Abstract
Patients with idiopathic REM sleep behavior disorder (iRBD) are at very high risk of developing neurodegenerative synucleinopathies, which are disorders with prominent autonomic dysfunction. Several studies have documented autonomic dysfunction in iRBD, but large-scale assessment of autonomic symptoms has never been systematically performed. Patients with polysomnography-confirmed iRBD (318 cases) and controls (137 healthy volunteers and 181 sleep center controls with sleep diagnoses other than RBD) were recruited from 13 neurological centers in 10 countries from 2008 to 2011. A validated scale to study the disorders of the autonomic nervous system in Parkinson's disease (PD) patients, the SCOPA-AUT, was administered to all the patients and controls. The SCOPA-AUT consists of 25 items assessing the following domains: gastrointestinal, urinary, cardiovascular, thermoregulatory, pupillomotor, and sexual dysfunction. Our results show that compared to control subjects with a similar overall age and sex distribution, patients with iRBD experience significantly more problems with gastrointestinal, urinary, and cardiovascular functioning. The most prominent differences in severity of autonomic symptoms between our iRBD patients and controls emerged in the gastrointestinal domain. Interestingly, it has been reported that an altered gastrointestinal motility can predate the motor phase of PD. The cardiovascular domain SCOPA-AUT score in our study in iRBD patients was intermediate with respect to the scores reported in PD patients by other authors. Our findings underline the importance of collecting data on autonomic symptoms in iRBD. These data may be used in prospective studies for evaluating the risk of developing neurodegenerative disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia characterized by loss of the normal atonia of REM sleep, such that patients appear to act out dream content [1]. In the past few years, some research showed that patients with idiopathic RBD (iRBD) are at very high risk of developing a neurodegenerative synucleinopathy, such as Parkinson’s disease (PD), dementia with Lewy bodies (DLB), or multiple system atrophy (MSA) [2–4].
This provides a rare opportunity to directly test potential prodromal markers of disease and to assess the evolution of prodromal symptoms and signs. Several studies have documented autonomic dysfunction in iRBD, with abnormal cardiac beat-to-beat variability [5–7], metaiodobenzylguanidine (MIBG) scintigraphy [8], and orthostatic blood pressure changes, as well as symptoms of constipation, urinary dysfunction, and erectile dysfunction [9, 10]. Interestingly, PD, DLB, and MSA are disorders with prominent autonomic dysfunction. However, large-scale assessment of autonomic symptoms has never been systematically performed.
Recently, a prospective study in 91 iRBD patients [11] demonstrated that patients who develop PD and DLB from iRBD have clear abnormalities of autonomic function years before the diagnosis of disease. The estimated prodromal interval was approximately 10–20 years on regression analysis (and at least 5 years according to direct observation), and progression was relatively slow during prodromal periods. This study used only rudimentary questionnaires for autonomic symptoms.
In 2004, Visser and colleagues [12] developed a new validated scale to study the disorders of the autonomic nervous system in PD patients: the Scale for Outcomes in PD-Autonomic (SCOPA-AUT). The SCOPA-AUT consists of 25 items assessing the following domains: gastrointestinal (7), urinary (6), cardiovascular (3), thermoregulatory (4), pupillomotor (1), and sexual dysfunction (2 items for men and 2 items for women).
In the present study examining a large multicenter, clinic-based cohort, we compared the SCOPA-AUT results of iRBD patients with those of control subjects.
Methods
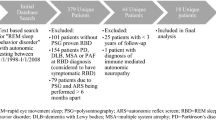
Patients with polysomnography-confirmed iRBD were recruited from 13 international RBD study group centers from 2008 to 2011. All cases met International Classification of Sleep Disorders–2 criteria for RBD (i.e., enhanced REM muscle tone on polysomnography with a history of dream enactment or complex behaviors during REM sleep on polysomnography) [13]. All cases had neurologic examination to confirm the absence of dementia (as defined as Mini-Mental State Examination <24 with functional impairment due to cognitive decline) [14] and Parkinsonism (according to UK Brain Bank criteria) [15]. Each center also recruited controls, frequency-matched 1:1 on age (within 5 years) and sex (10 % tolerance outside perfect matching was allowed). There were two groups of controls: sleep center controls, i.e. patients referred to the sleep center for other sleep problems (e.g., apnea, restless legs, insomnia, and hypersomnia), and normal volunteers. All sleep center controls had a polysomnogram documenting the absence of RBD and REM-without atonia, as did 67 of the 129 volunteer controls. All participants provided written informed consent to participate, and the research ethics board of each center gave approval for the study.
