Abstract
Cognitive impairment in multiple sclerosis (MS) is common, debilitating and burdensome. Key evidence from trials was reviewed to enable recommendations to be made to guide clinical practice and research. Behavioural and pharmacological interventions on cognition reported in published studies were reviewed. Most studies evaluating behavioural treatment for impairment in learning and memory, deficits of attention and executive function have demonstrated some improvement. Controlled studies in relapsing remitting MS indicate interferon (IFN) β-1b and IFN β-1a were associated with modest cognitive improvement. The effects of symptomatic therapies such as modafinil and donepezil are inconsistent. Most studies yielding positive findings have significant methodological difficulties limiting the confidence in making any broad treatment recommendations. There are no published reports of glatiramer acetate, natalizumab and fingolimod being effective in improving cognition in controlled trials. The effects of disease modifying therapies in other forms of MS and clinically isolated syndrome have not yielded positive results. Data linking behavioural therapy, symptomatic treatment or disease modifying treatment, to either reducing cognitive decline or improving impaired cognition are limited and inconsistent. The treatment and prevention of cognitive impairment needs to remain a key research focus, identifying new interventions and improving clinical trial methodology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impairment of cognitive function in multiple sclerosis (MS) is estimated to affect 40–60 % of patients [1–4]. While severe dementia is rare, it has been estimated that up to 20 % of patients develop at least a mild form of dementia [3–5]. Cognitive impairment has been detected in all the disease subtypes [6] although it progresses over time [7] and is most frequent and severe in secondary progressive MS (SPMS) [6, 8] Cognitive deficits are detected in approximately one-third of patients with early relapsing remitting MS (RRMS) [9], 20–30 % of patients with clinically isolated syndromes (CIS) [10, 11] and even and in some patients with radiologically isolated syndrome [12, 13]. The extent of cognitive impairment noted in a subset of patients with so-called benign MS (low EDSS with disease duration of over 15 years) [14] brings into question the appropriateness of the term “benign”.
Typically, not all domains of cognitive functioning are impaired in MS. Although the profile of cognitive deficits varies among patients, memory (long-term, explicit, episodic), complex attention, information processing speed and executive functions are most commonly involved; language, semantic memory and attention span are rarely involved [7, 15, 16]. The pathophysiological changes that underpin the development and progression of cognitive impairment in MS patients are complex, highly variable, and incompletely understood [17]. The correlation between magnetic resonance imaging (MRI) findings and cognitive performance in MS is consistently robust, but only one-third to one-half of the variance can be explained by MRI findings [15]. Cognitive reserve, a behavioural adaptation acquired through experience which improves cognitive performance in increase phenotypic expression in the presence of disease, could explain the high interindividual variability in cognitive deficits in MS and the limited correlation with MRI findings [18].
Cognitive dysfunction in MS presents a considerable burden to patients and to society, due to the negative impact on function, including maintaining employment, activities of daily living, social activity, and the capacity to benefit from in-patient rehabilitation [7]. In some individuals with MS the impact of cognitive impairment can be profound, even if physical functioning remains relatively intact. Interventions to ameliorate or reduce cognitive impairment, as part of a comprehensive rehabilitation programme, may benefit patient function and quality of life.
To diagnose and quantify the extent of cognitive impairment, appropriate assessments are essential but often difficult. Patient report is unreliable and highly correlated with depressive symptomatology [19, 20]. Unfortunately, routine neurological examinations for MS are too insensitive to yield valid information on cognitive function. For example, the expanded disability status scale (EDSS), does not include an adequate assessment of cognitive dysfunction. The development of the Multiple Sclerosis Functional Composite (MSFC) which includes the Paced Auditory Serial Addition Test (PASAT) [21] was a step forward towards incorporating a sensitive measure of cognition into a standardized rapid MS assessment tool.
The challenge with more detailed and comprehensive performance-based cognitive evaluations is that while they are the most reliable, they can be time consuming and impractical in many clinical settings. Screening patients to identify those with the highest likelihood of dysfunction would be ideal, but validated screening tools have yet to be developed or applied. One assessment approach is to use test batteries that range from 30 to 90 min in duration. The goal of these batteries is to capture the core features of MS-associated cognitive dysfunction. The Brief Repeatable Neuropsychological Battery (BRNB) [22] assesses those domains most commonly impaired in MS and is most widely used in clinical and research settings [7]. The Minimal Assessment of Cognitive Function in MS (MACFIMS), developed for a similar purpose, is a more recent battery created by expert consensus and published in 2002 [23]. These batteries differ in the specific auditory/verbal memory and visual/spatial memory tests employed, but assess similar domains, and are comparable in their overall sensitivity to disease status [24].
Despite the availability of such batteries, the assessment of cognitive function in research studies of MS is far from optimal. Methodological shortcomings include the variability of the domains assessed and the instruments used, the handling of common confounds such as fatigue and depression, and the inclusion of heterogeneous groups of patients in whom selection criteria for cognitive impairment were either applied inconsistently across studies or not applied at all. Examples of some of these methodological issues are shown in Table 1.
There is little information to guide clinicians on how to interpret the benefit, or lack thereof, of interventions designed to improve cognition in MS. Given the prevalence of cognitive impairment in MS, its adverse effects on daily function, and the fragmented nature of what is known about interventions to treat the condition, we thought it germane to review key evidence from trials with a view to providing interpretation and recommendations to guide practice and further research. Interventions including cognitive rehabilitation, the effects of symptomatic treatments and the effects of disease modifying treatments (DMTs) will be discussed. This is not a systematic review of all available literature that has ever addressed the topic, but rather a review of research that formed the basis of presentations on the topic given at the conference “Cognition Disorders in Multiple Sclerosis” which was held in October 2011.
Cognitive rehabilitation
Effective cognitive rehabilitation programmes in clinical settings do not only employ techniques designed solely to improve specific domains of cognitive function, but also typically include psychotherapy for addressing emotional issues and interventions designed to improve related factors such as behavioural and personality difficulties. While some integrated cognitive rehabilitation programmes exist for individuals with MS in clinical settings, few have been systematically evaluated, although there are exceptions, e.g., Jønsson et al. [25]. As specific cognitive interventions are an important component of a comprehensive rehabilitation programmes an understanding the impact on specific interventions on those domains of function that are of greatest clinical relevance in MS is important. Of particular interest are learning and memory, information processing speed, attention and executive function.
