Abstract
Objectives
To determine the impact of genetic muscle disorders and identify the sociodemographic, illness, and symptom factors influencing quality of life.
Methods
Adults (aged 16–90 years) with a confirmed clinical or molecular diagnosis of a genetic muscle disorder identified as part of a nationwide prevalence study were invited to complete an assessment of the impact of their condition. Quality of life was measured using the World Health Organization Quality of Life questionnaire. Impact was measured via the prevalence of symptoms and comparisons of quality of life against New Zealand norms. Multivariate regression models were used to identify the most significant predictors of quality of life domains.
Results
490/596 participants completed the assessment (82.2% consent rate). Quality of life was lower than the general population on physical (t = 9.37 p < 0.0001, d = 0.54) social (t = 2.27 p = 0.02, d = 0.13) and environmental domains (t = 2.28 p = 0.02, d = 0.13), although effect sizes were small. No difference was found on the psychological domain (t = − 1.17 p = 0.24, d = 0.07). Multivariate regression models (predicting 42%–64% of the variance) revealed personal factors (younger age, being in employment and in a relationship), symptoms (lower pain, fatigue, and sleep difficulties), physical health (no need for ventilation support, fewer activity limitations and no comorbidities), and psychosocial factors (lower depression, anxiety, behavioural dyscontrol and higher self-efficacy, satisfaction with health care and social support) contributed to improved quality of life.
Conclusions
A range of factors influence the quality of life in adults diagnosed with a genetic muscle disorder and some may serve as targets for multi-faceted intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic muscle disorders primarily present with muscle weakness, affecting a person’s ability to perform specific tasks and maintain independence [1]. A number of other symptoms can also have a significant bearing on a person’s experience. For example, respiratory muscle weakness might compromise sleep, and pain and fatigue can impact both motivation and ability to participate in daily life [2]. Combined, these challenges may reduce access to education, employment, and social activities and quality of life (QoL) [2, 3].
QoL is a multidimensional concept reflecting a subjective sense of well-being and life satisfaction [4]. The World Health Organization (WHO) defines QoL as an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns [5]. With advances in the treatment of muscle disorders, it is possible that treatments may prolong life, but have little impact on a person’s QoL. Consequently it is important to understand the factors associated with QoL to enable treatments to have optimal impact [2]. Studies have consistently shown that people living with genetic muscle disorders have significantly reduced QoL compared to the general population [3]. However, the range of potential factors influencing QoL in people with genetic muscle disorders currently remains unclear.
A previous systematic review revealed that QoL was linked to pain, symptom severity, fatigue and mood, and possibly linked to age, sleep quality and sex [2]. However, there was inconclusive evidence for links with cognition and employment, and no exploration of the roles that satisfaction with care, self-efficacy and social support play in relation to QoL outcomes. Two other studies have shown that although genetic muscle disorders are heterogenous in their symptom profile and rate of progression, there are no significant differences in overall QoL between different specific diagnoses [6, 7]. The review noted that evidence was highly limited by small sample sizes, lack of statistical analysis, lack of breadth in terms of conditions studied and potential sample selection bias (e.g. only included those actively seeking specialist care) [2]. This need for large-scale studies across a disease spectrum has yet to be addressed [2]. This information is crucial to guide implementation of interventions aimed at improving QoL. This study's main objective was to identify the key predictors of QoL in a large cohort of adults with a wide range of genetic muscle disorders recruited from a national prevalence study.
Methods
Study population
This population-based study drew upon a nationwide prevalence sample of adults and children with a confirmed diagnosis of a genetic muscle disorder, residing in New Zealand (NZ) on the point prevalence date of 01/01/2015. Full details and prevalence findings have been published elsewhere [8]. In brief, cases were ascertained using multiple overlapping sources of information, including health care and community services as well as self and family referrals. Genetic muscle disorders included all types of muscular dystrophy (Duchenne, Becker, Emery-Dreifuss, limb-girdle, facioscapulohumeral, myotonic, oculopharyngeal, distal and congenital), myotonia congenita, paramyotonia congenita, central core disease, nemaline myopathy, myotubular myopathy, hereditary inclusion body myopathy, periodic paralysis and Pompe’s disease [9]. The study team verified all diagnoses by reviewing medical records including the results of any clinical investigations and genetic tests.