The SCOPA-AUT scale was administered to all the patients and controls.
In addition, current use of drugs that may interfere with autonomic function was assessed in all subjects, and the following categories were defined for the analysis: (a) clonazepam; (b) dopaminergic drugs; (c) antidepressant drugs; (d) cardiovascular drugs.
Statistical analysis
The various domains on the SCOPA-AUT scale were analyzed using the Mann–Whitney U test. Pearson’s correlation coefficients were used to examine the correlation between SCOPA-AUT and age (in all subjects) and duration of RBD (in iRBD patients). To evaluate the association between the various domains on the SCOPA-AUT scale and iRBD, unadjusted odds ratios (ORs) were calculated. Adjusted ORs were then obtained from logistic regression, adjusting for age, sex, and use of drugs.
The proportions of subjects reporting a symptom (i.e. score greater than zero) for each item were compared using the Pearson Chi square test.
Poisson (P) regression, a special case of the generalized linear model (GLM) framework, was applied for modelling count data to evaluate drug effect on subscales and on total scores of the SCOPA-AUT scale. Zero-inflated poisson (ZIP) regression was used to model count data in those subscales with an excess of zero counts.
Statistical analyses were performed using SPSS-18 for Windows (SPSS, Chicago, IL, USA) and R statistical software (version 2.15.3; www.r-project.org).
Results
Demographics
A total of 318 patients with iRBD (259 men, 59 women) who had also completed SCOPA-AUT questionnaires were recruited from 13 centers in 10 countries. The number of controls was 318 (244 men, 74 women, p = 0.144). Controls were slightly younger than the patients (67.3 ± 9.8 vs 66.2 ± 9.8 years, p = 0.048). The controls consisted of healthy volunteers (137, 43.1 %) and sleep center controls with sleep diagnoses other than RBD (181, 56.9 %). Ninety-two controls (28.9 %) had sleep apnea syndrome (OSA).
Autonomic symptoms
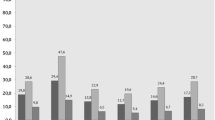
RBD patients had substantially more autonomic symptoms compared to control subjects as measured by the SCOPA-AUT total score (p < 0.0001) (Table 1). Differences between iRBD patients and control subjects were most noteworthy for gastrointestinal, urinary, and cardiovascular symptoms. The smallest differences between patients and control subjects were found for symptoms of the thermoregulatory and pupillomotor domain.
The association between gastrointestinal, urinary, and cardiovascular symptoms and iRBD remained statistically significant when adjusted for age, sex, and medication use (Table 2).
Interestingly, when OSA subjects were excluded from the control group, gastrointestinal (3.53 ± 2.84 vs 1.66 ± 1.97; p < 0.0001) and urinary (4.79 ± 3.11 vs 3.67 ± 3.09; p < 00001) symptom scores remained significantly greater in iRBD compared to controls, but not the cardiovascular domain score (0.68 ± 1.15 vs 0.52 ± 0.92).
In controls, age was positively correlated with scores in the urinary domain (R = 0.164; p = 0.004), as well in the sexual domain for men (R = 0.221; p = 0.003).
In iRBD patients, age showed a significant positive correlation with the gastrointestinal domain (R = 0.239; p = 0.0001), as well as with total scores of SCOPA-AUT (R = 0.126; p = 0.031).
Concerning the duration of iRBD, a positive correlation was found with gastrointestinal symptoms (R = 0.168; p = 0.016).