Learning and memory
Learning and memory has received the greatest research attention and may have the greatest impact on everyday life for people with MS. A number of papers have been published over the last two decades, especially more recently, on behavioural rehabilitation of learning and memory in MS patients. However, most studies suffer from significant methodological problems (see Table 1) [26]. A recent evidence-based review yielded only 16 papers, mostly from class II to class IV evidence [27], precluding conclusions about clear treatment benefits. This evidence-based review [27] was a systemic review employing strict inclusion and exclusion criteria for selecting the studies to be included in the review process, and therefore did not include several of the studies we cited in addressing this topic. The recent Cochrane review on the rehabilitation of memory in MS identified only eight studies, involving 521 patients in total, that met their standards for methodological rigour [28]. They concluded that there is no evidence to support the effectiveness of memory rehabilitation on memory function or functional abilities in patients with MS, but noted that this conclusion was made because of the limited quality of some of the primary studies reviewed in this area [28]. Despite methodological problems, there are several published studies that do report significant improvement in neuropsychological performance following behavioural treatment (Table 2).
Targeted interventions
Many of the interventions studied have been targeted on focused behavioural interventions designed to increase learning efficiency, as impaired acquisition of new information has been shown to be the primary problem in the learning and memory problems associated with MS [29–31]. Some of these targeted interventions have shown consistent support for improving learning and memory in MS across several studies and laboratories. For example, the use of self-generated learning (where patients generate the right answer versus being told what to remember) to improve the acquisition of new learning has been shown to improve recall and everyday functional activity, such as financial management and meal preparation [32], as well as the recall of names, appointments and object locations [33, 34].
Other targeted intervention techniques include spaced learning (spreading learning trials over time versus consecutive trials) [35] and spaced retrieval (also known as the ‘testing effect’) [18]. In the latter study, learning and memory impaired MS patients were required to study three sets of word pairs (in a within–group design); one word pair set was studied twice consecutively (massed trial), another set was studied twice but spaced over time (spaced trial), and the third set was studied only once, but was then tested. During subsequent recall, word pair retention was significantly better when material was tested compared to either the massed trial or the spaced trial, with patients recalling about twice as many word pairings as the massed studied material. A recent study examined whether utilizing two of these behavioural interventions (i.e., self-generation and spaced learning) was better than a single intervention alone (i.e., spaced learning). It demonstrated that the combined intervention achieved almost 50 % greater recall than the single technique alone [36]. A double-blind, placebo-controlled randomized clinical trial (RCT) designed to improve new learning by training use of context and imagery, to improve the strength of encoding, resulted in significantly improved recall on neuropsychological testing as well as self-report of everyday activities [37]. A recent study using this intervention showed increased activation in a variety of brain regions using functional MRI only in MS subjects who received training in context and imagery compared to placebo controls [38]. Taken together, these behavioural techniques, designed to improve information acquisition, have consistently resulted in significant improvement in learning and memory performance in persons with MS.
Non-specific interventions
In contrast to targeted interventions, several studies have employed "non-specific cognitive training" to improve learning and memory in MS patients. For example, Brenk et al. [39] claim that short-term, home-based, computer-delivered, non-specific training improved performance in several cognitive areas, including learning and memory. However, a non-treatment MS control group was not included, and having cognitive impairment was not an inclusion criterion in this study. In contrast, Tesar et al. [40] also utilized a computer-based non-specific training intervention and did not show improvement in memory performance.
Comparative studies
Several studies directly compared interventions targeting the treatment of learning and memory to non-specific interventions. In a single-blind RCT, Hildebrandt et al. [41] compared computer-based memory rehabilitation (home-based programme) with a non-intervention control group. The treatment group performed significantly better than the control group on verbal learning and delayed verbal recall as well as working memory performance. Mendozzi et al. [42] compared the efficacy and specificity of direct computer-assisted memory retraining with non-specific retraining and a no-training control group. Of the 11 tests administered before and after training, improvements were observed in seven tests for the specific memory retraining group, one for the non-specific retraining group, and none for the no-treatment control group. In contrast to these two RCTs, Jønsson et al. [25] compared a “specific cognitive treatment” and psychotherapy with “non-specific mental stimulation”. The overall results showed no group differences in verbal and visual memory following treatment, but the treatment group did show improvements in visual memory at 6 months’ follow-up, an overall less-than-impressive effect. Taken together, targeted interventions can result in significant improvement in learning and memory, but the nature of the “targeted” programme may be important. For instance, the results of specific memory training of Hildebrandt et al. [41] and Mendozzi et al. [42] were much more impressive than those of Jønsson et al. [25] whose programme involved training not only for memory, but concentration, visuospatial and orientation training.
Overall, despite the lack of well-designed studies and the multiple methodological limitations of those studies that have been performed, there appears to be moderate support for behavioural interventions for the treatment of impaired learning and memory in individuals with MS. Targeted interventions designed to specifically address problems in learning and memory are most beneficial compared with generalized cognitive interventions that have little support overall.
Processing speed
In contrast to work in learning and memory, there are no behavioural studies specifically designed to improve processing speed in persons with MS, despite the fact that it is the most prevalent problem in people with MS [43] and its putative importance in underlying the observed deficits in other domains of cognition. In contrast to MS, studies of the effects of nonpharmacological interventions on processing speed have been undertaken in other cognitive disorders such as Alzheimer’s disease [44] and aging [45]. While the reason for the lack of behavioural intervention studies for processing speed in MS is unclear, there are a series of well-designed studies in aging populations which clearly show significant improvement in processing speed and everyday functional activity [45]. Such studies provide a framework from which studies in persons with MS can be investigated. The need for studies designed to improve processing speed in MS is clear and a fruitful area for future research.
