Abstract
Inherited peripheral neuropathies (IPN) are one of the most frequent inherited causes of neurological disability characterized by considerable phenotypic and genetic heterogeneity. Based on clinical and electrophysiological properties, they can be subdivided into three main groups: HMSN, dHMN, and HSN. At present, more than 50 IPN genes have been identified. Still, many patients and families with IPN have not yet received a molecular genetic diagnosis because clinical genetic testing usually only covers a subset of IPN genes. Moreover, a considerable proportion of IPN genes has to be identified. Here we present results of WES in 27 IPN patients excluded for mutations in many known IPN genes. Eight of the patients received a definite diagnosis. While six of these patients carried bona fide pathogenic mutations in known IPN genes, two patients had mutations in genes known to be involved in other types of neuromuscular disorders. A further group of eight patients carried sequence variations in IPN genes that could not unequivocally be classified as pathogenic. In addition, combining data of WES and linkage analysis identified SH3BP4, ITPR3, and KLHL13 as novel IPN candidate genes. Moreover, there was evidence that particular mutations in PEX12, a gene known to cause Zellweger syndrome, could also lead to an IPN phenotype. We show that WES is a useful tool for diagnosing IPN and we suggest an expanded phenotypic spectrum of some genes involved in other neuromuscular and neurodegenerative disorders. Nevertheless, interpretation of variants in known and potential novel disease genes has remained challenging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inherited peripheral neuropathies (IPN) represent one of the most frequent inherited causes of neurological disability with an estimated prevalence of 1 in 2,500 [1]. Age at disease onset is usually within the first two decades of life but may be later as well. Clinical hallmarks comprise foot deformities, slowly progressive weakness and wasting of the distal parts of the lower limbs leading to gait disturbances, and usually distally pronounced sensory deficits. Involvement of the small hand muscles may also be present to a variable degree [2, 3]. All modes of inheritance have been described. Traditionally, IPN are subdivided into three main groups based on clinical and electrophysiological properties: the hereditary motor and sensory neuropathies (HMSN), i.e., the classical form, which is also known as Charcot–Marie–Tooth (CMT) syndrome. HMSN is further subdivided into the demyelinating form (HMSN1, CMT1) characterized by a considerable reduction of nerve conduction velocities (NCV; motor median nerve <38 m/s), an axonal variant (HMSN2, CMT2) with normal or slightly slowed NCV but low compound motor action potential amplitudes (CMAP), and an intermediate form (intermediate HMSN, ICMT) with NCV in the intermediate range (motor median nerve 25–45 m/s) [2, 3]. Patients lacking sensory disturbances both clinically and electrophysiologically have been classified as distal hereditary motor neuropathies (dHMN) [4, 5]. However, an overlap between dHMN and HMSN (mainly HMSN2) has been observed even within families [6, 7]. If patients present with predominant sensory (and autonomic) abnormalities and no or milder motor disturbances, the disease is called hereditary sensory (and autonomic) neuropathy (HSN, HSAN) [8, 9]. Furthermore, the IPN phenotype may be complicated by various additional neurological and/or non-neurological features [10–12].
Molecular genetic studies have demonstrated marked genetic heterogeneity of the IPN with more than 50 genes identified so far ([3] and are in part listed by http://www.molgen.ua.ac.be/CMTMutations/). However, many patients and families with IPN have not yet received a molecular genetic diagnosis. One explanation is that clinical genetic testing usually does not include all known IPN genes (mostly due to restricted funds and limited availability of tests). Moreover, further genetic heterogeneity has been suggested and additional causative genes have to be elucidated [13].
Next-generation sequencing techniques including whole-exome sequencing (WES) and whole-genome sequencing (WGS) have now opened promising possibilities to find the disease-causing mutation in patients harboring any Mendelian disease. Recent studies have also demonstrated the diagnostic and scientific impact of WES and WGS in IPN patients [14–18].
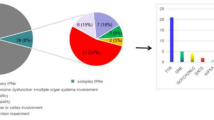
In the present study, WES was carried out in 27 patients with presumed rare or novel forms of IPN excluded for mutations in numerous known IPN genes (Supplementary Table 1). Thereby, eight patients (29.6 %) received an accurate diagnosis, while potentially disease-causing mutations were identified in another eight cases (29.6 %). In three families (11.1 %), we identified interesting new candidate genes for IPN. Another three probands (11.1 %) carried variants of unknown significance in genes known to cause other neuromuscular disease, but still in five patients (18.5 %) neither mutations in known IPN genes nor any strong candidate variants could be sorted out.
Patients and methods
Clinical and electrophysiological studies
Twenty-seven index probands who had received a diagnosis of IPN were included in this study. Clinical and electrophysiological studies were performed using standard methods as described previously [19]. Patients were subdivided according to the mode of inheritance and their phenotype, which either resembled HMSN or dHMN. Cases exhibiting IPN, but also additional neurological and/or non-neurological signs or symptoms, were classified as “complicated” IPN.
Ten patients showed dominant or autosomal dominant inheritance (HMSN-D1–D6; dHMN-D1–D4). Autosomal recessive inheritance was suspected in seven probands, all exhibiting an HMSN phenotype (HMSN-R1–R7). In ten patients, the disease occurred apparently sporadic. Four of these showed a “classical” HMSN-phenotype (HMSN-S1–S4), four had a complicated form of HMSN (HMSN-SC1-4) and two were classified as dHMN (dHMN-S1-S2) (Tables 1, 2, 3).
The study was approved by the local ethical committees of the Medical Universities of Vienna and Graz.
