Abstract
Introduction
Fatigue (F) is a common, inexplicable, and disabling symptom in multiple sclerosis (MS) patients. The purpose of this study was to evaluate a possible correlation between fatigue and morpho-volumetric features and site of dilated perivascular spaces (dPS), visible on 3T magnetic resonance (MR) in fatigued multiple sclerosis patients (FMS).
Methods
We studied 82 relapsing remitting (RR) FMS patients and 43 HC, matched for age, sex, and education. F was assessed by the Fatigue Severity Scale (FSS). To evaluate a possible correlation between degree of F and characteristics of dPS, patients were divided in two groups: more (mFMS) (FSS ≥ 5; n = 30) and less fatigued (lFMS) (FSS ≥ 4; n = 52), compared to a matched healthy control (HC) subject group. The MR study was performed with 3T scanner by SpinEcho T1, Fast-SpinEcho DP-T2, FLAIR, and 3D FSPGR T1 sequences. dPS volumes were measured with Medical Image Processing Analysis and Visualization (MIPAV); Global Cerebral Atrophy (GCA), expressed as Brain Parenchymal Fraction (BPF), was assessed by FSL SIENAX.
Results
The t test showed significantly increased dPS number (p = 0.021) in FMS patients (mFMS p = 0.0024 and lFMS p = 0.033) compared to HC. Pearson correlation revealed a significant correlation between dPS number and FSS (r = 0.208 p = 0.051). Furthermore, the chi-squared test confirms the intragroup (HC, mFMS, lFMS) differences about dPS location (p = 0.01) and size (p = 0.0001).
Conclusion
Our study confirms that PS in MS patients presents with different volumetric and site characteristics as compared to HC; moreover, F severity significantly correlates with dPS number, site, and size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatigue (F) is defined by the UK MS Society as an “unmotivated, overwhelming feeling of tiredness [1], affecting both mental and physical domains [2, 3]” and by the MS Council as “a subjective lack of physical and/or mental energy that is perceived, by the individual or caregiver, to interfere with usual and desired activities” in MS patients [2–4]. Primary F may depend on inflammation, demyelination, or axonal loss [4] and is a common symptom in MS patients [4, 5]. F also occurs, almost invariably, in patients with infectious disorders (human immunodeficiency virus), systemic diseases (rheumatoid arthritis, systemic lupus erythematosus, chronic fatigue syndrome) [4, 6, 7] and neurologic diseases (Parkinson’s disease, stroke) [4–7]. Sometimes, F is described as the first symptom (31 % [2]) in MS patients; it can be daily and to be present for years, it may worsen over the day or in case of hot [4] or hot-humid weather [1]. F occurs in up to 50–80 % [3] of MS patients [1, 6, 8, 9] independently on depressed mood [3] and it is a major complaint of MS patients [10] and up to 40 % [6] of them refers F as their most troublesome [2] and disabling symptom [1, 8–11]. Despite the clinical relevance of F [1], its pathogenesis is still unclear [1, 7, 12]. Autoimmune dysfunction of endogenous vasoactive neuropeptides (VNs) such as vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide has been postulated as the cause for some fatigue-related conditions, characterized by immune impairment, including MS [13, 14]. The VN dysfunction would lead to the alterations of both the adenosine monophosphate cyclic (AMP-c) levels, required for the integrity of blood–brain barrier (BBB) and blood spinal cord barrier (BSCB) [13] and, accordingly, to the development of MS relapses and the correlated symptom of F [13]. Perivascular spaces (PS), also known as Virchow-Robin space (VRS), are specific sites for inflammatory process in MS patients [15], both for inflammatory cells transit (lymphocytes and macrophages) [16] and for hormones, cytokine, and VN activities [13]. The PS is also a critical place where the immunological impairment of VNs occurs [13], here—hence follows the dilatation of PS themselves, observed in MR brain parenchyma studies, in various MS disease phases [15–17]. Based on the interrelation among PS, NVs, inflammatory-autoimmune processes, and F, the aim of this study was to assess the possible correlation between F level and PS dilatation in FMS patients, compared to HC.