Attention
Attention encompasses a variety of cognitive processes involved with the processing of information. Several studies have evaluated the effects of computerized attention training packages, which have the advantages of a being a reliably administered and reproducible intervention. One of the earliest studies [46] used a computerized assessment of the MS patients’ attention skills at baseline. Only those with attention deficits on computer assessment were recruited to the study. A computerized training package was then selected for each patient to target one of their two weakest attention domains. The results showed that specific training of individual impaired domains of attention (alertness, divided attention, vigilance, or selective attention) uniquely improved the target domain and not other aspects of attention [46]. A small randomized, controlled trial (RCT) [40] allocated half the MS patients to computer-based treatment targeting their two most impaired cognitive areas, being taught everyday compensation strategies, and self-control techniques. Patients also received out-patient multidisciplinary rehabilitation that did not address cognition, structured according to individual needs. The MS control group only received the multidisciplinary rehabilitation. The authors do not report results separately for those patients who received training in attention; however, overall the treated group did no better than the control group on tests of attention [40].
One of the largest and best designed studies of attention training in MS was a RCT in which MS patients were selected if they had both self-reported impairments in attention and impairments on neuropsychological tests [47]. Participants were randomized to either memory and attention computer retraining (treatment group) or to visual construction and visual-motor coordination computer training (control group). Both groups received 16 training sessions across 8 weeks. Approximately 45 % of patients improved in both groups, with no treatment effect on tests of attention [47].
More recently, Mattioli et al. [48] investigated the use of intensive computer-assisted training of attention, information processing and executive function in 20 MS patients with objectively confirmed deficits compared with 10 control patients and reported significant improvements in all three cognitive domains after 3 months of training carried out three times a week. Another small study [39] utilized non-specific cognitive training tasks on paper that were distributed weekly for 6 weeks for participants to complete at home several times a day, and compared MS patients with healthy control subjects. At baseline, the patients were significantly worse than the control group on some computer assessments of attentional skills (but not on memory or executive tests), and both groups showed significant improvements on some parts of the computerized assessment of attention. However, there was no group effect of treatment, with both groups improving to a similar extent.
Sastre-Garriga et al. [50] used functional magnetic resonance imaging (fMRI) in a cognitive rehabilitation study of 15 MS patients with impaired attention and/or memory compared with five healthy controls. After 5 weeks of computer-aided and non–computer-aided exercises designed to target attention and other frequently affected cognitive domains, significant improvements were observed on the backward version of the digit span test and on a composite score of neuropsychological outcomes. Patients also showed an increase in their brain fMRI activity compared with controls during rehabilitation, primarily in cerebellar brain regions. There was, however, no correlation observed between cognitive improvements and regional increases in brain activation.
Overall, the studies of moderately intensive attention training yielded contradictory results. In addition, access to and individual suitability of retraining programmes restricts their usefulness. It seems safe to conclude that they are unlikely to cause harm and, if sufficiently precisely targeted, may bring improvement.
Executive function
Executive function processes are involved in planning, problem solving, judgement, reasoning, and organisation. When asked to choose and complete several simple cognitive tasks from an array, to maximize points scored within a given time, MS patients do significantly worse than healthy controls [51]. Because of their superordinate, supervisory role, executive function processes are involved in many aspects of everyday life, especially those that are not routine. Executive function processes could in principle be improved by direct training and, because of their involvement in all novel and challenging tasks, could also be improved by cognitive training of other skills.
There are few retraining programmes that have specifically targeted executive function. Fink et al. [52] evaluated the efficacy of an executive function intervention programme in a double-blind, placebo-controlled, “pseudo-randomized” study involving 40 MS patients. Patients in the intervention group completed textbook exercises for executive functioning for 25–30 min per day, four times per week, with weekly feedback from a psychologist for 6 weeks. Executive function (and verbal learning) improved significantly in the intervention group compared with the placebo and untreated groups after 6 weeks.
Tesar et al. [40] did not separately report the outcomes of patients who received computer-based executive skills training, but overall the MS treated group showed improvement on a test of executive functioning, compared to the MS control group receiving non-specific rehabilitation, and the advantage was maintained at a 3-month follow up. It is worth noting that the general compensatory strategy package that all the treatment groups received included building up routines of behaviour and ‘problem-solving and planning’, which could explain the improvement in executive test scores [40]. Solari et al. [47] utilized a computer programme designed to train attention and memory skills. However, the one test that showed superior performance after training was a test of executive function. The authors suggest that this may be explicable by regression to the mean since the control arm was significantly better at baseline than the intervention group [47]. Mattioli et al. [48] also investigated the use of intensive computer-assisted training on executive function and reported significant improvements in this domain after 3 months of training for three times a week.
Although there is no body of convincing evidence that training executive processes results in specific improvements in executive functions, the evidence hints at general cognitive training, inevitably involving executive processes, may improve them.
Symptomatic drug treatments
The two strategies for assessing the effects of medication to ameliorate MS-associated cognitive impairment have been either to add cognitive measures to the pivotal trials of DMTs for RRMS (based on the assumption that improving the disease course will help cognition) or to focus on symptomatic therapies that may enhance specific domains of cognitive functioning.
In contrast to the DMT clinical trials, studies applying the strategy of using cognitive enhancing medications in MS have specified inclusion criteria relative to cognitive performance and have focused on improving performance in specific cognitive domains. Given that the core neuropsychological deficits in MS are a slowing of information processing speed [43, 59], and defective anterograde episodic memory [30, 60], it is not surprising that efforts to treat MS-associated cognitive impairment with medication have targeted these domains. As shown in Table 3, treatment studies that have addressed cognition using neuropsychological tests as either primary or secondary outcomes show wide variability in the medications tested, research designs, and patient sample sizes.
Stimulants
Slowed mental processing often coexists with impairments in various aspects of complex information processing, such as divided attention, working memory, or in lay terms “multi-tasking”. Multiple sclerosis patients seldom have problems allocating attention resources, but many suffer from marked limitations in attention capacity. It is therefore reasonable to consider central nervous system (CNS) stimulant medication for patients with this constellation of impairments. Negative results were reported by Geisler et al. [61] on the effects of amantadine, although there was a trend for benefit on the Symbol Digit Modalities Test (SDMT) [62], which may be the most sensitive [4] and reliable [63] of the tests available for MS research. Two studies reported positive effects following single-doses of the stimulants, methylphenidate [64] and l-amphetamine [65] when outcomes were administered shortly after administration. However, the l-amphetamine effects were not replicated in a continuous dosing, larger-sample study [66]. A re-examination of the effects of l-amphetamine on patients selected for memory impairment compared with those with normal memory performance showed more promising findings [67]. It was noted that some memory effects were seen in both previous studies [65, 66], especially on visual memory outcomes. Although the retrospective analysis proved positive, it is difficult to draw any firm conclusions from a subgroup analysis such as this. The effects of other stimulants such as lisdexamfetamine are currently under investigation.
