Wood LD (1990) Effect of lactic acidosis on canine hemodynamics and left ventricular function. Am J Physiol 258(4 Pt 2):H1193–H1199
Wahbi K, Larue S, Jardel C et al (2010) Cardiac involvement is frequent in patients with the m.8344A>G mutation of mitochondrial DNA. Neurology 74(8):674–677
Anan R, Nakagawa M, Miyata M et al (1995) Cardiac involvement in mitochondrial diseases. A study on 17 patients with documented mitochondrial DNA defects. Circulation 91(4):955–961
Fukuhara N (1995) Clinicopathological features of MERRF. Muscle Nerve 3:S90–S94
Yilmaz A, Gdynia HJ, Ponfick M et al (2012) Cardiovascular magnetic resonance imaging (CMR) reveals characteristic pattern of myocardial damage in patients with mitochondrial myopathy. Clin Res Cardiol 101(4):255–261
Barkovich AJ, Good WV, Koch TK, Berg BO (1993) Mitochondrial disorders: analysis of their clinical and imaging characteristics. AJNR Am J Neuroradiol 14(5):1119–1137
Kendall BE (1992) Disorders of lysosomes, peroxisomes, and mitochondria. AJNR Am J Neuroradiol 13(2):621–653
Ito S, Shirai W, Asahina M, Hattori T (2008) Clinical and brain MR imaging features focusing on the brain stem and cerebellum in patients with myoclonic epilepsy with ragged-red fibers due to mitochondrial A8344G mutation. AJNR Am J Neuroradiol 29(2):392–395
Chuang CS, Lo MC, Lee KW, Liu CS (2007) Magnetic resonance spectroscopy study in basal ganglia of patients with myoclonic epilepsy with ragged-red fibers. Neurol India 55(4):385–387
Orcesi S, Gorni K, Termine C et al (2006) Bilateral putaminal necrosis associated with the mitochondrial DNA A8344G myoclonus epilepsy with ragged red fibers (MERRF) mutation: an infantile case. J Child Neurol 21(1):79–82
Scalais E, Nuttin C, Seneca S et al (2007) Infantile presentation of the mitochondrial A8344G mutation. Eur J Neurol 14(11):e3–e5
Tsao CY, Herman G, Boué DR et al (2003) Leigh disease with mitochondrial DNA A8344G mutation: case report and brief review. J Child Neurol 18(1):62–64
Howell N, Kubacka I, Smith R et al (1996) Association of the mitochondrial 8344 MERRF mutation with maternally inherited spinocerebellar degeneration and Leigh disease. Neurology 46(1):219–222
Hirano M, Pavlakis SG (1994) Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): current concepts. Child Neurol 9(1):4–13
Tanji K, Gamez J, Cervera C et al (2003) The A8344G mutation in mitochondrial DNA associated with stroke-like episodes and gastrointestinal dysfunction. Acta Neuropathol 105(1):69–75
Zaganas I, Latsoudis H, Papadaki E, Vorgia P, Spilioti M, Plaitakis A (2009) A8344G tRNALys mutation associated with recurrent brain stem stroke-like episodes. J Neurol 256(2):271–273
Mathews PM, Andermann F, Silver K, Karpati G, Arnold DL (1993) Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology 43(12):2484–2490
Filosto M, Tomelleri G, Tonin P et al (2007) Neuropathology of mitochondrial diseases. Biosci Rep 27(1–3):23–30
Kaufmann P, Shungu DC, Sano MC et al (2004) Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology 62(8):1297–1302
Mancuso M, Petrozzi L, Filosto M et al (2007) MERRF syndrome without ragged-red fibers: the need for molecular diagnosis. Biochem Biophys Res Commun 354(4):1058–1060
Lombes A, Mendell JR, Nakase H et al (1989) Myoclonic epilepsy and ragged-red fibers with cytochrome c oxidase deficiency: neuropathology, biochemistry, and molecular genetics. Ann Neurol 26(1):20–23
Acknowledgments
This study was partially supported by Telethon Grant GUP09004.
Conflicts of interest
The authors declare no conflict of interests.
Ethical standard
This study has been approved by the appropriate ethics committee and has therefore been performed in the accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catteruccia, M., Sauchelli, D., Della Marca, G. et al. “Myo-cardiomyopathy” is commonly associated with the A8344G “MERRF” mutation. J Neurol 262, 701–710 (2015). https://doi.org/10.1007/s00415-014-7632-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7632-0