Abstract
We reviewed the literature on how muscle disease affects quality of life compared to healthy controls, and the factors that influence the effects of muscle disease on quality of life. We also wanted to know whether quality of life differed between muscle diseases. We searched online databases and identified 26 relevant studies. The quality of each study was assessed, results sections analysed and a database of factors associated with quality of life developed. We graded the level of evidence supporting the association between each factor and quality of life as inconclusive, moderate or high. Compared to controls, muscle disease compromised quality of life in all areas of functioning. There was little evidence to suggest that quality of life differed significantly between muscle diseases. There was a high level of evidence suggesting that disease severity, pain, fatigue, and mood significantly affect quality of life. There was a moderate level of evidence suggesting that illness perceptions, coping strategies, age and gender affect quality of life. Several factors had an inconsistent level of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle diseases (MD) cause chronic and progressive muscle weakness leading to an insidious decline in mobility. They are distinguished from other neuromuscular conditions as they involve muscle tissue selectively, and are separate from anterior horn cell diseases or neuromuscular junction disorders. Although gene therapies and enzyme replacement treatments are now being used or developed for MD, there are no currently available effective medical interventions for MD. Indeed, it is possible that treatments may prolong life with disability at some considerable financial cost but with no discernable improvements in a patient’s quality of life (QoL). Thus, a more relevant marker for treatment success in MD may be quality rather than quantity of life. Subsequently, healthcare providers are increasingly demanding evidence that such treatments improve QoL.
QoL is a subjective psychosocial concept that people instinctively understand but which is challenging to measure. Nevertheless, there is a recognised methodology for the construction of a questionnaire to capture aspects of QoL. Most start from the World Health Organization (WHO) definition of QoL as, “an individual’s perception of their position in life in the context of the culture and value systems in which they live, in relation to their goals, expectations, standards and concerns” [1]. Many QoL questionnaires are multi-dimensional; assessing physical concerns, functional ability, social functioning and occupational functioning, but they may differ in the emphasis that they give to these dimensions. Some QoL measures are generic, meaning that they are applicable to any disease. Frequently used generic measures of QoL in MD include the Short Form-36 (SF-36) [2], sickness impact profile (SIP) [3] and Nottingham health profile (NHP) [4]. Disease specific QoL measures also exist and are argued to be more relevant and sensitive to the idiosyncrasies of individual diseases. However, at present there exists only one MD specific QoL measure for adults: the individualised neuromuscular quality of life questionnaire (INQoL) [5].
Measuring the factors which affect QoL in MD is particularly useful as it may identify ways to improve QoL in the absence of direct treatment for muscle weakness. This prospect arises from the recognition that symptoms other than weakness, such as pain and fatigue which are more treatable, have a considerable impact on QoL [6–8]. Studies of QoL in MD have identified several interventions which may improve QoL for these patient groups, including fatigue management [6], and tailored orthoses and rehabilitative strategies for pain resulting from posture or gait [9]. Furthermore, evidence from across studies of QoL in chronic illness suggest that disease severity may not have as great an affect on QoL as would be assumed intuitively [10]. Indeed it has been reported that over 50% of patients with moderate to severe disabilities experience either an excellent, or good, QoL [11]. This counter-intuitive preservation of QoL in chronic illness and disability has been called the “disability paradox” [10]. Thus, it seems, QoL does not have a simple relationship to disease severity but is influenced by a variety of other psychosocial factors [12, 13], and these factors may also be treatable.
For these reasons it becomes important to measure the impact of MD on QoL and to understand the other factors which may influence QoL in these illnesses. To date there has been no attempt to systematically review literature on QoL in adults with MD. This review aims to: (1) assess the direct effect of MD on QoL by comparison with healthy adult populations; (2) assess QoL across MD illness groups; (3) identify the factors which are associated with QoL in MD.
Method
Procedure
Online databases PsychINFO, EMBASE, MEDLINE and the Cochrane Database of Systematic Reviews were searched from their earliest available date until the end of February 2010. Abstracts of studies were read and, if suitable, a full-text copy was retrieved and assessed against inclusion/exclusion criteria. The review used a subject and text word strategy with muscle disease, neuromuscular, muscular dystrophy and quality of life, psychosocial functioning, well-being as the primary search terms.
The reference sections of all retrieved articles were searched to identify further studies suitable for inclusion. Studies were included based on the inclusion/exclusion criteria shown below. Study quality was then assessed; the cut-off for high quality was set at ≥19/22 (86%) available marks.
Results sections were analysed and a database of factors associated with QoL in MD compiled. This allowed all studies that concerned a certain factor to be grouped together and to be considered in relation to each other. When describing the strength of correlations the following categories were used: “weak” correlation = 0 < |r| < 0.3; “moderate” correlation = 3 < |r| < 0.7; “strong” correlation = |r| > 0.7. Findings were considered alongside quality assessment as listed in Table 2 and levels of evidence determined for each factor (see Table 1).