Genetic studies
Sanger sequencing
Prior to WES, up to 20 known IPN genes were excluded for mutations in the probands using Sanger sequencing techniques (Supplementary Table 1). Sequence variations detected by WES that were considered to be associated with the disease were confirmed by Sanger sequencing.
Whole-exome sequencing
Sequencing was performed on a HiSeq 2000 system (Illumina, San Diego, CA, USA) after in-solution enrichment of exonic and adjacent intronic sequences [SureSelect Human all Exon 50 Mb v3 and v4 kits (Agilent, Santa Clara, CA, USA)] and indexing of samples for multiplex-sequencing (Multiplexing Sample Preparation Oligonucleotide Kit, Illumina). We performed 100-bp paired-end runs yielding on average 8.8 Gb of sequence for 35 samples (the 27 index probands and for eight families an additional affected individual was included). The average read depth was 110 with 90 % of the targeted regions covered at least 20-fold. Read alignment was performed with BWA (version 0.6.1) to the human genome assembly hg19. Single-nucleotide variants and small insertions and deletions were called with SAMtools (version 0.1.18). We filtered variants to exclude HapMap-SNPs present in dbSNP-135 with an average heterozygosity greater than 0.02 and those present in more than four of 2,246 in-house exomes from individuals with unrelated diseases. Variant annotation was performed with custom scripts.
Linkage analysis
To perform linkage analysis, genomic DNA samples from all available affected and unaffected individuals and spouses of families HMSN-D5, HMSN-D6, HMSN-R4, and dHMN-D2 were hybridized to GeneChip Human Mapping 10 K 2.0 and 250 K Nsp SNP arrays (Affymetrix, Santa Clara, CA, USA) using the protocols recommended by the manufacturer. Parametric multipoint LOD scores and haplotypes were obtained with the ALOHOMORA program [20] based on linkage analysis from Merlin [21] under the assumption of an autosomal dominant or autosomal recessive, fully penetrant model. Pedigrees of the families, possible disease intervals, and maximum LOD scores detected for each family are shown in Supplementary Fig. 1a–1d.
Biochemical studies
Very long chain fatty acids (VLCFA) and phytanic acid concentrations in plasma were determined by GC/MS with deuterated internal standards as their methyl or dimethylsilyl derivatives according to established methods [22, 23]. Pipecolic acid was analyzed with a modified commercially available method designed for amino acid analysis by liquid chromatography/triple quadrupole mass spectrometry with pipecolic acid-D9 as internal standard (Phenomenex, Torrance, CA, USA).
Results
Clinical and electrophysiological findings in the index patients and results of WES are summarized in Tables 1, 2, and 3. Applying filtering as indicated above revealed up to 377 variants (108–377, mean 163) for each individual. First, we focused on significant SNVs in genes known to be involved in the pathogenesis of IPN. This approach resulted in 15 missense variants in 13 IPN genes (Table 4). Subsequently, we searched for variants in genes known to cause other neuromuscular or neurodegenerative diseases. Thereby we detected seven interesting variants in five genes (Table 5). Moreover, we identified three interesting novel candidate genes for IPN (Table 6). Variants were further evaluated in detail to define their potential impact on the disease (Tables 4, 5, 6). Whenever possible, further affected and unaffected family members were screened to confirm or exclude segregation.
SNVs in IPN genes regarded to be disease causing
In eight index cases we identified mutations which we regarded to be disease causing. These mutations affect amino acid residues that are well conserved among species. Most of them are predicted to be probably or possibly damaging by Polphen2 and SIFT (Tables 4, 5) and segregated within the families. Patient HMSN-D2 carried the c.1 A>T (p.M1L) missense variant in GJB1 (gap junction protein, beta 1, 32 kDa) that was also present in his less severely affected mother. The same mutation had been detected previously in another unrelated Austrian HMSN patient (unpublished data) exhibiting a similar phenotype thus making causality of the disease highly likely. In patient HMSN-D2 this variant had been missed by conventional Sanger sequencing in a laboratory offering genetic testing. In two patients (HMSN-R2 and HMSN-R7) displaying a classical HMSN2 phenotype we identified two novel homozygous mutations in GAN (gigaxonin) (c.1312 G>A, p.V438I; c.305 T>C, p.I102T). Segregation within the family could be confirmed for the c.305 T>C (p.I102T) mutation. The mutation in SPTLC1 (serine palmitoyltransferase, long chain base subunit 1) (c.992 C>A, p.S331Y, patient HMSN-SC1) resulted in an unexpected severe sensory motor neuropathy. The detailed phenotype has been reported recently [24].
Patient HMSN-SC2, exhibiting a severe HMSN phenotype with unrecordable NCV in the upper and lower limbs, was homozygous for the c.1066 C>T (p.R356X) mutation in SBF2 (SET binding factor 2). In addition to prominent distal muscle weakness and wasting, the patient presented with moderate scoliosis and a hoarse voice due to unilateral vocal fold paralysis. The c.1415 A>G (p.H472R) mutation in GARS (glycyl-tRNA synthetase), which had already been reported previously [25], was identified in family dHMN-D1 and was also present in the similarly affected father.
SNVs in non-IPN genes regarded to be disease causing
Proband HMSN-SC3 was initially diagnosed as HMSN2 because of prominent pes cavus, distal muscle wasting, and axonal neuropathy being the most striking feature at the beginning of the disease. However, with progression of the disease complications like bilateral juvenile cataracts, cerebellar and pyramidal tracts signs, and abnormalities of lipid metabolism became evident as well. WES identified a known homozygous mutation in CYP27A1 (cytochrome P450, family 27, subfamily A, polypeptide 1) (c.1016 C>T, p.T339M), the gene responsible for cerebrotendinous xanthomatosis [26, 27]. The advanced clinical presentation fits well with the latter diagnosis although initial predominant peripheral neuropathy may be an unusual finding. The mutation in REEP1 (receptor accessory protein 1) (c.304-2 A>G; family dHMN-D4) and the associated phenotype has been reported elsewhere [16].