Procedure
All identified cases in the prevalence study were invited to participate in an assessment to determine the impact of their disorder on their QoL. Written, informed consent was obtained prior to conducting the assessment. Assessments were conducted face-to-face with a research assistant, over the phone, or self-completed online or on paper. The flexible mode of assessment administration was designed to allow for participant preference, and therefore to enhance recruitment, facilitate data completeness/response rate and increase feasibility if practical difficulties made an in-person assessment too challenging [10]. The team of researchers conducting the assessments were based across the South and North Islands of NZ to facilitate the participation of those residing in both rural and urban areas. Participants were able to have a support person with them during the assessment and/or to complete the assessment over several sessions if preferred. The use of automated questionnaire logic also enabled the omission of measures if they did not need to be completed, e.g. if not employed, the work measures were not asked. Patients were able to decline to answer any question and/or stop the assessment at any time if they needed to do so. An open question on completion of the interview asked how participants found the assessment and if anything of importance had been omitted. This was also a place for the Research Assistant to note any comments, e.g. difficulty answering questions. Responses to the online questionnaires were entered directly into the study database using SurveyMonkey (www.surveymonkey.com), with self-completed forms entered manually into the database. Only data for those aged ≥ 16 years of age were extracted for this analysis due to the need to use age-standardised measures for those aged < 16 years reported elsewhere [11].
Outcome measure
The World Health Organization Quality of Life questionnaire (WHOQOL-BREF) measures QoL [12] is based on the WHO definition of QoL. This measure includes 26 items representing four different QoL domains (physical health, psychological health, social relationship, and environment). Subscales scores were according to the WHOQOL-BREF manual [13] and converted into a linear measure based on the Rasch model [14]. Higher scores indicate better QoL. NZ normative data were obtained to enable comparison to the general population [14].
Measures of potential predictors
Participants completed questions including date of birth, sex, the ethnicity with which they most associated, educational level, relationship status, employment status, annual income, comorbidities, and the age of symptom onset of their genetic muscle disorder. Participants rated their overall satisfaction with health care services on a scale from (0) very dissatisfied to (10) very satisfied.
Limitation in daily activities was assessed using the ACTIVLIM measure [15]. Participants were asked to estimate the difficulty level they experienced in performing 18 everyday activities without help on a 3-point scale (impossible, difficult, easy). The online scoring algorithm was used to convert the raw scores into a linear measure accounting for item difficulty [16]. The ACTIVLIM has demonstrated excellent test–retest reliability (0.93), content validity, concurrent validity with the Functional Independence Measure (FIM) (p = 0.85) 43 with evidence of responsiveness to change over time.44 High scores indicate fewer activity limitations.
The McGill Pain Questionnaire Short Form version 2 was used to assess pain. This measure lists 22 common descriptors of pain including sensory, affective, intermittent and neuropathic aspects of pain. Participants were asked to rate the intensity they experience each descriptor of pain on a scale from 0 = none to 10 = severe. A total pain score was calculated as the mean of all of the items, with higher scores indicative of more pain. Due to the non-normal distribution, the pain was classified as 0 = no pain and 1 = some degree of pain experienced in the regression model.
The Fatigue Severity Scale assessed levels of extreme tiredness [17]. Nine items assess the impact of fatigue. The extent to which a person agrees or disagrees with each item is recorded on a scale from 1 = strongly disagree to 7 = strongly agree. A total score of all summed items ranges from 9 to 63, with higher scores indicative of higher levels of perceived fatigue. Scores of > 36 indicate the person is experiencing problematic levels of fatigue [17].
The NeuroQol was used to assess levels of self-reported cognitive functioning and emotional and behavioural dyscontrol (e.g. pathologic laughing and crying, affective lability, irritability, disinhibition, and aggression) [18]. The NeuroQol was developed specifically for people living with neurological conditions [19]. Participants rated how they felt over the past seven days on a five-point scale from 1 = never to 5 = always. Total scores for each scale were calculated and converted to standardised T scores, with a mean of 50 and standard deviation of 10.
The Multidimensional Scale of Perceived Social Support [20] was used to assess levels of perceived social support from family and friends. The scale contains 12 items, with participants asked to rate the extent to which they agree with 12 statements such as ‘I can count on my friends when things go wrong’ and ‘there is a special person who is a real source of comfort to me”. Responses were rated on a seven-point scale from 1 = very strongly disagree to 7 = very strongly agree. A mean total score from across all items was calculated (ranging from 12 to 84), with higher scores indicating higher perceived levels of social support.