Inclusion/exclusion criteria
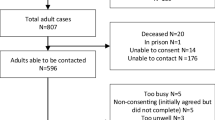
Studies were considered for inclusion if they: (a) assessed QoL in acquired and/or hereditary MD populations; (b) included adult populations (≥19 years); (c) used at least one domain from a QoL measure; (d) either grouped together MD as an umbrella term and measured QoL for the whole group, or grouped together muscle diseases e.g., proximal muscle disease or myotonias; (e) were published in the English language. Studies with samples which included other types of neuromuscular disease were included if it was possible to see MD results in isolation Fig. 1).
Study quality
Using a standard quality checklist [14] the extent to which each study fulfilled a pre-existing set of criteria was rated. For each item the same rating scale was applied (yes = 2, partially = 1 and no = 0). All areas were summed and an overall percentage mark was ascertained by dividing the total marks achieved by the total available (see Table 2). Some items were removed from the checklist as they were not applicable to this review.
A sub-set of all the studies included was assessed independently by two reviewers (CG, SDK) and inter-rater reliability was found to be moderate (Cohen’s kappa = 0.41). Discrepancies in assessment between the two reviewers were discussed and the quality of a sub-set of studies was then re-assessed by each reviewer resulting in an improved reliability (Cohen’s kappa = 0.56). One reviewer (CG) then assessed the quality of all studies against the standard quality checklist.
Results
Description of studies included
The electronic search yielded 10,187 potentially relevant articles; of these, 26 studies fulfilled the inclusion and exclusion (see Fig. 1). Across studies, 12 different QoL measures were used. The most popular measures used were the SF-36 (n = 12), SIP (n = 8), the Kaasa test (n = 4) and INQoL (n = 2). All others (TAAQoL, PGWBI, AIMS 1, DLQI, QoLP, SQoL, Skindex-16, NHP) were used once. Total sample size was n = 4,085 (range: n = 18–1,092). The average sample size of was n = 151.3. Quality scores ranges from 55 to 100% (see Table 1).
QoL in MD compared to healthy populations
All 12 studies which compared people with MD to healthy controls reported poorer scores for the MD groups on one or more QoL domains [6, 7, 9, 13, 15–22], with five of these studies reporting significantly lower QoL across all sub-scales of the QoL measure [6, 7, 15, 21, 22].
QoL across MD groups
Six of the 11 studies which compared QoL across different MDs reported no significant between-group differences [19, 21, 23, 25–27]. However, group differences were reported in the two studies with the largest sample sizes: Rose et al. (submitted) [7] and Sansone et al. [27], both using INQoL, reported that QoL was poorer in a limb-girdle muscular dystrophy (LGMD) [7] and a MyoDys group [27], respectively.
Factors affecting QoL in MD
Patterns of associations between QoL domains and factors studied in relation to QoL are described in Table 3.
Disease severity
Eleven studies assessed relationships between severity or function and QoL [7–9, 13, 15, 16, 21, 24, 25, 27, 28]. Of these, two assessed severity using manual muscle testing [8, 27], and nine assessed function using measures of function. Of the nine which assessed function, two studies assessed group differences between more and less functionally impaired groups. Both reported poorer QoL in the more functionally impaired group on at least one QoL domain, but with no significant differences in emotional functioning [16, 28]. Seven studies [7, 9, 13, 15, 21, 24, 25] assessed associations between measures of function and QoL; all reported strong and/or significant associations between physical domains and function. Of these seven, two studies [24, 25] reported significant correlations of weak to moderate strength between function and SIP psychosocial. Of the five studies which reported individual domain scores, all [7, 9, 13, 15, 21] noted no significant correlation between function and emotion/psychological functioning measures. However, four of these studies [7, 9, 13, 15] noted significant correlations between QoL social functioning domains and function. Only one [21] noted a significant, but weak, correlation between QoL measures of pain and function.
Results from the two studies assessing manual muscle testing, observed the following: Sansone et al. [27] reported that muscle strength (Medical Research Council) was moderately correlated with INQoL total score. However, Kalkman et al. [8] reported that MRC was not significantly associated with “impairment” (a composite physical QoL score derived from the SIP domains, “body care and movement”, “home management”, “communication”, “work limitations”, “recreation and pastimes” and “impairments with eating”).