SNVs of unknown significance in known IPN genes
A further eight patients carried novel mutations in known IPN genes (Table 4). The significance of these variations has remained unclear so far for several reasons. Mutations in DCTN1 (dynactin 1) have been reported in patients with dHMN and amyotrophic lateral sclerosis [28, 29] but not in HMSN2 as is the phenotype of family HMSN-D1 carrying the c.2009 A>T (p.Y670F) mutation, which has not yet been reported. Only one family without clinical symptoms but slow NCV has been reported carrying a mutation in ARHGEF10 (Rho guanine nucleotide exchange factor (GEF) 10) [30]. The two patients of family HMSN-D3 exhibit very mild intermediate HMSN and both carry a c.604 A>C (p.N202H) variant. We also identified mutations in AARS (alanyl-tRNA synthetase) and YARS (tyrosyl-tRNA synthetase), two further IPN genes [31, 32]: case HMSN-S4 carried a YARS mutation c.820 G>A (p.E274K) and proband dHMN-S1 carried an AARS mutation c.1823 C>T (p.T608M). Although all these variants alter well-conserved amino acid residues, we noted that a considerable number of rare missense variants affecting almost invariable residues occur in DCTN1, ARHGEF10, AARS, and YARS in our controls (Supplementary Table 2) and in other databases (http://evs.gs.washington.edu/EVS/; https://genomics.med.miami.edu/). Thus, caution seems warranted when evaluating these variants in these genes.
Mutations in HSJ1 (DnaJ (Hsp40) homolog, subfamily B, member 2) have been described in one recessive dHMN family only [33]. However, the phenotype in family HSMN-R1 carrying a c.14 A>G (p.Y5C) mutation resembles HMSN2. To confirm the pathogenicity of this mutation, functional studies are currently ongoing.
Mutations in MFN2 (mitofusin 2) have been reported in both autosomal dominant and autosomal recessive HMSN [34–37]. The heterozygous c.776 G>A (p.R259H) MFN2 mutation in family HMSN-R6 presenting with a mild HMSN2 phenotype affects a highly conserved residue but does not segregate within the family. If this MFN2 variant was a true mutation, two carriers (III/4 and IV/1) would have remained asymptomatic (Supplementary Fig. 2). Individuals III/4 and IV/1 are neurologically and electrophysiologically normal except for diminished tendon reflexes in the lower limbs in III/4 and very mild pes cavus foot deformity in IV/1.
Moreover, we identified compound heterozygosity for PRX (periaxin) (c.604 T>C, p.V525A and c.4004 G>A, p.R1335Q) in a patient with a complex phenotype including dysarthria, cerebellar signs, and hypermobility of joints (HMSN-SC4). While the c.4004 G>A (p.R1335Q) mutation is novel and involves a residue moderately conserved among species, the c.604 T>C (p.V525A) variant is a known rare polymorphism affecting a weakly conserved amino acid [38].
Finally, the c.117 G>C (p.K39N) variant in GDAP1 (ganglioside-induced differentiation associated protein 1) changes a well-conserved amino acid, segregates within the small family dHMN-D2, and is one of only three variants located with the possible linkage regions detected (Supplementary Fig. 1d). However, a dHMN phenotype is unusual in context with dominant GDAP1-associated neuropathy.
Identification of IPN candidate genes
Linkage analysis was most helpful in selecting possible candidate genes in small IPN families. Results are summarized in Supplementary Fig. 1a–1d. WES in families HMSN-D5 and HMSN-D6 (Supplementary Fig. 1a and 1b) both classified as HMSN1 based on NCV studies did not reveal a variation in the known IPN genes. Also, the two families did not share variants within a single gene. Therefore, we scrutinized all variants located within the regions of possible linkage in each family. Given the structure of the pedigrees six chromosomal regions in family HMSN-D5 and more than ten loci in family HMSN-D6 reached the corresponding maximum LOD scores of 1.5 and 1.2, respectively. Combining data of linkage analysis and WES revealed only one candidate gene for family HMSN-D5 (SH3BP4 gene (SH3-domain binding protein 4): c.47 G>A, p.R16H) and two for family HMSN-D6 (ITPR3 gene (inositol 1,4,5-trisphosphate receptor, type 3): c.4271 C>T, p.T1424M and TEC gene (tec protein tyrosine kinase): c.700 G>A, p.V234I) (Supplementary Fig. 1a and 1b). All three variants affected well-conserved amino acid residues (Supplementary Fig. 1a and 1b). In line with data fom the linkage screens, segregation of these variants was confirmed by Sanger sequencing.
Family HMSN-R3 consists of two affected brothers exhibiting severe early onset intermediate HMSN leading to wheelchair dependence after age 30. The parents were unaffected by history. By filtering for X-chromosomal, homozygous, and compound heterozygous variants, only a variant on the X-chromosome affecting the KLHL13 gene (kelch-like family member 13) (c.1127 T>C, p.L376S) was detected. This variant was also present in the affected brother.