Materials and methods
Patients
The study sample consisted of 82 RRMS according to the revised McDonald criteria and of 43 age- and sex-matched HC [18]; inclusion criteria for MS patients were age 18–55, absence of relapses and steroid therapy for at least 3 months prior to the study entry, presence of significant fatigue (score on the Fatigue Severity Scale > 36), absence of previous head trauma, and claustrophobia. The study was approved by the local ethical committee in agreement with the ethical standards expressed in the Declaration of Helsinki of 1964 and a signed informed consent was obtained by all participants. All patients underwent a neurological evaluation, including assessment of disability by the Expanded Disability Status Scale (EDSS). MS patients were grouped into two subgroups: less (lF; 52) and more fatigued (mF; 30).
Table 1 summarizes demographic characteristics of MS patients, HC, and of dVRS features.
MR protocol
The MR study was performed on a high-field magnet 3Tesla Signa HDXT (General Electric, Milwaukee, WI, USA), with head coil with following sequences: Axial FSE PD-T2 (TR = 3080 ms, TE = 121/26 ms, FOV 240 × 240/z, matrix = 384 × 256, NEX = 2, 44 slices, thickness = 3 mm, gap interval = 0), FLAIR (TR = 9002 ms, TE = 121 ms, TI = 2500 ms, FOV = 240 × 240/z, matrix 448 × 224, NEX = 1, 44 slices, thickness = 3 mm, gap = 0), SE T1 (TR = 400 ms, TE = 9 ms/fr, FOV 24 × 24/z, matrix 384 × 256, 44 slices, thickness = 3 mm, gap = 0), and FSPGR Sagittal T1 3D (TE = 2.8, TR = 7, TI = 650, FOV = 26 × 24, 4, 166 slices, voxel = 1.2 × 1, 2 × 1 mm, gap = 0) sequences. The sequences were those already used by Millis et al. in a study published in 2006 [19].
The MR data was double-blind analyzed, by two expert radiologists (RC) and (AS) with 20-year experience, by a Software Analysis, the Medical Image Processing Analysis and Visualization (MIPAV—Center for Information Technology, National Institutes of Health, Bethesda, MD).
Through this semi-automatic post-processing applicative system, the dPS of all subjects (patients and controls) were visualized, identified, and measured in the sequences T1, T2, and FLAIR, based on their signal, morphology, and size.
We identified and manually circumscribed the dPS of each subject (patients and healthy controls), placing a volume of interest (VOI) through the semi-automated delineation of the contours, and we evaluated the number, area, volume, and site of the dPS, to assess their modifications in agreement with previous studies [15, 20].
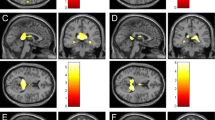
In particular, we considered dPS atypical locations (Fig. 1a), a part the three characteristic locations [21] that are
a Axial FLAIR, SE T2 and SE T1 MR study, shows typical site dPS I type on the right side. b Axial FLAIR, SE T2 and SE T1 MR study, shows dPS atypical site on the right temporal lobe close to the temporal horn of the ventricle. c Axial SE T2 MR study shows dPS II type. d Axial SE T2 RM image shows dPS in the midbrain (type III)
-
Type I: along lenticulostriate arteries through the basal ganglia (Fig. 1b).
-
Type II: they are in the path of the medullary perforating arteries when they enter in the gray matter and heading toward the white matter (Fig. 1c)
-
Type III: appears in the midbrain mainly in the cerebral peduncles (Fig. 1d).
Previously adopted criteria for dPS detection [15] were the following: (a) morphology: punctate or tubular areas more often on the course of the anatomical perforating arteries [15, 22] clearly visible on FSPGR 3D T1 sequence; (b) signal: areas isointense to cerebrospinal fluid (CSF) [15] on T2 and T1, hypo-isointense on FLAIR sequences, without any signal changes in the surrounding brain parenchyma [15, 20, 22]; (c) mass effect: not present [15, 22].
The estimation of brain atrophy was obtained on lesion-filled 3D T1 images, by means of the tool of FSL, SIENA. In the current study, the cross-sectional version of this tool (SIENAX) was used and the brain parenchymal fraction (BPF), which is the ratio of brain parenchymal volume to intracranial volume, was computed. BPF indicates global cerebral atrophy (GCA).