Additionally, two studies assessed measures of respiration and cardiac function in relation to QoL [19, 26]. Ahlstrom et al. (2004) [26] reported significant associations between Forced Vital Capacity and SIP total score (moderate correlation), SIP physical composite score (moderate correlation) and SIP psychosocial composite score (weak correlation), but PCO2 levels (a measure of respiration) were not associated with any indices of the SIP. Abnormal ECG was moderately associated with SIP total score and SIP physical composite score but not SIP psychosocial score. Indices of the Kaasa test were not significantly correlated with any measure of respiration or cardiac function.
Bostrom and Ahlstrom [19], reported poorer functioning for MD patients on just the “energy” item (of 18 items) of the SQoL between MD patients using a ventilator and those who were not. Ahlstrom et al. [26], reported that use of a ventilator was moderately associated with SIP total score, SIP physical composite score, and SIP psychosocial composite score.
Fatigue
Five studies assessed fatigue in relation to QoL [6, 8, 26, 27, 29]. Two assessed QoL differences between fatigued and non-fatigued MD groups [6, 29]. Laberge et al. [29] reported significant differences between a MyoDys group without fatigue or excessive daytime sleepiness (EDS) and a MyoDys group with both fatigue and EDS on all domains of the SF-36. Kalkman et al. [6] also observed that severely fatigued patients had significantly lower scores on all SF-36 domains. Two studies [6, 27] observed significant associations between QoL and standard fatigue scales. One study [8] measuring fatigue with the checklist individual strength fatigue scale (CIS-Fatigue) reported that SF-36 “physical functioning”, “social functioning” and “bodily pain” were significant predictors of fatigue whilst “mental health” was not a significant predictor of fatigue.
Pain
In total, five studies assessed the relationship between pain and QoL [6, 9, 21, 30, 31]. Of these, one study assessed associations between SF-36 “bodily pain” and the other SF-36 indices [31]. Moderate correlations were observed with all other SF-36 indices but were greatest for “social functioning”, “vitality” and “general health”.
Relationships between pain scales and QoL were assessed in four studies [9, 21, 30, 31]. One study reported that pain in the last week was significantly associated with “psychosocial functioning” [30]; and another reported that arthralgia was a significant negative predictor for “physical functioning”, “bodily pain” and “social functioning” [21]. In a study with MyoDys patients, Padua et al. [9] reported that SF-36 “bodily pain” was moderately associated with age, Beck’s Depression Inventory (BDI), and both SF-36 physical composite score and SF-36 mental composite score, but not associated with fragment size (genetic measure) or severity (as measured by the clinical severity scale). Standard pain scales were also strongly related to QoL: visual analogue scale pain (VAS) showed significant relationships with both physical (moderate correlation) and mental composite scores (moderate correlation); the SF-36 and Portenoy-6 questions (ID pain) showed significant relationship with just the physical composite scale. Both VAS and ID Pain were moderately correlated with SF-36 “social functioning”.
Age
Seven studies investigated the effect of age on QoL [9, 13, 15, 20, 21, 24, 25]. Two studies stratified age into younger and older age-groups [20, 25]. One reported poorer QoL in the older group on the majority of domains [25] and, one reported no significant difference between age-groups on just the “bodily pain” domain of the SF-36 [20].
Three studies assessed associations between QoL domains and age [9, 13, 15] with a mixed pattern of results; Padua et al. [9] correlated age with SF-36 domains and reported moderate correlations between age and all domains, whereas Sadjadi et al. [13] reported only weak correlations between age and SF-36 domains. Antonini et al. [15] reported moderate correlations between age and “physical functioning” and “vitality”. A pattern common to all correlational studies was that “physical functioning” was the domain most strongly associated with age. In a predictor study, age was a significant predictor for the “mental health” domain [21].
Duration of disease
Four studies assessed the effects of duration of illness [13, 15, 20, 27] on QoL, with two [20, 27] of these stratifying age into groups to assess between-group differences, Oksuz et al. [20] into those of less or more than 8 years duration and, Sansone et al. [27], into four categories: 1–5, 6–10, 11–20, >21 years. Oksuz et al. [20] reported no significant differences on any NHP domains. Sansone et al. [27] found that with the exception of “locking” and “pain” domains, all INQoL sub-scales deteriorated between groups as duration of symptoms increased.
In studies which assessed correlations, Sadjadi et al. [13] reported only very weak correlations between duration of illness and QoL domains. Antonini et al. [15] reported strong and significant negative correlations between duration of illness and “physical functioning” “role physical” “general health” “vitality” and “social functioning”.
Age of onset
Age at onset was considered in one study [19]. Those with onset before the age of 20 years assessed their “relationship to own children” worse than both those with onset between 20 and 39 years and those with onset between 40 and 56 years. Those aged 20–39 years at onset had a significantly better assessment of “energy” than the group with the latest age at onset (40–56 years). The onset <20 years group were poorer on “personal economy” than the onset between 20 and 39 years group and reported poorer “energy” than the 40–56 years group.