Mutations in genes known to cause other neuromuscular diseases
In the remaining families, we did not find potentially relevant variants in any of the known IPN genes. However, family HMSN-R4 was compound heterozygous for two well-conserved variants in PEX12 (peroxisomal biogenesis factor 12), a gene previously reported to cause a severe autosomal recessive infantile disease, called Zellweger syndrome [39–41]. While the c.538 C>T (p.R180X) mutation had already been reported in Zellweger patients [40], the c.569 C>T (p.S190L) variant is novel but affects a strongly conserved residue. Testing of further family members revealed segregation with the phenotype. PEX12 was the only gene in the regions suggestive for linkage harboring two mutations (Supplementary Fig. 1c). As patients with Zellweger syndrome have elevated levels of VLCFA and phytanic acid, we tested serum of our patients for these acids. While VLCFA and phytanic acid were normal, we repeatedly found elevated levels of pipecolic acid in both patients but not in the healthy parents. In cultured fibroblasts of the patients, 70–75 % of catalyze activity was particle bound while in control fibroblasts 95–100 % are associated with peroxisomes.
In patient dHMN-D3, we detected a novel mutation in RYR1 (ryanodine receptor 1 (skeletal)) (c.1655 G>A, p.R552Q), a gene which is involved in malignant hyperthermia and central core disease [42]. This mutation was also present in the affected father.
Patient HMSN-S2 was compound heterozygous for two SNVs in GAA (glucosidase, alpha; acid) (c.1912 G>T, p.G638W; c.2749 C>T, p.L917F), the gene responsible for Pompe’s disease [43, 44].
Detection of mutations in genes causing other Mendelian diseases
In addition to dHMN, patient dHMN-S1 was affected with piebaldism, an autosomal dominant skin disorder that was present in three generations of the family. The genetic cause had not yet been identified. WES revealed a known heterozygous missense variant (c.1747 G>C, p.E583Q) in KIT (v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog), the gene most frequently involved in the pathogenesis of piebaldism [45].
In total, 4–47 (mean 22) SNVs in different genes causing dominant and recessive Mendelian diseases could be detected in every proband but these variants were not further evaluated.
Discussion
Among 27 patients who had already been screened negative for many known IPN genes and were thus highly enriched for mutations in rarer IPN genes, we identified the disease-causing mutation in eight cases (29.6 %) by WES. This result is in line with previous studies [17] and highlights the power of WES in the diagnosis of rare forms of IPN. In six of these subjects, mutations in known IPN genes (GAN, GARS, GJB1, SBF2, SPTLC1) were detected, while in two cases the responsible gene was previously reported to cause another neurodegenerative disease (CYP27A1, REEP1). To interpret the value of each candidate variant, we used prediction programs (Polyphen 2, Sift), considered alignment of sequences with multiple organisms, and tested for segregation of the variants within the families whenever possible. Variants in these eight patients were either predicted to be probably or possibly damaging and targeted strongly conserved amino acid residues (Tables 4, 5). Interestingly, we found a mutation in GJB1, a gene that is frequently mutated in HMSN and therefore included in routine diagnosis as was the case in patient HMSN-D2. This demonstrates that WES may detect variants that have been missed by conventional Sanger sequencing. It is noteworthy that two patients with classical HMSN2 carried mutations in GAN. None of the patients had cerebellar signs or characteristic hair changes as described previously [46–48]. A “pure” HMSN2 phenotype due to GAN mutations is unusual and unexpected. We therefore suggest that sequence analysis of GAN should more often be included in routine diagnosis in childhood-onset HMSN2 patients.
Notably, three of four individuals (i.e., 75 %) classified as sporadic and complicated IPN received a definite diagnosis. In patient HMSN-SC2, we found a novel homozygous nonsense mutation in SBF2. Mutations in SBF2 are known to cause severe recessive HMSN1, which may be accompanied by glaucoma [49–51]. The latter feature was not observed in our patient. However, a hoarse voice was noted and could be explained by unilateral vocal fold paralysis, thus expanding the phenotypic spectrum of SBF2-associated IPN. Vocal fold paralysis is common in dominant HMSN2C caused by mutations in TRPV4 (transient receptor potential cation channel, subfamily V, member 4) [52] and has also been reported in patients with recessive HMSN4A due to GDAP1 mutations [53]. Patient HMSN-SC3 initially presented with pes cavus, gait abnormalities, and an axonal neuropathy in the lower limbs. Other features like ataxia, cataracts, elevated serum lipid levels, and dementia, which are typically seen in patients with cerebrotendinous xanthomatosis due to CYP27A1 mutations developed later, thus postponing the distinct diagnosis on a clinical basis [26]. This case demonstrates that in particular conditions, WES may serve to establish a diagnosis on the basis of the genotype leading to re-assessment of the phenotype. Finally, the complicated, syndromic phenotype of patient HMSN-SC1, which has already been reported in detail, could surprisingly be explained by a distinct mutation in SPTLC1 [24].
In a further eight subjects (29.6 %), we identified mutations in known IPN genes (DCTN1, ARHGEF10, HSJ1, MFN2, YARS, PRX, GDAP1, AARS). Most of these targeted well-conserved amino acid residues and were predicted to be probably or possibly damaging (Table 4). However, interpretation of clinical significance turned out to be challenging for several reasons. For some of these genes, well-conserved SNVs not reported in any database and absent in our large series of 2,246 controls are also frequently found in individuals not afflicted with IPN (Supplementary Table 2). This raises the question of how to sort out true disease-causing mutations. Testing of further family members to confirm segregation is an attractive option, but this requires availability and cooperation of larger families. As functional studies are usually expensive and time-consuming and not always readily available, systematic comparison of SNV in IPN databases will become a promising strategy to provide a correct diagnosis for patients. Currently, this service is already offered at https://genomics.med.miami.edu. Patients carrying sequence variants of unknown significance in IPN genes should be invited to genetic counseling and have to be informed that a final diagnosis based on results available by WES is currently challenging. Although dominant mutations in GDAP1 have been reported in IPN families [54], we were cautious to define the c.117 G>C (p.K39N) mutation in our family dHMN-D2 as definitively disease-causing. To make any firm conclusions, additional patients with dHMN carrying heterozygous GDAP1 mutations have to be identified.