Statistical analysis
The Student t test was used to find out significant differences among the different groups (HC, MS, lFMS, mFMS) in dVRS parameters (number, area, volume, atypical site) and for brain atrophy (p values, lower than 0.05, were considered statistically significant).
Pearson correlation coefficient was used to estimate correlations among different dVRS parameters (number, area, volume, atypical site, and brain atrophy). Results were considered statistically significant when p < 0.05.
Chi-squared test was used to examine differences among groups (HC, MS, lFMS, mFMS) depending on variable features (dVRS number, site, size).
Results
Student t test showed significant differences in dPS number between MS patients and HC (p = 0.021), and between lFMS (p = 0.033) and mFMS patients (p = 0.0024) as compared to HC. The dPS number was not significantly different between lFMS and mFMS patients (p = 0.196). Both lFMS and mFMS had a significant difference in dPS > 3 mm compared to HC (lFMS p = 0.0162 and mFMS p = 0.0054, respectively). The HC did not show dPS >4 mm, without intragroup (lFMS ad mFMS) significant difference. Significant differences were found for the dPS area and volume between HC and the whole group of MS patients (lFMS and mFMS) (p = 0.004), as well as for lFMS (p = 0.03), and for mFMS patients (p = 0.001). There were no significant differences between lFMS and mFMS patient group for dPS area (p = 0.27) and volume (p = 0.27). HC group (43 subjects) showed 4 dPS in atypical site in 4 subjects (frequency of atypical site in HC 9 % and of HC with atypical site 9.52 %). In the lFMS group (52 patients), we observed 28 dPS in atypical site in 19 subjects (frequency of atypical site in lFMS 54 % and of lFMS with atypical site 26.92 %). In the mFMS group (30 patients), we observed 19 dPS in atypical site in 10 subjects (frequency of atypical site in mFMS 63 % and of mFMS with atypical site 23.33 %). The Student t test showed significant differences for atypical site between FMS and HC (p = 0.030), and between lFMS and HC (p = 0.029), but not significant differences between lFMS and mFMS (p = 0.723) (Fig. 2).
Student t test showed significant differences for atypical site. dVRS dilated Virchow-Robin spaces, AS atypical site, HC health control subject (blue); lFMS low fatigued multiple sclerosis patient (red); mFMS more fatigued multiple sclerosis patient (green). The graphics show quantitative differences among subject groups (lFMS, mFMS, HC). We evaluated the percentage of subjects respect to dVRS total number (a), with minor axis >3 mm (b), minor axis >4 mm (c), and AS (d) located for each group
The Pearson correlation coefficient showed significant correlation between dPS number and F in FMS patients (r = 0.208; p = 0.0559) (Fig. 3).
Pearson correlation coefficient showed significant correlation between dPS number and F in FMS patients. FSS Fatigue Severity Scale, MS multiple sclerosis patients, dVRS dilated Virchow-Robin spaces, r 2 coefficient of determination linear regression, Pearson correlation analysis between FSS score and dVRS number in all MS patients. FFS score directly related to dVRS number (r = 0.208; p = 0.051)
Chi-squared test showed significant differences among different group (HC, lFMS, mFMS) depending on dPS number, site (p = 0.01), and size (p = 0.0001).
The various dPS features did not correlate with CGA, expressed as BPF, either for HC or for FMS subject. In FMS patients, dPS number and F (measured as mean FSS score) were not influenced by CGA degree, expressed as BPF (Pearson correlation: for lFMS patients, dPS versus BPF = r = −0.0826, p = 0.560 and FSS versus BPF r = −0.1114, p = 0.431 and for mFMS patients, dPS versus BPF = r = −0.07, p = 0.71 and FSS versus BPF r = −0.068, p = 0.718.)