Genetics
Three studies considered a genetic marker of illness severity in relation to QoL: for FSHD [9] and MyoDys [15]. Both reported no significant correlations between QoL domains and genetic marker. In a multiple regression, one of these studies [9] reported fragment size to be a significant predictor of SF-36 “physical functioning” in FSHD patients.
Coping
Three studies measured associations between QoL and coping [30, 32, 33]. Ahlstrom and Sjoden [32] correlated SIP with the mental adjustment to cancer (MAC) scale. “Hoplessness/helplessness” coping was moderately associated with QoL. Similar moderate associations were evident between QoL and “Anxious preoccupation”, “Minimization”, “Social comparison”. “Establishment of control over everyday life”, “Performs the task with aid of an appliance or other technical resource”, “Accepts help or leaves it to others”, “Stoic acceptance” and “Has tried alternative treatment” was weakly associated with Kaasa “happiness”. Nätterlund et al. [33] reported similar moderate associations between helplessness/hoplessness (MAC scale) and SIP indices. Here, also “Fatalism” and “Avoidance” coping strategies were moderately associated with QoL [33]. Miro et al. [30] reported a moderate association between "Catastrophizing" (Coping Skills Questionnaire) and SF-36 “mental health”.
Mood
All six studies which correlated measures of mood with QoL reported significant associations between mood and QoL on at least half of the domains investigated [7–9, 13, 15, 25]. Piccinnini et al. [25] reported that SIP physical composite score, SIP psychosocial composite score and SIP total score were all significantly correlated with Psychological General Well-Being Index (PGWBI) “anxiety” and “depression” sub-scales. Antonini et al. [15] reported that four (of eight) SF-36 dimensions were significantly associated with measures of affect and anxiety. Kalkman et al. [8] reported significant but weak correlations between a sub-set of the mainly physical domains from the SIP and measures of mood.
Rose et al. (submitted) [7] observed that all domains of the INQoL were at least moderately correlated with HADS Depression (HADS-D), whilst most were correlated with HADS Anxiety (HADS-A). HADS-D was also the best predictor of overall QoL and was the best predictor of “emotional”, “social” and “fatigue” domains. Padua et al. [9] reported that all SF-36 sub-scales were significantly correlated with BDI and Sadjadi et al. [13] reported that the majority of SF-36 sub-scales were correlated with BDI. The four strongest associations between BDI and SF-36 indices were the same in two studies [9, 13]: “general health”, “vitality”, “social function” and “mental health”—these were all moderately correlated with BDI score. Additionally, Sadjadi et al. [13], reported that up to 14% of the effect of disease severity on some SF-36 sub-scales was mediated through BDI scores.
Employment
Four studies assessed the relationship between employment and QoL [20, 21, 31, 34]. Of these, two looked for QoL differences between employed and unemployed groups [20, 34]. Oksuz et al. [20], using the NHP reported that employed MD patients experienced significantly less “pain”, “social isolation” and better “sleep” and had an overall better QoL score than unemployed MD patients. Minis et al. [34] investigated group differences on four domains of the SF-36 between employed and unemployed patients in MyoDys and FSHD groups. For the MyoDys group, significant differences were reported between employed and unemployed groups on “physical functioning” and “social functioning”, but not “bodily pain” or “vitality”. For the FSHD group significant differences were reported between the groups for “physical functioning”, “vitality” and “bodily pain” but not “social functioning”.
Two studies assessed correlations between employment measures and QoL. Ponyi et al. [21] reported that no measure of QoL (SF-36) was significantly related to work status, and Abresch et al. [31] found only a weak correlation between “bodily pain” and global ratings of satisfaction with employment. One study used QoL domains to predict employment status [34], finding that “physical functioning” (from the SF-36) was the only significant predictor.
Cognition
Three studies assessed cognitive functioning in relation to QoL [8, 15, 27]. Antonini et al. [15] reported that only the Stroop colour-word interference task was significantly associated with QoL indices; specifically for SF-36 “physical functioning”, “general health” and “vitality”—these correlations were of moderate strength. Similarly, Kalkman et al. [8] observed significant, but weak, correlations for a FSHD group on 1 (of 6) tests of reaction time and movement time. No significant association in the MyoDys group for any test of movement time or reaction time from the complex reaction time test battery was reported. Sansone et al. [27] observed a moderate correlation between Mini-Mental State Examination score and INQoL total score.
Sleep
Two studies correlated measures of QoL with measures of sleep [8, 31]. Abresh et al. [31] reported a weak correlation between “bodily pain” and self-reported sleep disturbance. Kalkman et al. [8] correlated measures of sleep functioning with “functional impairment”—a composite measure of mainly physical sub-scales of the SIP. Here the Symptom Checklist-90 “sleep” domain was significantly associated with “functional impairment” in both MyoDys (weak correlation) and FSHD (moderate correlation) groups. There were no significant correlations between “functional impairment” and daily general sleep quality or Non-restorative sleep for either group.