As has been shown in previous studies [16], a combination of linkage analysis and WES was helpful in identifying novel candidate genes for IPN in this study. Combining linkage and WES data of four families dramatically reduced the number of SNVs and identified interesting candidate genes (Supplementary Fig. 1a–1d). In family HMSN-D5, a mutation in SH3BP4 (c.47 G>A, p.R16H) was the only variant located within the regions of suggestive linkage. SH3BP4 functions in transferrin receptor internalization at the plasma membrane through a cargo-specific control of clathrin-mediated endocytosis [55]. It interacts with DNM2 (dynamin 2) a protein which is also involved in the pathogenesis of IPN [56]. In family HMSN-D6, two unknown SNVs remained within the regions of potential linkage. The most interesting candidate gene is ITPR3 [57]. It encodes a receptor for inositol 1,4,5-trisphosphate, a second messenger that mediates the release of intracellular calcium. ITPR3 is expressed in distinct cellular domains of the Schwann cells, particularly in dense patches in the paranodal region. Notably, connexin 32 (Cx32), a gap junction protein responsible for HMSN X, is expressed in close proximity with ITPR3. It has therefore been speculated that Schwann cell Ca2+ signals control the function of the gap junctions, or that the gap junctional channels serve as conduits for rapid radial spread of Ca2+ signals initiated during action potential propagation [58]. Sequencing of SH3BP4 and exon 32 of ITPR3 in more than 30 HMSN1 families did not reveal further mutations, indicating that these genes might—if so—be a rare cause of IPN. In family HMSN-R3, we suspected autosomal recessive inheritance as the parents were reported to be unaffected. However, no homozygous or compound heterozygous mutations could be detected. Instead, we identified a mutation in KLHL13, which is located on chromosome X and encodes a BTB (Bric-a-brac–Tramtrack–Broad complex) and kelch domain-containing protein [59].
Of particular interest is family HMSN-R4 with severe intermediate HMSN. Autosomal recessive inheritance is likely as the parents were clinically and electrophysiologically normal after age 80. The only gene harboring biallelic mutations and being located in one of the potential linkage regions was PEX12. Segregation of the variants within the family was confirmed (Supplementary Fig. 1c). While one of the mutations (c.538 C>T, p.R180X) has already been reported in Zellweger’s disease, the second (c.569 C>T, p.S190L) was novel. Testing for metabolic consequences of the two mutations yielded normal values for VLCFA and phytanic acids but considerably elevated levels of pipecolic acids in both affected probands but not in the heterozygous parents. Furthermore, in cell lines from both patients, a considerable fraction of catalase activity is cytosolic. Functional studies are ongoing to further explain these results. However, no firm conclusions can be drawn that particular recessive mutations in PEX12 can produce an IPN phenotype. It cannot be ruled out that the PEX12, SH3BP4, ITPR3, and KLHL13 variants represent harmless variants and that large insertions, deletions, chromosomal rearrangements, or intronic variants not detected by WES are the true disease-causing mutation in these families. Therefore, identification of mutations in PEX12, SH3BP4, ITPR3, and KLHL13 in further IPN families as well as functional studies are needed to confirm whether these are indeed genes for IPN.
Finally, given the example of patient dHMN-S1 presenting with dHMN and a dominant skin disease due to a KIT c.1747 G>C (p.E583Q) mutation, this study demonstrates that WES is a suitable method for screening patients harboring more than one Mendelian disease.
In summary, we confirm that WES is an efficient tool in the diagnosis of IPN but interpretation of variants in known and potential novel disease genes has remained challenging. We suggest an expanded phenotypic spectrum of some genes involved in other neuromuscular and neurodegenerative disorders and introduce three novel IPN candidate genes. Based on the results obtained in this study, we conclude that WES should be preferred to IPN/CMT panels if patients present with a complicated phenotype and/or if many IPN genes have already been excluded.