Modafinil, an agent designed to improve excessive sleepiness, has been examined for its effects on aspects of cognitive dysfunction in MS. A recent double-blind, placebo-controlled RCT involving 121 patients with MS and fatigue found that modafinil had no convincing effects on fatigue or cognitive dysfunction [68]. In this study, there was a significant improvement the SDMT with modafinil but not in the PASAT, which actually improved significantly in the placebo group. In a double-blind, placebo-controlled RCT of 21 patients with MS by Lange et al. [69], a total of 18 patients (eight in the treatment arm) were tested using the D2 Alertness Test [70], which measures focusing of attention. While modafinil-treated patients showed relative improvement on the D2 test and subjectively reported fatigue, another larger study involving 115 patients did not replicate the benefit on fatigue [71], and the small sample size and potential for regression to the mean in the original study limit the interpretation of the findings. Another study with modafinil suggested a positive treatment effect on other neuropsychological tests, but this study was not placebo controlled [72]. Hence, the cognitive enhancing effects of modafinil on attention in MS patients remain uncertain.
Potassium channel blockers
In demyelinated axons, abnormal potassium currents contribute to impaired action potential duration and amplitude [73]. Potassium channel blockers could conceivably facilitate neuronal function in regions important for attention or processing efficiency. Pilot work with 3,4 diaminopyridine [74] and 4 aminopyridine [75, 76], which included some cognitive testing, showed largely negative results. However, the study methodologies were weak, and there is now renewed interest in this class of medications. Research with dalfampridine to improve cognitive function is also underway.
Acetylcholinesterase inhibitors
The neuropharmacology of episodic memory involves cholinergic transmission, and there is a vast literature on acetylcholinesterase inhibitors and improved memory in Alzheimers’ disease. Krupp and colleagues [77] reported that donepezil improves cognitive performance and subjective ratings of memory over 24 weeks. However, the sample was small and there were a few noteworthy methodological shortcomings in the study (e.g., treatment groups not matched on disease course, lack of independent clinician rates) leading the investigators to conduct a larger, better controlled, multicentre, replication study [78]. Unfortunately, the results of this study were negative. The positive donepezil findings were not replicated in another study examining the effects of a similar acetylcholinesterase inhibitor, rivastigmine [79].
Overall, these studies suggest only possible benefits of symptomatic drug treatments on cognitive impairment in MS. Some positive results have been reported, but these have often been followed by replication failure. There are many challenges associated with clinical trial design. Methodological issues relevant to all symptomatic therapy trials include variability in the degree of impairment required for inclusion, optimizing primary and secondary outcomes, determining realistic effect sizes and hence sample size, and standardizing treatment duration. Requiring too cognitively impaired patients could adversely affect recruitment. However, enrolling patients without sufficient impairment might obscure an otherwise positive treatment effect [67]. Several studies with improved clinical designs and potentially more effective treatments are underway and could lead to more promising therapeutic option.
Disease modifying treatments
The DMTs have the potential to positively influence the cognitive outcome of the patients by acting on some key pathogenic mechanisms of MS-related cognitive impairment. In particular, all the approved DMTs reduce the accumulation of irreversible nervous damage, as shown by the positive effects on T2 and T1 lesion load, and some of them have also effects on the brain atrophy [82]. The decrease of the ongoing inflammatory activity may also contribute to better cognitive performances. Moreover, moreover, it has been speculated that some of the DMTs may also exert a direct neuroprotective/neurotrophic effect [82].
However, evidence in the field is limited. Interpretation of available data is complicated by issues largely related to methodological problems of study design and execution (Table 1). In DMT clinical trials, cognitive assessment is often limited to just the PASAT, administered in the context of the MSFC. In most of the published studies, the cognitive outcome represents a secondary endpoint and therefore patient inclusion criteria and sample size calculations may not be appropriate to assess cognitive outcomes.
Relapsing-remitting MS
Interferon (IFN) β can have an indirect effect on cognition since it reduces immune mediated inflammation and demyelinization thus preserving function. One of the earliest RCTs [83] evaluated the effects on cognition of low-dose IFN β-1b (50 mg, n = 8), high dose IFN β-1b (250 mg, n = 9) and placebo (n = 13) in a small group of 30 relapsing–remitting MS (RRMS) patients from one centre. A focused neuropsychological assessment was conducted between the second and fourth years, and therefore baseline neuropsychological status was not known. High-dose IFN β-1b therapy was associated with better performance on only one test of 13 examined, a measure of delayed visual recall, although group differences in visual memory were also observed at baseline. This finding was related to a reduced MRI lesion burden (r = 0.43, p = 0.03), although the main effect on the test remained after controlling for MRI changes. In another, small, open-label study [84] of IFN β-1b 250 mg in RRMS patients (n = 46), a benefit was suggested in the treated group (n = 23, EDSS <5.5) on measures of attention, visuospatial learning and recall after 1 year of treatment.
The effects of intramuscular (IM) IFN β-1a on cognition were evaluated as part of a multicentre, phase III RCT conducted in the USA [85]. A comprehensive (at baseline and week 104) and also a brief neuropsychological battery (every 6 months) were administered to a large subgroup of 166 patients with RRMS. After adjusting for baseline performance, IFN β-1a had a significant beneficial effect on tests of information processing, learning/memory, as well as a positive trend on tests of visuospatial abilities and problem solving. Interestingly, the brief battery revealed a clear practise effect in both arms, with a significant difference favouring patients on active treatment. Moreover, IFN β-1a significantly increased the time to sustained deterioration in the PASAT processing rate. In interpreting the study’s findings, a few issues should be considered. Data from only the 60 % of the baseline group were included in the analysis of change over 2 years, a few outcome measures were determined post hoc, and it is not specified how statistically significant effects were taken into account in the analysis. It is also difficult to extrapolate the study findings to everyday life due to the extremely extensive and lengthy neuropsychological assessment, which took approximately 3 h.