Illness perceptions
Two studies assessed illness perceptions in relation to QoL [7, 30]. Miro et al. [30] found significant associations between the SF-36 “mental health” and the survey of pain attitudes (SOPA). Here, several beliefs about pain were weakly to moderately correlated with SF-36 “mental health”.
Rose et al. (submitted) [7] investigated associations between QoL and the Illness Perceptions Questionnaire (IPQ-R) [35]. Perceptions about “consequences”, “identity” and “emotional representations” were moderately correlated with most indices of the INQoL. These perceptions were also moderately correlated with overall QoL. Many illness perceptions were also predictors of QoL indices: IPQ-R Consequences was the best predictor of INQoL “body image”; IPQ Identity was the best predictor of INQoL “pain”.
Gender
Six, [9, 16, 21, 27, 28, 31] of eight [9, 16, 20, 21, 27, 28, 31, 36], studies which assessed QoL in males compared to females reported that being female was either associated with a worse QoL, or that females with MD experienced significantly worse QoL than males with MD.
Discussion
QoL in MD compared to healthy control groups
Several studies found that all QoL domains were worse in an MD group compared to healthy controls [6, 7, 15, 21, 22]. However, whilst it was apparent that the physical domains of QoL measures were affected by having MD, an affect on psychosocial indices was reported less consistently. This finding may be due to the relative complexity of psychosocial well-being. Koch [37] criticises QoL measurement for being to simplistic and failing to include in an ecologically valid way, familial, financial, individual, interpersonal, marital, professional, physical and social attributes; perhaps these complexities are played out in the psychosocial indices of QoL measures and thus manifest in large volatile personal shifts in QoL and therefore, at group level, less consistent patterns or smaller effect sizes. It should be noted that whilst, ostensibly, this finding of poorer QoL in MD populations sits in contrast to the disability paradox [10], the disabilty paradox emphasizes, from the viewpoint of a healthy person, an unexpectedly high QoL—here this may still be the case.
QoL across different MDs
Taken together the studies suggest that there is little difference in QoL between MD groups. This conclusion is borne out by a qualitative study of illness experience across different MDs [38] which concluded that “… the illness experience was similar irrespective of the particular diagnosis. There were similar reactions to learning of the diagnosis, a similar feeling of uncertainty about the future, and similar accompanying psychosocial consequences of having a hereditary disease.”
Equally however, this finding may be due to sample sizes being too small to detect group differences. In two recent studies, with large sample sizes, MyoDys [27] or LGMD [7] were found to have a greater impact upon QoL than other MDs.
Factors with a high level of evidence
Disease severity was consistently associated with the physical indices of QoL measures and also social functioning. It is likely that increased severity limits the opportunity to socialise. However, interestingly, psychological or emotional functioning and experienced pain appeared to be relatively independent of disease severity, which likely reflects the relatively complex nature of psychological/emotional functioning. Similar findings have also been reported in a study of people with Duchenne Muscular Dystrophy [39]. Here QoL was found to be independent of the degree of disability and respiratory impairment. This finding is important, as based upon judgements of severity the under-rating of QoL by healthcare professionals can occur and this has an impact upon care decisions [40].
Significant pain is a frequently reported complaint in MD [41, 42]. More than half of FSHD patients report at least moderate pain on a VAS pain scale [9] and this systematic review uncovered a high level of evidence linking experienced pain with aspects of QoL. Whilst both psychosocial and physical domains were associated with pain, there appears to be an intimate association between social functioning and pain. This association is expected since experienced pain would restrict activity and reduce motivation, and therefore, the opportunity to socialise. However, it has been reported that lack of perceived social support is associated with pain experience and SF-36 “mental health” [30]; this suggests that mental health or beliefs about level of support received from others may mediate or moderate the relationship between social functioning and pain.
There are high levels of experienced fatigue in MD [8, 43]. In this systematic review, fatigue was associated with poorer QoL and consistently found to be associated with poorer physical functioning. An association with psychosocial indices was also apparent, though this was a less consistent finding.
Mood was strongly associated with QoL in MD and has been evidenced to be associated with most QoL indices. Measures of mood were the best predictors of QoL in two studies [7, 13] ahead of measures of severity. These findings are mirrored in Parkinson’s disease where several studies have found depression to be strongly associated with QoL [44–46]. Mood may also mediate some of the effect of MD on the physical indices of QoL measures [13]. Studies have reported increased prevalence of depression in BMD [47] and MyoDys [48].