Table 3 shows the proportion of subjects in the two groups with a score greater than zero in each item. Sialorrhea, constipation, lightheadedness standing up, and erection problems in men were the commonest autonomic symptoms in iRBD.
Concerning the current use of drugs that may interfere with autonomic function, 39.7 % of iRBD and only 0.9 % of controls were taking clonazepam (Table 4). The percentage of iRBD patients under dopaminergic treatment was low, and very similar to that in control subjects. The percentage of subjects taking antidepressant drugs was 17.3 in the iRBD group and 9.1 for controls. In particular, the more frequently used antidepressant drugs were SSRIs (11.9 % in the iRBD group and 3.8 % for controls). Therapy with cardiovascular drugs was reported in 44.0 % of iRBD patients and in 48.4 % of control subjects.
Use of antidepressants was associated with higher gastrointestinal domain scores in both the iRBD and control groups (β = 0. 359; p < 0.0001 and β = 0.394; p = 0.0035, respectively), whereas higher urinary domain scores (β = 0.206; p = 0.0039) and higher cardiovascular domain scores (β = 0.567; p = 0.0064) appeared only in the iRBD group.
Use of cardiovascular drugs was associated with higher urinary domain scores for both patients and controls (β = 0.120; p = 0.033 and β = 0.126; p = 0.046, respectively), but were associated with higher total SCOPA-AUT scores only in the control group (β = 0.122; p = 0.002).
Discussion
In this study we evaluated autonomic symptoms using a reliable and valid instrument that includes items considered relevant by patients and specialists [12]. Our results show that compared to a group of control subjects with a similar overall age and sex distribution, patients with iRBD experience significantly more problems with gastrointestinal, urinary, and cardiovascular functioning.
It is well known that iRBD has been associated with synucleinopathies such as PD, DLB, or MSA, often preceding the full clinical expression of these Parkinsonian syndromes for years to decades [16, 17]. Moreover, synucleinopathies are frequently associated with neurovegetative impairment that often precedes motor symptoms. In PD, manifestations of autonomic dysregulation are frequently encountered including orthostatic hypotension, reduced heart rate variability, and impairment of sudomotor, gastrointestinal, and urinary functions [18]. In DLB, recurrent syncopes represent supporting evidence for the diagnosis and may precede the manifestations of cognitive decline [19]. In MSA, autonomic failure may dominate the clinical picture, as in the form previously identified as the Shy–Drager syndrome [20].
In 1996, it was first reported [21] that patients with iRBD not only have a reduced tonic and phasic heart rate variability during sleep, but the majority of these patients also have an impairment in one or more tests assessing sympathetic or parasympathetic functions during wakefulness compared to age and sex-matched healthy controls. In agreement with these results, Fantini and colleagues [22] found a reduced cardiac activation related to PLMS during stage two sleep in patients with iRBD compared to age-matched and sex-matched patients with restless leg syndrome (RLS).
More recently, Postuma and colleagues [23] found that a systolic blood pressure drop was impaired in patients with iRBD compared to controls. Studies with 123I-MIBG scintigraphy, a marker of post-ganglionic cardiac sympathetic denervation, have shown substantial and consistent abnormalities in iRBD [24–26].
Frauscher and colleagues [27] showed that autonomic dysfunction in iRBD was intermediate between controls and patients with PD in blood pressure regulation during an orthostatic standing test, and similar to PD in Valsalva testing. This last finding corresponds well to results of a cardiac 123I-MIBG scintigraphic study that demonstrated even worse results in iRBD than in early PD patients [28].
Another recent study evaluated the heart rate response to arousal or to leg movements during sleep in PD patients with and without RBD and patients with iRBD compared with healthy controls [7]. The heart rate response associated with arousal or leg movement from all sleep stages was analyzed from 10 heart beats before the onset of the sleep event to 15 heart beats following onset of the sleep event. The heart rate response to arousal was significantly lower in both PD groups compared with the control group and the iRBD group: the value in the iRBD group was intermediate with respect to the control and PD groups.