The effects IFN β-1a on cognitive function in early, mildly disabled RRMS patients were also addressed in a large, multicentre, post-marketing study [86]. The COGIMUS study [86] was a prospective cohort study including 459 early RRMS patients treated with IFN β-1a s.c. 22 or 44 mcg. The patients were assessed through the BRNB and the Stroop test at baseline and at 12 monthly intervals for 3 years. At baseline there were no differences between the two dose groups in demographic and clinical characteristics or in the proportions of patients impaired on more than three tests. Data on cognitive function at 3 years were available for 318 patients of the original cohort (72.1 %; 22 mcg, n = 153; 44 mcg, n = 165) and showed a 32 % risk reduction of developing impairment in three or more tests for patients on high dose compared with those on the lower dose.
The effect of glatiramer acetate (GA) on cognition was also evaluated as part of a phase III US RCT [87]. Two hundred and forty-eight patients were tested at baseline and after 1–2 years using the BRNB. At baseline, neuropsychological test performance was similar in both arms, with mean scores falling within the range of normal performance with the exception of the word list generation test. Both arms showed a significant improvement in cognitive performance because of the practise effect. No differences were detected between the treatment groups for any of the neuropsychological tests. No significant interactions were observed between the effects of treatment and either time or baseline level of impairment. Both the low level of baseline cognitive abnormalities and the strong practise effects may explain the absence of an effect of GA on cognitive function despite the fact that the trial showed a significant effect on disease activity. A subgroup of 153 patients (65 %) was re-examined 10 years after inclusion into the clinical trial [88]. Attention tests and the PASAT showed a significant decline in patients who originally received either GA or placebo. However, other tests had not deteriorated significantly, despite the long-term follow-up. The Z score of the BRNB revealed a decline of more than 0.5 of a standard deviation of the mean in only 19 % of participants. There were no differences between patients originally in the placebo arm or the GA arm.
In the assessment of the effects of natalizumab on cognitive functioning in the AFFIRM and SENTINEL phase III three clinical trials of RRMS patients [89, 90] the PASAT was the only instrument used. Thus far, no results from this assessment have been published. The impact of natalizumab on cognitive functioning was also investigated in a small post-marketing study of RRMS patients (n = 17) [91] and the results suggested natalizumab had a positive effect on neuropsychological performance.
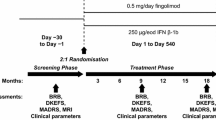
In a 24 month, RCT of oral fingolimod compared with placebo in patients with RMRS a significant effect on the MSFC was observed in both active groups compared with placebo [92] although no data for the PASAT component have been published yet.
Secondary progressive MS
Published evidence dealing with secondary progressive MS (SPMS) are limited to one study. The IMPACT trial [93], which was performed to determine whether IM IFN β-1a reduced disability progression in 217 secondary progressive MS (SPMS) patients (EDSS between 3.5 and 6.5), demonstrated an overall MSFC benefit driven predominantly by the 9HPT and, to a lesser extent, the PASAT3 (p = 0.061). No results have been published on cognitive function form the other three large trials of IFN β-1b [93, 94] or subcutaneous IFN β-1a [95].
Primary progressive MS
Trials in primary progressive MS have failed to demonstrate any benefit on cognitive performance. No cognitive assessments were performed in the pilot trial of IM IFN β-1a. [96]. In one study of IFN β-1b, 73 patients were assessed with the BRNB at baseline, at 12 months and at 24 months [97]. No significant differences between IFN β-1b and placebo groups were observed at any time point in any of the cognitive domains tested. A total of 943 patients with primary progressive multiple sclerosis were randomized to GA or placebo in a 3-year, double-blind RCT [98]. The trial was stopped after an interim analysis by an independent data safety monitoring board indicated no discernible treatment effect on the primary outcome. Although the MSFC was performed no results from the PASAT are reported.
Clinically isolated syndromes
The effect of IFN β-1b on cognition in patients with clinically isolated syndromes (CIS) has been assessed in the phase III, BENEFIT RCT [99] and its extension at 3 [100] and 5 years [101]. The mean MSFC score improved over the 5 years in most patients, and there was no significant difference between those who had received IFN β-1b during the initial 2-year trial and those who received it only during the extension trial (delayed treatment) (p = 0.608). Improvement of the overall MSFC score was largely due to improvement in the PASAT, and this was more pronounced in the early treatment group compared with the delayed treatment group; the difference between these groups increased during the course of the study until year 5 (year 3, p = 0.064; year 5, p = 0.005).
In summary, the effect of DMTs on cognition has not been adequately studied and methodological limitations render it difficult to draw any firm conclusions. Nevertheless, most of the studies with DMTs have shown weak positive effects on cognition. On the basis of studies focusing on CIS and early RRMS patients, it is hypothesized that early treatment will help preserve intact cognitive functioning and delay the development of cognitive impairment. Studies to test this hypothesis are needed.
Summary
Cognitive impairment in MS is important and is associated with meaningful functional impairment and adverse effects on quality of life. The fact that cognitive impairment and associated disability can predate the onset of physical disability amplifies the importance of managing this aspect of the disease and maximizing clinical outcomes. Management of cognitive impairment may encompass slowing of further deterioration of impairment or improvement in already impaired cognition. Currently, data linking interventions to either slow cognitive decline or improve already impaired cognitive function are limited and at times, have yield inconsistent results. Further, linking any changes as a result of specific interventions with actual functional outcomes, or even surrogate proxy outcome measures, is currently a theoretical construct and requires validation using appropriate research studies and endpoints. Brief assessment of cognitive impairment should be incorporated in future clinical trials. Recently, based on literature review and expert opinion, a Brief International Cognitive Assessment for MS has been proposed (BICAMS) which focuses on measures of processing speed, visual-spatial and verbal memory; validation studies of this instrument are currently ongoing in different countries [102]. Based on the findings described above we have proposed a summary of current challenges and recommendations that we hope can inform and guide the clinical and research communities (Table 4).