The Student t test showed a significantly higher dPS number both for lFMS and mFMS patients as compared to HC; instead, no significant differences in dPS number were found between lFMS and mFMS patients. Furthermore, both lFMS and mFMS patients have significantly higher number of dPS >3 mm than HC. The HC do not show dPS >4 mm and there are no significant differences for dPS >4 mm number between lFMS and mFMS patients. The FMS shows significantly higher number of dPS atypical site than HC, without significant intragroup differences (lFMS and mFMS patients). With regard to the intragroup differences (lFMS and mFMS patients), both Pearson correlation coefficient test and chi-squared test showed a significant correlation, respectively, about dPS number for the first, site and size, for the second one. In fact, the Pearson correlation coefficient test showed significant correlation between dPS number and F in FMS patients (r = 0.208; p = 0.055) and chi-squared test showed significant differences among different groups (HC, lFMS, mFMS) depending on dPS site (p = 0.01) and size (p = 0.0001).
Discussion
Despite the high clinical relevance of F in MS patients, there are only a few studies about the possible pathophysiology [2, 4, 10, 11]. Since 1999, Bakshi et al. [5] hypothesized the probable causes of F in MS patients. The more important role to explain F, in MS patients, is played by “central” abnormalities [3, 11], such as demyelination and axonal loss or immune actions in the central nervous system (CNS) [4].
There is growing evidence suggesting that F can be caused by cortico-subcortical interconnection damage in critical sites of the CNS such as the cortical-spinal tract [3], due to disseminated demyelination [11]. MR studies have greatly contributed to the analysis of the pathophysiology of F in MS patients [3]. The correlation between F and conventional MR features is debated [6, 9, 23]. Scientific literature on MS-associated F is characterized by ambiguous results [1]. Some studies showed moderate [1, 3] to absent [3, 5, 7] correlations between global or regional lesion load, atrophy [3, 8], and F severity, probably due to the low sensibility and specificity of diagnostic procedures [1].
Other studies found a positive correlation between lesion load and F, highlighting a particular role of lesion in specific locations (parietal lobe [1], internal capsule and peri-trigonal white matter [6, 9]). Other MR brain studies showed increased T1 relaxation of the deep gray matter, significantly correlating with F [24]. Previous traditional MR studies did not show significant differences in brain atrophy between fatigued and not fatigued patients [5, 7]. Most recent longitudinal studies describe as the worsening of F, in the first 2 years, is significantly associated with progressive brain atrophy in the following 6 years in MS patients [4]; the results suggest that F predicts brain atrophy as opposed to being a consequence of the demyelination process [4]. Other studies suggest that F is significantly correlated to a destructive pathological process involving both white matter (WM) and gray matter (GM) [3, 9, 25].
More recent studies suggest that F may be caused by GM pathology, involving both cortex (particularly parietal lobe) and basal ganglia (thalamus and nucleus caudatus) [1], suggesting that the involvement of GM may contribute to the development of F in MS patients [1] .
Positron emission tomography (PET) and magnetic resonance spectroscopy studies revealed widespread axonal and basal ganglia dysfunction [3, 4] and impaired interactions between functionally related cortical and subcortical regions in MS fatigued patients [2, 4, 8, 25].
A PET study revealed the reductions in cerebral glucose metabolism (CMRGlu) in various cortical and subcortical areas in MS patients with severe F [2, 8, 12].
MR spectroscopy studies reported a significant association between F, brain atrophy [8], and diffuse periventricular reduction of the putative neuronal marker N-acetyl aspartate (NAA) in high-fatigued MS patients, compared to a low fatigued ones [11, 13, 26].
The physiopathology of F may rely on the dysfunction of cortical and subcortical circuits (thalamus, basal ganglia, and frontal cortex) affected by MS lesions or disturbed in their function by the products of inflammation that could be the main substrate of F [3]. VRS or PS are perivascular compartments surrounding small vessels within the CNS; they are filled by interstitial fluid, with functional connections to sub-arachnoid space, and they contain VNs [13] (Fig. 4).
PS were defined (as in previous publications [17, 27, 28]) as small, sharply delineated structures of CSF signal intensity, on MR brain studies, that follow the orientation of the perforating vessels and run perpendicular to the brain surface, characterized by specific morphology and signal on MR studies [17]. PS is defined as dilated, if the minimum axis is more than 2 mm [15].