The General Self-Efficacy Scale assessed participants’ belief in their own ability to succeed despite difficult demands in life [21]. This measure consists of eight items on a scale from 1 = strongly disagree to 5 = strongly agree. A total mean score of all items was calculated, with higher scores indicating higher perceived self-control.
The Hospital Anxiety and Depression Scale [22] contains two subscales, each containing seven items, measuring levels of anxiety and depression. Participants rate each item, which indicates how they have been feeling over the past week on a scale of 0 (not at all) to 3 (very often). A total score for each subscale ranging between (0–21) was calculated with scores of 0–7 classified as being within the normal range, 7–11 classified as mild and scores of > 11 as moderate to severe.
The Pittsburgh Sleep Quality Index [23] assessed levels of perceived sleep quality in significant others. Participants were asked to rate the quality of different components of sleep such as falling asleep within 30 min, waking up during the night and feeling tired during the day during the last month on a scale 0 (not during past month) to 3 (three of more times per week). A total score was calculated to represent overall sleep quality ranging, with scores ≥ 5 indicative of poor sleep quality requiring intervention.
Data analysis
Descriptive statistics including means and standard deviations were used to describe the participant sample based on medical records data as part of the prevalence study on age, sex and diagnosis. Normality of variables was determined using histograms and analysis of skewness and kurtosis. Variables which were highly skewed with a kurtosis score > 2 were considered to be non-normal. Chi-square or t-tests were used to determine any differences between adults who completed the assessment and those who did not and to explore any differences in QoL between our sample and the NZ general population. Cohen’s d effect size statistics were calculated to show the magnitude of difference, with effect sizes of < 0.2 considered trivial, 0.20–0.59 small, 0.60–1.19 moderate and > 1.20 large [24].
Predictive models were developed by first running simple regression models for each of the QoL domains. Potential predictors were considered for inclusion in the multiple regression model if the p-value was < 0.2. This criterion enabled exploration of a range of potential contributors to the model in this exploratory study, whilst excluding variables with low likelihood of contributing to the model with a very weak association with the outcome creating unnecessary ‘noise’. Spearmans correlations between each of the predictors were checked. If there was a moderate or high correlation between the predictors (r ≥ 0.5), only the predictor with the most significant contribution to the model was included. The remaining predictors were then entered using a stepwise selection procedure.
Results
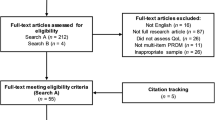
There were 596 adult participants identified in the prevalence study for whom there were valid contact details, and a researcher was able to talk to the participant about the study. Of these, 490 (82.2%) consented to take part in this study and completed the assessment (Fig. 1). The majority of assessments (441, 90.0%) were conducted in-person with a researcher. A further nine (1.8%) were completed over the phone, 28 (5.7%) self-completed online, and 12 (2.4%) self-completed via postal questionnaire. The battery of assessments was found to be manageable and comprehensive by the majority of participants. Only one participant, who had a learning disability, reported finding the assessment long and difficult to complete.
Table 1 shows the overall characteristics of the study population. The sample mean age was 45.6 ± 16.3 years (16–90). Half of the participants had at least one comorbid condition. The most commonly reported comorbid conditions were asthma, diabetes, cardiovascular conditions, and mental health conditions.
In order to check for generalizability of the findings, key sociodemographics of this sample were compared with those who did not consent to the assessment using the initial prevalence sample data. There were no significant differences between adults who completed the assessment and those who did not with regards to age (t = 0.92, p = 0.36) and diagnosis (χ2 = 6.29, p = 0.18). However, there was a significantly lower proportion of males than females who completed the outcome assessment (χ2 = 6.39, p = 0.01).
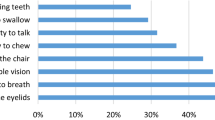
Nearly three-quarters of participants reported moderate to severe muscle weakness (n = 386, 78.8%) and fatigue (n = 369, 75.3%). More than half reported moderate to severe sleep difficulties (n = 281, 57.3%) and nearly half of the sample reported balance difficulties (n = 234, 47.8%). Nearly one-third (n = 149, 30.4%) reported moderate to severe levels of pain. Less than 20% of the sample reported difficulties with vision (n = 88, 18.0%) and/or speech (n = 65, 13.2%). Less than 5% (n = 23) of adults had cognitive difficulties (defined as 2SD below the norm mean) on the NeuroQol. Twenty-one adults (4.3%) met the criteria for moderate to severe anxiety, and seven (1.4%) for depression on the HADS. Three-quarters of the sample (76.1%) reported good or very good QoL.