As with these previous studies, the SCOPA-AUT cardiovascular domain score in our studyof iRBD patients (0.74 ± 1.22 vs 0.49 ± 0.90 of controls) was intermediate with respect to the scores reported in PD patients by Visser and colleagues [12] (1.2 ± 1.3 in PD vs 0.3 ± 0.6 in controls). This is consistent with the concept that iRBD can be a manifestation of synuclein-associated neurodegenerative disorders like PD. A recent study [29] found that PD patients with clinical RBD have more severe orthostatic hypotension than PD patients without RBD, supporting previous findings [30].
The most prominent differences in severity of autonomic symptoms between our iRBD patients and control subjects emerged in the gastrointestinal domain. Interestingly, it has been reported that an altered gastrointestinal motility can predate the motor phase of PD [31]. Neuropathological studies report Lewy-body-pathology in the dorsal motor nucleus of the vagal nerve (DMNV) prior to PD-related motor symptoms, and the DMNV modifies gastrointestinal motility by interacting with the enteric nervous system where Lewy body pathology has been described in PD [32, 33]. Interestingly, within established PD, it has been reported that constipation was more prevalent in PD patients with RBD than in those without [34].
Some authors recently investigated PD patients, iRBD patients, and controls that underwent standardized testing for gastric emptying with the (13)C-octanoate breath test [35]. Gastric emptying was significantly delayed in drug-naïve and in treated PD patients, but normal in patients with iRBD. The authors concluded that this finding might be explained by the fact that neurodegenerative changes in structures modulating gastric motility are not severe enough to cause a functional deficit that can be detected by the (13)C-octanoate breath test. On the other hand, in our iRBD patients the duration of the disorder showed a positive correlation with gastrointestinal symptoms.
Another recent study measured fasting and postprandial ghrelin serum concentrations in PD patients, iRBD patients, and controls [36]. Ghrelin, an orexigenic peptide, has multiple functions, which include promoting gastrointestinal motility. The delayed gastric emptying in PD could be related to disturbed excretion of ghrelin. Controls showed a decrease of mean fasting ghrelin serum concentrations in the early postprandial phase, followed by a recuperation starting 60 min after the test meal and a maximum at 300 min. This recuperation was less pronounced in PD and iRBD, and post hoc testing showed a difference between controls and PD patients (p = 0.002) and between controls and iRBD patients (p = 0.037).
In conclusion, this first large-scale assessment of autonomic symptoms in iRBD showed that iRBD patients experience significant problems with gastrointestinal, urinary, and cardiovascular functioning. This may determine decrements in health status. Moreover, our findings underline the importance of collecting prospective data to evaluate the possible association between autonomic symptoms in iRBD and the risk of developing neurodegenerative disorders, especially PD. Additionally, these findings suggest important potential treatment opportunities. For example, 58 % of iRBD patients reported constipation; constipation is readily treatable, and can result in a substantial impairment in quality of life, so vigilance for gastrointestinal autonomic problems in patients with iRBD is warranted. Similarly, one-third of patients with iRBD reported a symptom consistent with orthostatic hypotension, which is potentially treatable with medications such as fludrocortisone, domperidone, or physostigmine. This also implies a need for periodic reassessment of indications for antihypertensive medications in RBD patients, as they may no longer be needed once a patient has developed iRBD.