References
Peyser JM, Rao SM, LaRocca NG, Kaplan E (1990) Guidelines for neuropsychological research in multiple sclerosis. Arch Neurol 47:94–97
Rao S, Leo G, Bernardin L, Unverzagt F (1991) Cognitive dysfunction in multiple sclerosis: frequency, patterns, and predictions. Neurology 41:685–691
Rao SM (1997) Neuropsychological aspects of multiple sclerosis. In: Raine CS, McFarland HF, Tourtellotte WW (eds) Multiple sclerosis: clinical and pathogenetic basis. Chapman & Hall, London, pp 357–362
Benedict RH, Cookfair D, Gavett R et al (2006) Validity of the minimal assessment of cognitive function in multiple sclerosis. J Int Neuropsychol Soc 12:549–558
Benedict R, Bobholz J (2007) Multiple sclerosis. Semin Neurol 27(1):78–85
Amato MP, Portaccio E, Goretti B et al (2010) Cognitive impairment in early stages of multiple sclerosis. Neurol Sci 31(Suppl 2):S211–S214
Amato MP, Zipoli V, Portaccio E (2008) Cognitive changes in multiple sclerosis. Expert Rev Neurother 8:1585–1596
Benedict RH, Bruce JM, Dwyer MG, Abdelrahman N, Hussein S, Weinstock-Guttman B, Garg N, Munschauer F, Zivadinov R (2006) Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol 63(9):1301–1306
Amato MP, Portaccio E, Goretti B et al (2010) Relevance of cognitive deterioration in early relapsing-remitting MS: a 3-year follow-up study. Mult Scler 16:1474–1482
Zipoli V, Goretti B, Hakiki B et al (2010) Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Mult Scler 16:62–67
Reuter F, Zaaraoui W, Crespy L, Faivre A, Rico A, Malikova I et al (2011) Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. J Neurol Neurosurg Psychiatry 82(10):1157–1159
Lebrun C, Blanc F, Brassat D, Zephir H, de Seze J, CFSEP (2010) Cognitive function in radiologically isolated syndrome. Mult Scler 16(8):919–925
Amato MP, Hakiki B, Goretti B, et al (2012) Association of T1 lesion volume and neocortical atrophy with cognitive impairment in radiologically isolated syndromes. Neurology (in press)
Amato MP, Zipoli V, Goretti B et al (2006) Benign multiple sclerosis: cognitive, psychological and social aspects in a clinical cohort. J Neurol 253:1054–1059
Benedict RH, Zivadinov R (2011) Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol 7(6):332–342
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151
Comi G (2010) Effects of disease modifying treatments on cognitive dysfunction in multiple sclerosis. Neurol Sci 31(Suppl 2):S261–S264
Sumowski JF, Wylie GR, Chiaravalloti ND, DeLuca J (2010) Intellectual enrichment lessens the effect of brain atrophy on learning and memory in MS. Neurology 74:1942–1945
Benedict RH, Cox D, Thompson LL, Foley F, Weinstock-Guttman B, Munschauer F (2004) Reliable screening for neuropsychological impairment in multiple sclerosis. Mult Scler 10(6):675–678
Sonder J, Bosma L, van der Linden F, Knol D, Polman C, Uitdehaag B (2012) Proxy measurements in multiple sclerosis: agreement on different patient-reported outcome scales. Mult Scler 18(2):196–201
Rudick R, Antel J, Confavreux C et al (1997) Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann Neurol 42:379–382
Rao SM (1990) A manual for the brief, repeatable battery of neuropsychological tests in multiple sclerosis. Milwaukee, Wisconsin
Benedict RH, Fischer JS, Archibald CJ et al (2002) Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol 16:381–397
Strober L, Englert J, Munschauer F, Weinstock-Guttman B, Rao S, Benedict RH (2009) Sensitivity of conventional memory tests in multiple sclerosis: comparing the Rao Brief Repeatable Neuropsychological Battery and the Minimal Assessment of Cognitive Function in MS. Mult Scler 15:1077–1084
Jønsson A, Korfitzen EM, Heltberg A, Ravnborg MH, Byskov-Ottosen E (1993) Effects of neuropsychological treatment in patients with multiple sclerosis. Acta Neurol Scand 88:394–400
Solari A (2010) Methodological aspects of randomized controlled trials on cognitive interventions. Neurol Sci 31(2):279–282
O’Brien AR, Chiaravalloti N, Goverover Y, Deluca J (2008) Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil 89:761–769
das Nair R, Ferguson H, Stark DL, Lincoln NB (2012) Memory Rehabilitation for people with multiple sclerosis. Cochrane Database Syst Rev 14(3):CD008754
DeLuca J, Barbieri-Berger S, Johnson SK (1994) The nature of memory impairments in multiple sclerosis: acquisition versus retrieval. J Clin Exp Neuropsychol 16(2):183–189
DeLuca J, Gaudino EA, Diamond BJ, Christodoulou C, Engel RA (1998) Acquisition and storage deficits in multiple sclerosis. J Clin Exp Neuropsychol 20:376–390
Gaudino EA, Chiaravalloti ND, DeLuca J, Diamond BJ (2001) A comparison of memory performance in relapsing-remitting, primary progressive and secondary progressive, multiple sclerosis. Neuropsychiatry Neuropsychol Behav Neurol 14(1):32–44
Goverover Y, Chiaravalloti N, DeLuca J (2008) Self-generation to improve learning and memory of functional activities in persons with multiple sclerosis: meal preparation and managing finances. Arch Phys Med Rehabil 89:1514–1521
Basso MR, Lowery N, Ghormley C, Combs D, Johnson J (2006) Self-generated learning in people with multiple sclerosis. J Int Neuropsychol Soc 12:640–648
Basso MR, Ghormley C, Lowery N, Combs D, Bornstein RA (2002) Self-generated learning in people with multiple sclerosis: an extension of Chiaravalloti and DeLuca (2002). J Clin Exp Neuropsychol 30:63–69
Goverover Y, Hillary FG, Chiaravalloti N, Arango-Lasprilla JC, DeLuca J (2009) A functional application of the spacing effect to improve learning and memory in persons with multiple sclerosis. J Clin Exp Neuropsychol 31:513–522
Goverover Y, Basso MR, Wood H, Chiaravalloti N, DeLuca J (2011) Examining the benefits of combining two learning strategies on recall of functional information in persons with multiple sclerosis. Mult Scler 17(12):1488–1497
Chiaravalloti ND, DeLuca J, Moore NB, Ricker JH (2005) Treating learning impairments improves memory performance in multiple sclerosis: a randomized clinical trial. Mult Scler 11:58–68