References
Skre H (1974) Genetic and clinical aspects of Charcot–Marie–Tooth’s disease. Clin Genet 6:98–118
Dyck PJ, Chance P, Lebo R, Carney JA (1993) Hereditary motor and sensory neuropathies. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF (eds) Peripheral neuropathy, 3rd edn. Saunders, Philadelphia, pp 1094–1136
Reilly MM, Murphy SM, Laurá M (2011) Charcot–Marie–Tooth disease. J Peripher Nerv Syst 16:1–14
Harding AE (1993) Inherited neuronal atrophy and degeneration predominantly of lower motor neurons. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF (eds) Peripheral neuropathy, 3rd edn. Saunders, Philadelphia, pp 1051–1064
Rossor AM, Kalmar B, Greensmith L, Reilly MM (2012) The distal hereditary motor neuropathies. J Neurol Neurosurg Psychiatry 83:6–14
Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ, Jordanova A, Kremensky I, Christodoulou K, Middleton LT, Sivakumar K, Ionasescu V, Funalot B, Vance JM, Goldfarb LG, Fischbeck KH, Green ED (2003) Glycyl tRNA synthetase mutations in Charcot–Marie–Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet 72:1293–1299
Auer-Grumbach M, Schlotter-Weigel B, Lochmüller H, Strobl-Wildemann G, Auer-Grumbach P, Fischer R, Offenbacher H, Zwick EB, Robl T, Hartl G, Hartung HP, Wagner K, Windpassinger C, Austrian Peripheral Neuropathy Study Group (2005) Phenotypes of the N88S Berardinelli-Seip congenital lipodystrophy 2 mutation. Ann Neurol 57:415–424
Dyck PJ (1993) Neuronal atrophy and degeneration predominantly affecting peripheral sensory and autonomic neurons. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF (eds) Peripheral neuropathy, 3rd edn. Saunders, Philadelphia, pp 1065–1093
Auer-Grumbach M, Mauko B, Auer-Grumbach P, Pieber TR (2006) Molecular genetics of hereditary sensory neuropathies. Neuromol Med 8:147–158
Kiwaki T, Umehara F, Takashima H, Nakagawa M, Kamimura K, Kashio N, Sakamoto Y, Unoki K, Nobuhara Y, Michizono K, Watanabe O, Arimura H, Osame M (2000) Hereditary motor and sensory neuropathy with myelin folding and juvenile onset glaucoma. Neurology 55:392–397
Münch C, Rosenbohm A, Sperfeld AD, Uttner I, Reske S, Krause BJ, Sedlmeier R, Meyer T, Hanemann CO, Stumm G, Ludolph AC (2005) Heterozygous R1101K mutation of the DCTN1 gene in a family with ALS and FTD. Ann Neurol 58:777–780
Auer-Grumbach M, Weger M, Fink-Puches R, Papić L, Fröhlich E, Auer-Grumbach P, El Shabrawi-Caelen L, Schabhüttl M, Windpassinger C, Senderek J, Budka H, Trajanoski S, Janecke AR, Haas A, Metze D, Pieber TR, Guelly C (2011) Fibulin-5 mutations link inherited neuropathies, age-related macular degeneration and hyperelastic skin. Brain 134:1839–1852
Murphy SM, Laura M, Fawcett K, Pandraud A, Liu YT, Davidson GL, Rossor AM, Polke JM, Castleman V, Manji H, Lunn MP, Bull K, Ramdharry G, Davis M, Blake JC, Houlden H, Reilly MM (2012) Charcot–Marie–Tooth disease: frequency of genetic subtypes and guidelines for genetic testing. J Neurol Neurosurg Psychiatry 83:706–710
Lupski JR, Reid JG, Gonzaga-Jauregui C, Rio Deiros D, Chen DC, Nazareth L, Bainbridge M, Dinh H, Jing C, Wheeler DA, McGuire AL, Zhang F, Stankiewicz P, Halperin JJ, Yang C, Gehman C, Guo D, Irikat RK, Tom W, Fantin NJ, Muzny DM, Gibbs RA (2010) Whole-genome sequencing in a patient with Charcot–Marie–Tooth neuropathy. N Engl J Med 362:1181–1191
Montenegro G, Powell E, Huang J, Speziani F, Edwards YJ, Beecham G, Hulme W, Siskind C, Vance J, Shy M, Züchner S (2011) Exome sequencing allows for rapid gene identification in a Charcot–Marie–Tooth family. Ann Neurol 69:464–470
Beetz C, Pieber TR, Hertel N, Schabhüttl M, Fischer C, Trajanoski S, Graf E, Keiner S, Kurth I, Wieland T, Varga RE, Timmerman V, Reilly MM, Strom TM, Auer-Grumbach M (2012) Exome sequencing identifies a REEP1 mutation involved in distal hereditary motor neuropathy type V. Am J Hum Genet 91:139–145
Choi BO, Koo SK, Park MH, Rhee H, Yang SJ, Choi KG, Jung SC, Kim HS, Hyun YS, Nakhro K, Lee HJ, Woo HM, Chung KW (2012) Exome sequencing is an efficient tool for genetic screening of Charcot–Marie–Tooth disease. Hum Mutat 33:1610–1615
Zimoń M, Baets J, Almeida-Souza L, De Vriendt E, Nikodinovic J, Parman Y, Battaloğlu E, Matur Z, Guergueltcheva V, Tournev I, Auer-Grumbach M, De Rijk P, Petersen BS, Müller T, Fransen E, Van Damme P, Löscher WN, Barišić N, Mitrovic Z, Previtali SC, Topaloğlu H, Bernert G, Beleza-Meireles A, Todorovic S, Savic-Pavicevic D, Ishpekova B, Lechner S, Peeters K, Ooms T, Hahn AF, Züchner S, Timmerman V, Van Dijck P, Rasic VM, Janecke AR, De Jonghe P, Jordanova A (2012) Loss-of-function mutations in HINT1 cause axonal neuropathy with neuromyotonia. Nat Genet 44:1080–1083
Auer-Grumbach M, Strasser-Fuchs S, Robl T, Windpassinger C, Wagner K (2003) Late onset Charcot–Marie–Tooth 2 syndrome caused by two novel mutations in the MPZ gene. Neurology 61:1435–1437
Rüschendorf F, Nürnberg P (2005) ALOHOMORA: a tool for linkage analysis using 10K SNP array data. Bioinformatics 21:2123–2125
Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin–rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101