References
Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW (1986) Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep 9:293–308
Iranzo A, Molinuevo JL, Santamaria J, Serradell M, Martí MJ, Valldeoriola F, Tolosa E (2006) Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 5:572–577
Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J (2009) Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 72:1296–1300
Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M, Sanchez-Valle R, Vilaseca I, Lomeña F, Vilas D, Lladó A, Gaig C, Santamaria J (2013) Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 12:443–453
Valappil RA, Black JE, Broderick MJ, Carrillo O, Frenette E, Sullivan SS, Goldman SM, Tanner CM, Langston JW (2010) Exploring the electrocardiogram as a potential tool to screen for premotor Parkinson’s disease. Mov Disord 25:2296–2303
Postuma RB, Lanfranchi PA, Blais H, Gagnon JF, Montplaisir JY (2010) Cardiac autonomic dysfunction in idiopathic REM sleep behavior disorder. Mov Disord 25:2304–2310
Sorensen GL, Kempfner J, Zoetmulder M, Sorensen HB, Jennum P (2012) Attenuated heart rate response in REM sleep behavior disorder and Parkinson’s disease. Mov Disord 27:888–894
Miyamoto T, Miyamoto M, Suzuki K, Nishibayashi M, Iwanami M, Hirata K (2008) 123I-MIBG cardiac scintigraphy provides clues to the underlying neurodegenerative disorder in idiopathic REM sleep behavior disorder. Sleep 31:717–723
Postuma RB, Gagnon JF, Vendette M, Montplaisir JY (2009) Markers of neurodegeneration in idiopathic REM sleep behavior disorder and Parkinson's disease. Brain 132:2298–2307
Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J (2006) Potential early markers of Parkinson's disease in idiopathic REM sleep behavior disorder. Neurology 66:845–851
Postuma RB, Gagnon JF, Pelletier A, Montplaisir J (2013) Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Mov Disord 28:597–604
Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ (2004) Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord 19:1306–1312
American Academy of Sleep Disorders (2005) International Classification of Sleep Disorders–2. American Academy of Sleep Disorders, Westchester
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Boeve BF (2010) REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 1184:15–54
Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF (2010) REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 75:494–499
Jain S (2011) Multi-organ autonomic dysfunction in Parkinson's disease. Parkinsonism Relat Disord 17:77–83
Idiaquez J, Roman GC (2011) Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci 305:22–27
Wenning GK, Stefanova N (2009) Recent developments in multiple system atrophy. J Neurol 256:1791–1808
Ferini-Strambi L, Oldani A, Zucconi M, Smirne S (1996) Cardiac autonomic activity during wakefulness and sleep in REM sleep behavior disorder. Sleep 19:367–369
Fantini ML, Michaud M, Gosselin N, Lavigne G, Montplaisir J (2002) Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology 59:1889–1894
Postuma RB, Gagnon JF, Vendette M, Montplaisir JY (2009) Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain 132:3298–3307
Jennum P, Mayer G, Ju YE, Postuma R (2013) Morbidities in rapid eye movement sleep behavior disorders. Sleep Med 14:782–787
Labate A, Salsone M, Novellino F, Morelli M, Sturniolo M, Gambardella A, Quattrone A (2011) Combined use of cardiac m-i123-iodobenzylguanidine scintigraphy and (1)(2)(3)I-fp-cit single photon emission computed tomography in older adults with rapid eye movement sleep behavior disorder. J Am Geriatr Soc 59:928–929
Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K (2006) Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology 67:2236–2238
Frauscher B, Nomura T, Duerr S, Ehrmann L, Gschliesser V, Wenning GK, Wolf E, Inoue Y, Högl B, Poewe W (2012) Investigation of autonomic function in idiopathic REM sleep behavior disorder. J Neurol 259:1056–1061
Kashihara K, Imamura T, Shinya T (2010) Cardiac 123I-MIBG uptake is reduced more markedly in patients with REM sleep behavior disorder than in those with early stage Parkinson’s disease. Parkinsonism Relat Disord 16:252–255
Nomura T, Inoue Y, Kagimura T, Nakashima K (2013) Clinical significance of REM sleep behavior disorder in Parkinson’s disease. Sleep Med 14:131–135
Romenets SR, Gagnon JF, Latreille V, Panniset M, Chouinard S, Montplaisir J, Postuma RB (2012) Rapid eye movement sleep behavior disorder and subtypes of Parkinson’s disease. Mov Disord 27:996–1003