The immunological activity, within PS, may have a role in a number of neurological conditions [13] and PS have important connections with immune system [13]. PS, therefore, have significant implications about inflammatory control (lymphatic drainage of the head and neck and a role in leukocyte recruitment across the BBB), providing a surface for activity and immunological function toward VNs and hormones [13]. The PS dilatation, with and without cell cuffin (macrophages and lymphocytes), usually occurs around vessels including arterioles [29].
The perivascular accumulation of inflammatory cells, observed in dPS, is a prominent feature of MS pathology [30]. Post-mortem studies showed subtle and widespread abnormalities in the normal appearing WM (NAWM) of MS patients, that, among other features (diffuse astrocytic hyperplasia, patchy edema) and, above all include, perivascular cellular infiltration [26].
The F symptom, perceived in MS patients, as in other diseases with immunological impairment, recognizes, among other causes, an autoimmune VN deficit [13]. VNs are widely distributed in the CNS and they have critical roles (immune system modulation, BBB and BSCB influence) [13].
In the case of NV-immune disorder, the brain and spinal cord PS function and homeostasis would be influenced [13, 14]; so that the PS immune dysfunction and their dilatation may be related to NV auto-immunity [3].
Our study, in agreement with previous reports [13], confirms and highlights the correlation between F and dPS. Specially, we established the linear correlation between the FSS score and dPS number (Pearson correlation coefficient test r = 0.208, p = 0.051). The dPS number, indeed, was significantly higher in FMS patients (both lFMS and mFMS), compared to HC. Furthermore, both lFMS and mFMS patients had significantly higher number of dPS >3 mm compared to HC while no dPS >4 mm were detected in the HC group. Whereas several dPS >4 mm had been identified in FMS.
Also, FMS shows significantly higher number of dPS in atypical site than HC.
With regard to the intragroup differences (lFMS and mFMS patients), both Pearson correlation coefficient test and chi-squared test showed a significant correlation, respectively, for dPS number for the first test and for dPS site and size for the second one and F. In fact, the Pearson correlation coefficient test showed statistically significant correlation between dPS number and F in FMS patients (r = 0.208; p = 0.055) and chi-squared test showed statistically significant differences among different groups (HC, lFMS, mFMS) depending on dPS site (p = 0.01) and size (p = 0.0001).
Conclusions
Our data confirm the hypothesis that dPSS could be the site where chemical processes generate F. Particularly, we verified a significant correlation between dPSS number and F measured by FSS, supporting the hypothesis of the PS involvement in the pathogenesis of F and their role in determining F severity in MS patients. Moreover, the dPS >4 mm and their atypical site in FMS patients were significantly higher compared to HC group.
Finally, the Pearson correlation coefficient test and chi-squared test underline the linear relation between F, tested by FSS and dPS number, site, and size; these last evaluations enable us to define a further link between F severity and dPS features.
References
Induruwa I, Constantinescu CS, Gran B (2012) Fatigue in multiple sclerosis—a brief review. J Neurol Sci 323:9–15
Niepel G, Tench CR, Morgan PS, Evangelou N, Auer DP, Constantinescu CS (2006) Deep gray matter and fatigue in MS. A T1 relaxation time study. J Neurol 253:896–902
Leocani L, Colombo B, Comi G (2008) Physiopathology of fatigue in multiple sclerosis. Neurol Sci 29(Suppl 2):S241–S243
Kos D, Kerckhofs E, Nagels G, D’hooghe MB, Ilsbroukx S (2008) Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair 22(1):91–100
Bakshi R, Miletich RS, Henschel K, Shaikh ZA, Janardhan V et al (1999) Fatigue in multiple sclerosis: cross-sectional correlation with brain MRI findings in 71 patients. Neurology 53(5):1151–1153
Colombo B, Martinelli BF, Rossi P, Rovaris M, Maderna L, Filippi M, Comi G (2000) MRI and motor evoked potential findings in nondisabled multiple sclerosis patients with and without symptoms of fatigue. J Neurol 247:506–509