Chiaravalloti ND, Wylie G, Leavitt V, DeLuca J (2012) Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol 259(7):1337–1346
Brenk A, Laun K, Haase CG (2008) Short-term cognitive training improves mental efficacy and mood in patients with multiple sclerosis. Eur Neurol 60:304–309
Tesar N, Bandion K, Baumhackl U (2005) Efficacy of a neuropsychological training programme for patients with multiple sclerosis—a randomised controlled trial. Wien Klin Wochenschr 117:747–754
Hildebrandt H, Lanz M, Hahn HK et al (2007) Cognitive training in MS: effects and relation to brain atrophy. Res Neurol Neurosci 25:33–43
Mendozzi L, Pugnetti L, Motta A, Barbieri E, Gambini A, Cazzullo CL (1998) Computer-assisted memory retraining of patients with multiple sclerosis. Ital J Neurol Sci 19:S431–S438
DeLuca J, Chelune GJ, Tulsky DS, Lengenfelder J, Chiaravalloti ND (2004) Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J Clin Exp Neuropsychol 26:550–562
Acevedo A, Loewenstein DA (2007) Nonpharmacological cognitive interventions in aging and dementia. J Geriatr Psychiatry Neurol 20(4):239–249
Ball K, Edwards JD, Ross LA (2007) The impact of speed of processing training on cognitive and everyday functions. J Gerontol Ser B Psychol Sci Soc Sci 1:19–31
Plohmann AM, Kappos L, Ammann W et al (1998) Computer assisted retraining of attentional impairments in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 64:455–462
Solari A, Motta A, Mendozzi L et al (2004) Computer-aided retraining of memory and attention in people with multiple sclerosis: a randomized, double-blind controlled trial. J Neurol Sci 222:99–104
Mattioli F, Stampatori C, Zanotti D, Parrinello G, Capra R (2010) Efficacy and specificity of intensive rehabilitation of attention and executive functions in multiple sclerosis. J Neurol Sci 288:101–105
O’Brien A, Chiaravalloti N, Arango-Lasprilla JC, Lengenfelder J, DeLuca J (2007) An investigation of the differential effect of self-generation to improve learning and memory in multiple sclerosis and traumatic brain injury. Neuropsychol Rehabil 17:273–292
Sastre-Garriga J, Alonso J, Renom M et al (2011) A functional magnetic resonance proof of concept pilot trial of cognitive rehabilitation in multiple sclerosis. Mult Scler 17:457–476
Birnboim S, Miller A (2004) Cognitive strategies application of multiple sclerosis patients. Mult Scler 10:67–73
Fink F, Rischkau E, Butt M, Klein J, Eling P, Hildebrandt H (2010) Efficacy of an executive function intervention programme in MS: a placebo-controlled and pseudo-randomized trial. Mult Scler 16:1148–1151
Allen DN, Goldstein G, Heyman RA, Rondinelli T (1998) Teaching memory strategies to persons with multiple sclerosis. J Rehabil Res Dev 35:405–410
Brissart H, Leroy M, Debouverie M (2010) Cognitive rehabilitation in multiple sclerosis: preliminary results and presentation of a new program, PROCOG-SEP]. Rev Neurol (Paris) 166:406–411
Chiaravalloti ND, DeLuca J (2002) Self-generation as a means of maximizing learning in multiple sclerosis: an application of the generation effect. Arch Phys Med Rehabil 83:1070–1079
Shatil E, Metzer A, Horvitz O, Miller A (2010) Home-based personalized cognitive training in MS patients: a study of adherence and cognitive performance. Neuro Rehabil 26:143–153
Sumowski JF, Chiaravalloti N, DeLuca J (2010) Retrieval practice improves memory in multiple sclerosis: clinical application of the testing effect. Neuropsychology 24:267–272
Vogt A, Kappos L, Calabrese P et al (2009) Working memory training in patients with multiple sclerosis. Restor Neurol Neurosci 27:225–235
Archibald CJ, Fisk JD (2000) Information processing efficiency in patients with multiple sclerosis. J Clin Exp Neuropsychol 22:686–701
Rao SM (1989) On the nature of memory disturbance in multiple sclerosis. J Clin Exp Neuropsychol 11:699–712
Geisler MW, Sliwinski M, Coyle PK, Masur DM, Doscher C, Krupp LB (1996) The effects of amantadine and pemoline on cognitive functioning in multiple sclerosis. Arch Neurol 53:185–188
Smith A (1982) Symbol digit modalities test: manual. Western Psychological Services, Los Angeles
Benedict RH (2005) Effects of using same- versus alternate-form memory tests during short-interval repeated assessments in multiple sclerosis. J Int Neuropsychol Soc 11:727–736
Harel Y, Appleboim N, Lavie M, Achiron A (2009) Single dose of methylphenidate improves cognitive performance in multiple sclerosis patients with impaired attention process. J Neurol Sci 276:38–40
Benedict RH, Munschauer F, Zarevics P et al (2008) Effects of l-amphetamine sulfate on cognitive function in multiple sclerosis patients. J Neurol 255:848–852
Morrow SA, Kaushik T, Zarevics P et al (2009) The effects of l-amphetamine sulfate on cognition in MS patients: results of a randomized controlled trial. J Neurol 256:1095–1102
Sumowski JF, Chiaravalloti N, Erlanger D, Kaushik T, Benedict RH, Deluca J (2011) l-amphetamine improves memory in MS patients with objective memory impairment. Mult Scler 17(9):1141–1145
Möller F, Poettgen J, Broemel F, Neuhaus A, Daumer M, Heesen C (2011) HAGIL (Hamburg Vigil Study): a randomized placebo-controlled double-blind study with modafinil for treatment of fatigue in patients with multiple sclerosis. Mult Scler 17(8):1002–1009
Lange R, Volkmer M, Heesen C, Liepert J (2009) Modafinil effects in multiple sclerosis patients with fatigue. J Neurol 256:645–650
Brickenkamp R (2002) Test d2. Aufmerksamkeits-Belastungs-Test, 9th edn. The d2 test. Test of attention under pressure, 9th edn. Hogrefe, Göttingen
Stankoff B, Waubant E, Confavreux C et al (2005) Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology 64:1139–1143
Wilken JA, Sullivan C, Wallin M et al (2008) Treatment of multiple sclerosis-related cognitive problems with adjunctive modafinil: rationale and preliminary supportive data. Int J MS Care 10:1–10
Waxman SG (1982) Membranes, myelin, and the pathophysiology of multiple sclerosis. N Engl J Med 306:1529–1533