It should be noted that mood is not just a surrogate measure for QoL. In Sadjadi et al. [13] multiple regression analysis showed that mood contributes 14% to the effect on QoL, with the major determinant of QoL being the severity of the MD. Also, the profile of moods’ effects on QoL differs from that of disease severity and mood is only loosely correlated with the severity.
Factors with a moderate level of evidence
It appears that the coping strategies that people with MD employ may have a bearing upon QoL. There were, however, few studies that assessed this and none of those that did were high quality. More research is required to disentangle coping, mood and QoL in MD.
Older age appears to negatively affect physical functioning. However, age is negatively associated with QoL in healthy populations [49, 50] and no studies included in the systematic review considered their results in comparison to population norms. Whilst it appears that age affects QoL in MD, it is unclear as to whether this is different from any decline in QoL that would be expected in healthy populations.
A similar criticism could be levelled at the majority of studies which assessed the effect of gender on QoL in MD. These suggest, on the basis of direct comparison between male and female QoL scores, that being female is associated with poorer QoL in MD groups. However, healthy females may report lower QoL scores than healthy males [49, 50], thus it would not be unexpected that females with MD would evidence a worse QoL. One study [16] does present some evidence that males and females have differing patterns of QoL decline when compared to healthy male and female controls—most saliently, women with MD reported lower QoL in the domain of “sleep” than healthy female controls, whereas there was no significant difference between males with MD and healthy male controls on “sleep”.
There is evidence emerging that the beliefs people hold about their illness, or illness perceptions, are linked to QoL. Pain beliefs were strongly related to the mental health domain of the SF-36 in one study [30], and another, Rose et al. (submitted) [7], reported that weakness, body image, pain, fatigue and overall QoL were all predicted by perceptions about the consequences of the illness, or the emotional representation of, and symptoms attributed to, the illness. This finding has been mirrored in other chronic conditions, including end stage renal disease, irritable bowel syndrome, chronic respiratory disease, and head and neck cancer [51]. Encouragingly, illness perceptions in physical illness are amenable to change: a cognitive intervention aimed at changing adverse illness perceptions in myocardial infarction was able to expedite return to work and improve QoL [52].
Factors with an inconclusive level of evidence
There was scant or inconsistent evidence to support or refute the association between QoL in MD and cognitive functioning, genetic measures, age of onset, duration of disease and employment. To evaluate potential relationships between these factors, large-scale studies, across illness groups, using comprehensive multi-domain measures of QoL, are required.
Implications for care
Pain, fatigue and emotional problems are prevalent in MD populations and detrimental to QoL. Clinicians should be vigilant of their impact and conduct appropriate assessments. One encouraging finding is that many QoL sub-scales are related to coping mechanisms, mood and illness perceptions. Thus, cognitive therapy aimed at improving mood, equipping sufferers with effective coping strategies or helpful illness perceptions may represent an effective method of improving QoL. In addition, treatment of fatigue through physical exercise may be beneficial for MD patients, although this awaits empirical testing. Indeed, as pain and fatigue may be independent of disease severity [7], benefits yielded from treatment, in terms of improving QoL, may be higher in those with higher levels of pain or fatigue than expected for their disease severity.
Limitations
Our review may have missed relevant papers published in non-English journals. In all, 12 different measures of QoL were used. Direct comparison of sub-scales is difficult due to the lack of conceptual consistency between measures. We were therefore obliged to adopt a mostly dichotomised view of QoL as representing physical or psychosocial functioning broadly with the addition of pain and fatigue concepts.
Many of the studies that we reviewed used very small sample sizes, but few reported effect sizes in addition to performing null hypothesis significance testing. There were also few longitudinal studies, which would have been desirable given the dynamic nature of QoL variables.
The muscle disease umbrella term encompasses a diverse group of diseases, some of which like MyoDys and mitochondrial disease are multisystem diseases which may affect cognition, cardiovascular function and physical appearance. This makes direct comparisons of study populations difficult as we may not be comparing like with like. As some studies used a mixed sample of MDs they may have diluted out, or exaggerated, any possible between group differences.
Suggestions for future research
Review of literature assessing the factors which affect psychosocial or physical constructs not considered measures of QoL, for example measures of pain or fatigue, would present a useful area for future research. Larger scale studies of QoL in MD, with samples including a range of MD are required to get reliable and comparable data.
Conclusion
QoL is reduced in MD, with physical indices more consistently affected than psychosocial ones. Many factors may affect QoL in MD. Of note, disease severity and function were strongly correlated with physical and social functioning across studies but independent of psychological functioning; this highlights the need to see beyond disease severity and to assess QoL comprehensively, beyond physical measures and at an individual level, in healthcare decision making.