Van der Werf SP, Jongen PJ, Hommes OR, Bleijenberg G, Barkhof F, Hommes OR, Bleijenberg G (1998) Fatigue in multiple sclerosis: interrelations between fatigue complaints, cerebral MRI abnormalities and neurological disability. J Neurol Sci 160:164–170
Marrie RA, Fisher E, Miller DM, Lee JC, Rudick RA (2005) Association of fatigue and brain atrophy in multiple sclerosis. J Neurol Sci 228:161–166
Tedeschi G, Dinacci D, Lavorgna L, Prinster A, Savettieri G, Quattrone A et al (2007) Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci 263:15–19
Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM (2006) Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry 77:34–39
Tartaglia MC, Narayanan S, Francis SJ, Carlos Santos A, De Stefano N, Lapierre Y, Arnold DL (2004) The relationship between diffuse axonal damage and fatigue in multiple sclerosis. Arch Neurol 61:201–207
Krupp LB, Christodoulou C (2001) Fatigue in multiple sclerosis. Curr Neurol Neurosci Rep 1:294–298
Staines DR, Brenu EW, Marshall-Gradisnik S (2008) Review article postulated role of vasoactive neuropeptide-related immunopathology of the blood brain barrier and Virchow-Robin Spaces in the aetiology of neurological-related conditions. Mediat Inflamm 2008:1–5, 792428
Staines DR (2008) Are multiple sclerosis and amyotrophic lateral sclerosis autoimmune disorders of endogenous vasoactive neuropeptides? Med Hypotheses 70:413–418
Conforti R, Cirillo M, Saturnino PP, Gallo A, Sacco R, Negro A et al (2013) Dilated Virchow-Robin spaces and multiple sclerosis: 3T magnetic resonance study. Radiol Med. doi:10.1007/s11547-013-0357-9
Algin O, Conforti R, Saturnino PP, Ozmen E, Cirillo M et al (2012) Giant dilatations of Virchow–Robin spaces in the midbrain: MRI aspects and review of the literature. Neuroradiol J 25:415–422
MacLullich AMJ, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ (2004) Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry 75:1519–1523
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Millis RJ, Young CA, Smith ETS (2006) 3D MRI in multiple sclerosis: a study of three sequences at 3T. Br J Radiol 80:307–320
Wuerfel J, Haertle M, Waiczies H et al (2008) Perivascular spaces—MRI marker of inflammatory activity in the brain? Brain 131:2332–2340
Kwee RM, Kwee TC (2007) Virchow–Robin spaces at MR imaging. Radiographics 27:1071–1086
Etemadifar M, Hekmatnia A, Tayari N et al (2011) Features of Virchow–Robin spaces in newly diagnosed multiple sclerosis patients. Eur J Radiol 80:e104–e108
Codella M, Rocca MA, Colombo B, Martinelli-Boneschi F, Comi G, Filippi M (2002) Cerebral grey matter pathology and fatigue in patients with multiple sclerosis: a preliminary study. J Neurol Sci 194:71–74
Neema M, Stankiewicz J, Arora A, Guss ZD, Bakshi R (2007) MRI in multiple sclerosis: what’s inside the toolbox? Neurotherapeutics 4(4):602–617
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G et al (2002) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. NeuroImage 15:559–567
Filippi M, Rocca MA (2005) MRI evidence for multiple sclerosis as a diffuse disease of the central nervous system. J Neurol 252(Suppl 5):V/16–V/24. doi:10.1007/s00415-005-5004-5
Heier LA, Bauer CJ, Schwartz L et al (1989) Large Virchow–Robin spaces—MR clinical correlation. Am J Neuroradiol 10:929–936
Udaka F, Sawada H, Kameyama M (2002) White matter lesions and dementia—MRI-pathological correlation. Ann N Y Acad Sci 977:411–415
Gay FW (2006) Early cellular events in multiple sclerosis intimations of an extrinsic myelinolytic antigen. Clin Neurol Neurosurg 108:234–240
van Horssen J, Bo L, Vos CM, Virtanen I, de Vries HE (2005) Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role influx and transport of leukocytes. J Neuropathol Exp Neurol 64:722–729
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that this manuscript does not contain clinical studies or patient data.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Conforti, R., Cirillo, M., Sardaro, A. et al. Dilated perivascular spaces and fatigue: is there a link? Magnetic resonance retrospective 3Tesla study. Neuroradiology 58, 859–866 (2016). https://doi.org/10.1007/s00234-016-1711-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1711-0