Bever CT Jr, Anderson PA, Leslie J et al (1996) Treatment with oral 3,4 diaminopyridine improves leg strength in multiple sclerosis patients: results of a randomized, double- blind, placebo-controlled, crossover trial. Neurology 47:1457–1462
Bever CT Jr, Young D, Anderson PA et al (1994) The effects of 4-aminopyridine in multiple sclerosis patients: results of a randomized, placebo-controlled, double-blind, concentration-controlled, crossover trial. Neurology 44:1054–1059
Rossini PM, Pasqualetti P, Pozzilli C et al (2001) Fatigue in progressive multiple sclerosis: results of a randomized, double-blind, placebo-controlled, crossover trial of oral 4-aminopyridine. Mult Scler 7:354–358
Krupp LB, Christodoulou C, Melville P, Scherl WF, MacAllister WS, Elkins LE (2004) Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology 63:1579–1585
Krupp LB, Christodoulou C, Melville P et al (2011) Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology 76:1500–1507
Shaygannejad V, Janghorbani M, Ashtari F, Zanjani HA, Zakizade N (2008) Effects of rivastigmine on memory and cognition in multiple sclerosis. Can J Neurol Sci 35:476–481
Smits RC, Emmen HH, Bertelsmann FW, Kulig BM, van Loenen AC, Polman CH (1994) The effects of 4-aminopyridine on cognitive function in patients with multiple sclerosis: a pilot study. Neurology 44:1701–1705
Lovera JF, Frohman E, Brown TR et al (2010) Memantine for cognitive impairment in multiple sclerosis: a randomized placebo-controlled trial. Mult Scler 16(6):715–723
Gold R, Wolinsky JS, Amato MP, Comi G (2010) Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord 3(6):351–367
Pliskin NH, Hamer DP, Goldstein DS, Towle VL, Reder AT, Noronha A (1996) Improved delayed visual reproduction test performance in multiple sclerosis patients receiving interferon beta-1b. Neurology 47:1463–1468
Barak Y, Achiron A (2002) Effect of interferon-beta-1b on cognitive functions in multiple sclerosis. Eur Neurol 47:11–14
Fischer JS, Priore RL, Jacobs LD et al (2000) Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol 48:885–892
Patti F, Amato MP, Bastianello S et al (2010) Effects of immunomodulatory treatment with subcutaneous interferon beta-1a on cognitive decline in mildly disabled patients with relapsing-remitting multiple sclerosis. Mult Scler 16:68–77
Weinstein A, Scwid SI, Schiffer RB, McDermott MP, Giang DW, Goodman AD (1999) Neuropsychologic status in multiple sclerosis after treatment with glatiramer. Arch Neurol 56:319–324
Schwid SR, Goodman AD, Weinstein A, McDermott MP, Johnson KP (2007) Cognitive function in relapsing multiple sclerosis: minimal changes in a 10-year clinical trial. J Neurol Sci 255:57–63
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, AFFIRM Investigators (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354(9):899–910
Rudick RA, Stuart WH, Calabresi PA, SENTINEL Investigators et al (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354(9):911–923
Mattioli F, Stampatori C, Capra R (2011) The effect of natalizumab on cognitive function in patients with relapsing-remitting multiple sclerosis: preliminary results of a 1-year follow-up study. Neurol Sci 32:83–88
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362(5):402–415
Kappos L, Polman C, Pozzilli C, Thompson A, Beckmann K, Dahlke F, European Study Group in Final analysis of the European multicenter trial on IFNbeta-1b in secondary-progressive MS (2001) Interferon beta-1b in Secondary-Progressive MS. Neurology 57(11):1969–1975
Panitch H, Miller A, Paty D, Weinshenker B, North American Study Group on Interferon beta-1b in Secondary Progressive MS (2004) Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 63(10):1788–1795
Cohen JA, Cutter GR, Fischer JS et al (2002) Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 59:679–687
Leary SM, Miller DH, Stevenson VL, Brex PA, Chard DT, Thompson AJ (2003) Interferon beta-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology 60(1):44–51
Montalban X, Sastre-Garriga J, Tintoré M et al (2009) A single-center, randomized, double-blind, placebo-controlled study of interferon beta-1b on primary progressive and transitional multiple sclerosis. Mult Scler 15:1195–1205
Wolinsky JS, Narayana PA, O’Connor P et al (2007) Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol 61(1):14–24
Kappos L, Polman CH, Freedman MS et al (2006) Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 67(7):1242–1249
Kappos L, Freedman MS, Polman CH, BENEFIT Study Group et al (2007) Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 370(9585):389–397
Kappos L, Freedman MS, Polman CH et al (2009) Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 8(11):987–997
Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, Hämäläinen P, Hartung HP, Krupp L, Penner IK, Reder AT, Benedict RH (2012) Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler 18(6):891–898
Acknowledgments
The medical writer, who supported the development of this manuscript (Janet Bray PharmD), undertook initial literature research, development of summary tables, editing the text provided by the authors, and verification of references and other editorial aspects. This work was supported by the Serono Symposia International Foundation (SSIF), an independent, non-profit organization dedicated to the Continuing Medical Education.
Conflicts of interest
All authors were compensated by Serono Symposia International Foundation (SSIF) for their contribution as faculty and speakers for the conference on “Cognition Disorders in MS” held in Florence on 30 September and 1 October 2011, which included contributions to this position paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amato, M.P., Langdon, D., Montalban, X. et al. Treatment of cognitive impairment in multiple sclerosis: position paper. J Neurol 260, 1452–1468 (2013). https://doi.org/10.1007/s00415-012-6678-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6678-0