There may be utility in developing future interventions based on QoL research. For example, there was a high level of evidence supporting an association between QoL and mood and illness perceptions, and these factors may be targeted by a cognitive intervention. Pain and fatigue are also associated with QoL in MD and these problems should be taken into consideration by clinicians when managing MD.
References
World Health Organization. Available at: http://www.who.int/en/. Accessed May 19
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection. Med Care 30(6):473–483
Bergner M, Bobbitt RA (1981) The sickness impact profile: development and final revision of a health status measure. Med Care 19(8):787–805
Hunt SM, McKenna SP, McEwen J (1981) The Nottingham health profile: subjective health status and medical consultations. Soc Sci Med Part A Med Sociol 15(3-I):221–229
Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR (2007) Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL). Neurology 68(13):1051–1057
Kalkman JS, Schillings ML, van der Werf SP et al (2005) Experienced fatigue in facioscapulohumeral dystrophy, myotonic dystrophy, and HMSN-I. J Neurol Neurosurg Psychiatry 76(10):1406–1409
Rose MR, Sadjadi R, Weinman J, Akhtar T, Pandya S, Kissel JT, Jackson CE (2011) The role of disease severity, illness perceptions and mood on quality of life in chronic muscle disease. (Submitted)
Kalkman JS, Schillings ML, Zwarts MJ et al (2007) The development of a model of fatigue in neuromuscular disorders: a longitudinal study. J Psychosom Res 62(5):571–579
Padua L, Aprile I, Frusciante R et al (2009) Quality of life and pain in patients with facioscapulohumeral muscular dystrophy. Muscle Nerve 40(2):200–205
Albrecht GL, Devlieger PJ (1999) The disability paradox: high quality of life against all odds. Soc Sci Med 48(8):977–988
Gerhart KA, Koziao-McLain J, Lowenstein SR, Whiteneck GG (1994) Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med 23(4):807–812
Abresch RT, Seyden NK, Wineinger MA (1998) Quality of life. Issues for persons with neuromuscular diseases. Phys Med Rehabil Clinics North Am 9:233–248
Sadjadi R, Rose MR (2010) Muscle Study Group. What determines quality of life in inclusion body myositis? J Neurol Neurosurg Psychiatry 81:1164–1166
Kmet LM, Lee RC, Cook LS (2004) Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Alberta Heritage Foundation for Medical Research, Alberta
Antonini G, Soscia F, Giubilei F et al (2006) Health-related quality of life in myotonic dystrophy type 1 and its relationship with cognitive and emotional functioning. J Rehabil Med 38(3):181–185
Grootenhuis MA, de Boone J, van der Kooi AJ (2007) Living with muscular dystrophy: health related quality of life consequences for children and adults. Health Quality Life Outcomes 5:31
Ford C, Kidd A, Hammond-Tooke G (2006) Myotonic dystrophy in Otago, New Zealand. N Z Med J 119(1241):U2145
Bronner IM, van der Meulen MF, de Visser M et al (2006) Long-term outcome in polymyositis and dermatomyositis. Ann Rheum Dis 65:1456–1461
Bostrom K, Ahlstrom G (2005) Quality of life in patients with muscular dystrophy and their next of kin. Int J Rehabil Res 28(2):103–109
Oksuz C, Kilinc M, Yildirim SA (2009) Predictors of health-related quality of life in adult ambulatory independence neuromuscular disease patients. Neurosciences 14(4):355–359
Ponyi A, Borgulya G, Constantin T, Vancsa A, Gergely L, Danko K (2005) Functional outcome and quality of life in adult patients with idiopathic inflammatory myositis. Rheumatology 44:83–88
Sultan SM, Ioannou Y, Moss K, Isenberg DA (2002) Outcome in patients with idiopathic inflammatory myositis: morbidity and mortality. Rheumatology 41:22–26
Bostrom K, Natterlund BS et al (2005) "Sickness impact in people with muscular dystrophy: a longitudinal study over 10 years." Clin Rehabil 19(6):686–694
Ahlstrom G, Gunnarsson L (1996) Disability and quality of life in individuals with muscular dystrophy. Scand J Rehabil Med 28(3):147–157
Piccininni M, Falsini C, Pizzi A (2004) Quality of life in hereditary neuromuscular diseases. Acta Neurol Scand 109(2):113–119
Ahlstrom G, Gunnarsson L-G, Kihlgren A, Arvill A, Sjödén P-O (1994) Respiratory function, electrocardiography and quality of life in individuals with muscular dystrophy. Chest 106(1):173–179
Sansone VA, Panzeri M, Montanari M et al (2010) Italian validation of INQoL, a quality of life questionnaire for adults with muscle diseases. Eur J Neurol 17(9):1178–1187