Moser HW, Moser AB (1991) Measurement of saturated very long chain fatty acids in plasma. In: Hommes F (ed) Techniques in diagnostic human biochemical genetics. Wiley, New York, pp 177–191
Vreken P, van Lint AE, Bootsma AH, Overmars H, Wanders RJ, van Gennip AH (1998) Rapid stable isotope dilution analysis of very-long-chain fatty acids, pristanic acid and phytanic acid using gas chromatography-electron impact mass spectrometry. J Chromatogr B Biomed Sci Appl 713:281–287
Auer-Grumbach M, Bode H, Pieber TR, Schabhüttl M, Fischer D, Seidl R, Graf E, Wieland T, Schuh R, Vacariu G, Grill F, Timmerman V, Strom TM, Hornemann T (2013) Mutations at Ser331 in the HSN type I gene SPTLC1 are associated with a distinct syndromic phenotype. Eur J Med Genet 56:266–269
Sivakumar K, Kyriakides T, Puls I, Nicholson GA, Funalot B, Antonellis A, Sambuughin N, Christodoulou K, Beggs JL, Zamba-Papanicolaou E, Ionasescu V, Dalakas MC, Green ED, Fischbeck KH, Goldfarb LG (2005) Phenotypic spectrum of disorders associated with glycyl-tRNA synthetase mutations. Brain 128:2304–2314
Cali JJ, Hsieh CL, Francke U, Russell DW (1991) Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem 266:7779–7783
Guyant-Maréchal L, Verrips A, Girard C, Wevers RA, Zijlstra F, Sistermans E, Vera P, Campion D, Hannequin D (2005) Unusual cerebrotendinous xanthomatosis with fronto-temporal dementia phenotype. Am J Med Genet A 139A:114–117
Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, Brown RH Jr, Ludlow CL, Fischbeck KH (2003) Mutant dynactin in motor neuron disease. Nat Genet 33:455–456
Münch C, Sedlmeier R, Meyer T, Homberg V, Sperfeld AD, Kurt A, Prudlo J, Peraus G, Hanemann CO, Stumm G, Ludolph AC (2004) Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology 63:724–726
Verhoeven K, De Jonghe P, Van de Putte T, Nelis E, Zwijsen A, Verpoorten N, De Vriendt E, Jacobs A, Van Gerwen V, Francis A, Ceuterick C, Huylebroeck D, Timmerman V (2003) Slowed conduction and thin myelination of peripheral nerves associated with mutant rho Guanine-nucleotide exchange factor 10. Am J Hum Genet 73:926–932
Jordanova A, Irobi J, Thomas FP, Van Dijck P, Meerschaert K, Dewil M, Dierick I, Jacobs A, De Vriendt E, Guergueltcheva V, Rao CV, Tournev I, Gondim FA, D’Hooghe M, Van Gerwen V, Callaerts P, Van Den Bosch L, Timmermans JP, Robberecht W, Gettemans J, Thevelein JM, De Jonghe P, Kremensky I, Timmerman V (2006) Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot–Marie–Tooth neuropathy. Nat Genet 38:197–202
Latour P, Thauvin-Robinet C, Baudelet-Méry C, Soichot P, Cusin V, Faivre L, Locatelli MC, Mayençon M, Sarcey A, Broussolle E, Camu W, David A, Rousson R (2010) A major determinant for binding and aminoacylation of tRNA(Ala) in cytoplasmic Alanyl-tRNA synthetase is mutated in dominant axonal Charcot–Marie–Tooth disease. Am J Hum Genet 86:77–82
Blumen SC, Astord S, Robin V, Vignaud L, Toumi N, Cieslik A, Achiron A, Carasso RL, Gurevich M, Braverman I, Blumen N, Munich A, Barkats M, Viollet L (2012) A rare recessive distal hereditary motor neuropathy with HSJ1 chaperone mutation. Ann Neurol 71:509–519
Züchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, De Jonghe P, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schröder JM, Vance JM (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot–Marie–Tooth neuropathy type 2A. Nat Genet 36:449–451
Chung KW, Kim SB, Park KD, Choi KG, Lee JH, Eun HW, Suh JS, Hwang JH, Kim WK, Seo BC, Kim SH, Son IH, Kim SM, Sunwoo IN, Choi BO (2006) Early onset severe and late-onset mild Charcot–Marie–Tooth disease with mitofusin 2 (MFN2) mutations. Brain 129:2103–2118
Nicholson GA, Magdelaine C, Zhu D, Grew S, Ryan MM, Sturtz F, Vallat JM, Ouvrier RA (2008) Severe early-onset axonal neuropathy with homozygous and compound heterozygous MFN2 mutations. Neurology 70:1678–1681
Polke JM, Laurá M, Pareyson D, Taroni F, Milani M, Bergamin G, Gibbons VS, Houlden H, Chamley SC, Blake J, Devile C, Sandford R, Sweeney MG, Davis MB, Reilly MM (2011) Recessive axonal Charcot–Marie–Tooth disease due to compound heterozygous mitofusin 2 mutations. Neurology 77:168–173
Guilbot A, Williams A, Ravisé N, Verny C, Brice A, Sherman DL, Brophy PJ, LeGuern E, Delague V, Bareil C, Mégarbané A, Claustres M (2001) A mutation in periaxin is responsible for CMT4F, an autosomal recessive form of Charcot–Marie–Tooth disease. Hum Mol Genet 10:415–421
Chang CC, Lee WH, Moser H, Valle D, Gould SJ (1997) Isolation of the human PEX12 gene, mutated in group 3 of the peroxisome biogenesis disorders. Nat Genet 15:385–388
Okumoto K, Shimozawa N, Kawai A, Tamura S, Tsukamoto T, Osumi T, Moser H, Wanders RJ, Suzuki Y, Kondo N, Fujiki Y (1998) PEX12, the pathogenic gene of group III Zellweger syndrome: cDNA cloning by functional complementation on a CHO cell mutant, patient analysis, and characterization of PEX12p. Mol Cell Biol 18:4324–4336
Gootjes J, Skovby F, Christensen E, Wanders RJ, Ferdinandusse S (2004) Reinvestigation of trihydroxycholestanoic acidemia reveals a peroxisome biogenesis disorder. Neurology 62:2077–2081
Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P (2006) Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat 27:977–989
Martiniuk F, Mehler M, Pellicer A, Tzall S, La Badie G, Hobart C, Ellenbogen A, Hirschhorn R (1986) Isolation of a cDNA for human acid alpha-glucosidase and detection of genetic heterogeneity for mRNA in three alpha-glucosidase-deficient patients. Proc Natl Acad Sci USA 83:9641–9644
Vorgerd M, Burwinkel B, Reichmann H, Malin JP, Kilimann MW (1998) Adult-onset glycogen storage disease type II: phenotypic and allelic heterogeneity in German patients. Neurogenetics 1:205–211
Fleischman RA (1992) Human piebald trait resulting from a dominant negative mutant allele of the c-kit membrane receptor gene. J Clin Invest 89:1713–1717
Nalini A, Gayathri N, Yasha TC, Ravishankar S, Urtizberea A, Huehne K, Rautenstrauss B (2008) Clinical, pathological and molecular findings in two siblings with giant axonal neuropathy (GAN): report from India. Eur J Med Genet 51:426–435
Tazir M, Nouioua S, Magy L, Huehne K, Assami S, Urtizberea A, Grid D, Hamadouche T, Rautenstrauss B, Vallat JM (2009) Phenotypic variability in giant axonal neuropathy. Neuromuscul Disord 19:270–274
Buysse K, Vergult S, Mussche S, Ceuterick-de Groote C, Speleman F, Menten B, Lissens W, Van Coster R (2010) Giant axonal neuropathy caused by compound heterozygosity for a maternally inherited microdeletion and a paternal mutation within the GAN gene. Am J Med Genet A 152A:2802–2804
Azzedine H, Bolino A, Taïeb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, Gouider R, Ravazzolo R, Brice A, Laporte J, LeGuern E (2003) Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot–Marie–Tooth disease associated with early-onset glaucoma. Am J Hum Genet 72:1141–1153
Senderek J, Bergmann C, Weber S, Ketelsen UP, Schorle H, Rudnik-Schöneborn S, Büttner R, Buchheim E, Zerres K (2003) Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot–Marie–Tooth neuropathy type 4B2/11p15. Hum Mol Genet 12:349–356
Hirano R, Takashima H, Umehara F, Arimura H, Michizono K, Okamoto Y, Nakagawa M, Boerkoel CF, Lupski JR, Osame M, Arimura K (2004) SET binding factor 2 (SBF2) mutation causes CMT4B with juvenile onset glaucoma. Neurology 63:577–580
Zimoń M, Baets J, Auer-Grumbach M, Berciano J, Garcia A, Lopez-Laso E, Merlini L, Hilton-Jones D, McEntagart M, Crosby AH, Barisic N, Boltshauser E, Shaw CE, Landouré G, Ludlow CL, Gaudet R, Houlden H, Reilly MM, Fischbeck KH, Sumner CJ, Timmerman V, Jordanova A, De Jonghe P (2010) Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain 133:1798–1809
Sevilla T, Cuesta A, Chumillas MJ, Mayordomo F, Pedrola L, Palau F, Vílchez JJ (2003) Clinical, electrophysiological and morphological findings of Charcot–Marie–Tooth neuropathy with vocal cord palsy and mutations in the GDAP1 gene. Brain 126:2023–2033
Zimoń M, Baets J, Fabrizi GM, Jaakkola E, Kabzińska D, Pilch J, Schindler AB, Cornblath DR, Fischbeck KH, Auer-Grumbach M, Guelly C, Huber N, De Vriendt E, Timmerman V, Suter U, Hausmanowa-Petrusewicz I, Niemann A, Kochański A, De Jonghe P, Jordanova A (2011) Dominant GDAP1 mutations cause predominantly mild CMT phenotypes. Neurology 77:540–548
Tebar F, Sorkina T, Sorkin A, Ericsson M, Kirchhausen T (1996) Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J Biol Chem 271:28727–28730
Sidiropoulos PN, Miehe M, Bock T, Tinelli E, Oertli CI, Kuner R, Meijer D, Wollscheid B, Niemann A, Suter U (2012) Dynamin 2 mutations in Charcot–Marie–Tooth neuropathy highlight the importance of clathrin-mediated endocytosis in myelination. Brain 135:1395–1411
Maranto AR (1994) Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J Biol Chem 269:1222–1230
Toews JC, Schram V, Weerth SH, Mignery GA, Russell JT (2007) Signaling proteins in the axoglial apparatus of sciatic nerve nodes of Ranvier. Glia 55:202–213
Sumara I, Quadroni M, Frei C, Olma MH, Sumara G, Ricci R, Peter M (2007) A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev Cell 12:887–900
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249
Acknowledgments
We are grateful for the participation of the patients and families in this study. This work was supported by the Austrian Science Fund (FWF, P23223-B19), the University of Antwerp (UA), the Association Belge contre les Maladies Neuromusculaires (ABMM), the Medical Foundation Queen Elisabeth (GSKE), the agency for Innovation by Science and Technology (IWT), the Fund for Scientific Research Flanders (FWO-Flanders) and the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement number 2012-305121 “Integrated European–omics research project for diagnosis and therapy in rare neuromuscular and neurodegenerative diseases (NEUROMICS)”.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schabhüttl, M., Wieland, T., Senderek, J. et al. Whole-exome sequencing in patients with inherited neuropathies: outcome and challenges. J Neurol 261, 970–982 (2014). https://doi.org/10.1007/s00415-014-7289-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7289-8