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57:456–462
Braak H, de Vos RA, Bohl J, Del Tredici K (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1989) Lewy bodies in the enteric nervous system in Parkinson’s disease. Arch Histol Cytol 52:191–194
Nihei K, Takahashi A, Koto B, Mihara B, Morita Y, Isozumi K, Ohta K, Muramatsu K, Gotoh J, Yamaguchi K, Tomita Y, Sato H, Seki M, Iwasawa S, Suzuki N (2012) REM sleep behavior disorder in Japanese patients with Parkinson’s disease: a multicenter study using the REM sleep behavior disorder screening questionnaire. J Neurol 259:1606–1612
Unger MM, Möller JC, Mankel K, Schmittinger K, Eggert KM, Stamelou M, Stiasny-Kolster K, Bohne K, Bodden M, Mayer G, Oertel WH, Tebbe JJ (2011) Patients with idiopathic rapid-eye-movement sleep behavior disorder show normal gastric motility assessed by the 13C-octanoate breath test. Mov Disord 26:2559–2563
Unger MM, Möller JC, Mankel K, Eggert KM, Bohne K, Bodden M, Stiasny-Kolster K, Kann PH, Mayer G, Tebbe JJ, Oertel WH (2011) Postprandial ghrelin response is reduced in patients with Parkinson’s disease and idiopathic REM sleep behaviour disorder: a peripheral biomarker for early Parkinson’s disease? J Neurol 258:982–990
Conflicts of interest
The authors report no disclosures relevant to the manuscript. L. Ferini-Strambi has received honoraria for serving on scientific advisory boards from UCB, Mundipharma, Menarini. Full financial disclosure for the previous 12 months W. Oertel has received honoraria for consultancy and for serving on scientific advisory boards; travel support from UCB; and honoraria for consultancy and lecture fees from Teva, Novartis, GlaxoSmithKline, Boehringer Ingelheim, Orion Pharma, and Merck Serono. Y. Dauvilliers has received funds for speaking and board engagements with UCB, Cephalon, Jazz, and Bioprojet. R.B. Postuma has received research funds from the Canadian Institute of Health Research, Fonds de la Recherche en Sante Quebec, The Webster Foundation, and the Drummond Foundation, as well as funds for travel and speaker fees from Novartis Canada and Teva Neurosciences. A. Iranzo had speaking and board engagements with UCB and Sanofi-Synthelabo. I Arnulf received honoraria for a speaking engagement and board from UCB Pharma. I. Arnulf received honoraria from UCB Pharma and Jazz for a speaking engagement and consultancy. B. Hogl has received honoraria for speaking, serving on an advisory board, or consulting from UCB, Mundipharma, BI, GSK, Respironics, Sanofi, Lundbeck, and Jazz; and a grant to institution from UCB, and travel support from Habel Medizintechnik and Vivisol, Austria. K. Sonka served on a scientific advisory board for UCB Pharma, received lecture fees from Ipsen, Pfizer, UCB Pharma, and GlaxoSmithKline, and participated in clinical trials managed by UCB Pharma, Bioprojet, and Eisai; and received research support from Charles University in Prague (PRVOUK P26/LF1/4). B. Frauscher has had speaking engagements with UCB and received a competitive research grant from the Austrian Science Fund (KLI 236), grant of the Nationalbank of Austria (15127). M. Unger received grants from the Micheal J. Fox Foundation for Parkinson’s Research and from the International Parkinson Fonds Germany. K. Stiasny-Kolster has received honoraria for serving on the scientific advisory boards for Boehringer Ingelheim, and UCB, and has received honoraria for speaking engagements sponsored by UCB and Boehringer Ingelheim. J. Montplaisir received funds from Merck and GlaxoSmithKline, and has received honoraria for consultancy from Jazz, Merck, Valeant, Servier, and Impac laboratories. S. Marelli, R. Manni, T. Miyamoto, M.L. Fantini, M. Puligheddu, P. Jennum, J. Santamaria, M. Zucconi, M.V·P. Rancoita, S. Leu-Semenesceu, M. Terzaghi, M. Miyamoto, A. Desautels, C. Wolfson, and A. Pelletier have nothing to disclose.
Patient consent
Obtained.
Ethical standard
Each sleep center obtained approval from its local institutional review board.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferini-Strambi, L., Oertel, W., Dauvilliers, Y. et al. Autonomic symptoms in idiopathic REM behavior disorder: a multicentre case–control study. J Neurol 261, 1112–1118 (2014). https://doi.org/10.1007/s00415-014-7317-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7317-8