Hundley JL, Carroll CL, Lang W et al (2006) Cutaneous symptoms of dermatomyositis significantly impact patients’ quality of life. J Am Acad Dermatol 54:217–220
Laberge L, Dauvilliers Y, Begin P, Richer L, Jean S, Mathieu J (2009) Fatigue and daytime sleepiness in patients with myotonic dystrophy type 1: to lump or split? Neuromuscul Disord 19(6):397–402
Miro J, Raichle KA, Carter GT et al (2009) Impact of biopsychosocial factors on chronic pain in persons with myotonic and facioscapulohumeral muscular dystrophy. Am J Hospice Palliative Med 26(4):308–319
Abresch RT, Carter GT, Jensen MP, Kilmer DD (2002) Assessment of pain and health-related quality of life in slowly progressive neuromuscular disease. Am J Hospice Palliative Med 19(1):39–48
Ahlstrom G, Sjoden PO (1996) Coping with illness-related problems and quality of life in adult individuals with muscular dystrophy. J Psychosom Res 41(4):365–376
Nätterlund B, Gunnarsson L-G, Ahlström G (2000) Disability, coping and quality of life in individuals with muscular dystrophy: a prospective study over five years. Disabil Rehabil 22(17):776–785
Minis MA, Kalkman JS, Akkermans RP et al (2010) Employment status of patients with neuromuscular diseases in relation to personal factors, fatigue and health status: a secondary analysis. J Rehabil Med 42(1):60–65
Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D (2002) The revised illness perception questionnaire (IPQ-R). Psychol Health 17(1):1–16
Natterlund B, Ahlstrom G (2001) Activities of daily living and quality of life in persons with muscular dystrophy. J Rehabil Med 33(5):206–211
Koch T (2000) Life quality vs the ‘quality of life’: assumptions underlying prospective quality of life instruments in health care planning. Soc Sci Med 51(3):419–427
Natterlund B, Sjoden PO, Ahlstrom G (2001) The illness experience of adult persons with muscular dystrophy. Disabil Rehabil 23(17):788–798
Kohler M, Clarenbach CF, Böni L, Brack T, Russi EW, Bloch KE (2005) Quality of life, physical disability, and respiratory impairment in Duchenne muscular dystrophy. Am J Respir Crit Care Med 172(8):1032–1036
Gibson B (2001) Long-term ventilation for patients with Duchenne muscular dystrophy: physicians’ beliefs and practices. Chest 119(3):940–946
Jensen MP, Abresch RT, Carter GT, McDonald CM (2005) Chronic pain in persons with neuromuscular disease. Arch Phys Med Rehabil 86(6):1155–1163
Jensen MP, Hoffman AJ, Stoelb BL, Abresch RT, Carter GT, McDonald CM (2008) Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil 89(2):320–328
Schillings ML, Kalkman JS, Janssen HM, van Engelen BG, Bleijenberg G, Zwarts MJ (2007) Experienced and physiological fatigue in neuromuscular disorders. Clin Neurophysiol 118(2):292–300
Schrag A, Jahanshahi M, Quinn N (2000) What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 69(3):308–312
Schrag A, Jahanshahi M, Quinn N (2000) How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord 15(6):1112–1118
Ravina B, Camicioli R, Como PG et al (2007) The impact of depressive symptoms in early Parkinson disease. Neurology 69(4):342–347
Melo M, Lauriano V, Gentil V et al (1995) Becker and limb-girdle muscular dystrophies: a psychiatric and intellectual level comparative study. Am J Med Genet Neuropsychiatric Genet 60(1):33–38
Bungener C, Jouvent R, Delaporte C (1998) Psychopathological and emotional deficits in myotonic dystrophy. J Neurol Neurosurg Psychiatry 65(3):353–356
Brorsson B, Ifver J, Hays RD (1993) The Swedish health-related quality of life survey (SWED-QUAL). Qual Life Res 2(1):33–45
Michelson H, Bolund C, Nilsson B, Brandberg Y (2000) Health-related quality of life measured by the EORTC QLQ-C30–reference values from a large sample of Swedish population. Acta Oncol 39(4):477–484
Hagger MS, Orbell S (2003) A meta-analytic review of the common-sense model of illness representations. Psychol Health 18(2):141–184
Petrie KJ, Cameron LD, Ellis CJ, Buick D, Weinman J (2002) Changing illness perceptions after myocardial infarction: an early intervention randomized controlled trial. Psychosom Med 64(4):580–586
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00415-011-6156-0
Rights and permissions
About this article
Cite this article
Graham, C.D., Rose, M.R., Grunfeld, E.A. et al. A systematic review of quality of life in adults with muscle disease. J Neurol 258, 1581–1592 (2011). https://doi.org/10.1007/s00415-011-6062-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6062-5