In comparison to NZ norms, this sample had a higher proportion of male participants (52% vs 42%); however, there was no difference in mean age (45.6 years vs 49.7 years). Participants reported statistically significantly poorer QoL than the NZ general population on physical, social and environmental domains, although the effect sizes were small (Table 2). There were no significant differences between participants and the NZ general population in the psychological domain. Mean QoL scores and standard deviations by diagnosis are reported in supplementary table 1. Mean scores had a range of 4 points between highest and lowest scores across the different condition types suggesting little difference in QoL between genetic muscle disorders.
As shown in Table 3, a number of factors were predictive of QoL outcomes amongst adults living with genetic muscle disorders. Increased self-efficacy and lower depression were the only predictors that significantly contributed to improved QoL across all domain models. Other predictors contributed to some QoL domains but not others, e.g., age was only related to the physical domain. Factors that were significantly associated with QoL in the regression models are visually represented in Fig. 2, including personal factors (younger age, being employed, in a relationship), physical health and functioning (no need for ventilation support, lower activity limitations, fewer comorbidities), symptoms (lower levels of pain, fatigue, sleep difficulties) and psychosocial factors (mood, behavioural dyscontrol, and higher self-efficacy, satisfaction with health care and social support). Sex, educational level, diagnosis, and cognitive function were not predictive of QoL across any domains.
Discussion
Drawing upon a nationwide prevalence study, this study explored the impact of genetic muscle disorders on adults through establishing the prevalence and severity of symptoms and comparing QoL with NZ population norms. A range of personal factors, physical health and functioning, symptoms and psychosocial factors were associated with QoL in adults living with a genetic muscle disorder. Those with a genetic muscle disorder had poorer physical, environmental and social QoL than the general population, but the effect sizes were small. There were no significant differences observed between participants and norms on the psychological domain of QoL. The study highlights the need to consider both illness-related and personal/psychosocial factors within a multidisciplinary treatment approach.
As found in previous studies, muscle weakness and fatigue were the most commonly reported symptoms [2]. Additionally this study revealed that nearly half the sample reported difficulties with balance, suggesting the need for fall prevention advice and risk assessment to prevent further complications. Although pain and fatigue have been found to be associated with QoL within this population, this study revealed that these physical symptoms independently contributed to the physical domain of QoL but not to other domains. Indeed there were no differences observed in the psychological domain. This accords with previous findings in a study exploring adults with muscular dystrophy [6] using the SF-36, where differences between QoL ratings were lower on the physical component of QoL but not the psychological domain and suggests that adults living with genetic muscle conditions may be drawing on effective mechanisms or have developed personal resources (such as resilience) to reduce the impact of physical symptoms on social and psychological aspects of QoL. Further research to identify these coping mechanisms and personal resources and associations with QoL may help inform interventions to optimize QoL in others living with a genetic muscle condition experiencing psychological difficulties.
Self-efficacy was one personal resource factor explored within this study which was found to be independently predictive of QoL across all four domains. The link between self-efficacy and QoL has previously been identified in adults with muscular dystrophy [6]. It highlights the need for clinicians to involve patients in health care decision-making and plan to increase their belief in their ability to manage their condition. The progressive nature of some of these conditions can present an additional challenge. Tailored approaches to improve symptom self-management, problem solving and involvement in future care planning have been found to be effective in conditions such as cancer [25]. There is currently a paucity of high quality research exploring the effectiveness of psychosocial interventions on QoL and well-being in adults with neuromuscular disorders. Multi-site, randomized controlled trials with active controls, standardised outcome measurement, and longer-term follow-ups are required [3].
The results provide further evidence to support the links between age, pain, fatigue, symptoms, mood and sleep identified in a previous systematic review [2]. However, in contrast to previous studies that have found that females report poorer QoL than males [26], our study showed no association between sex and QoL. These contrasting findings may reflect the lower number of male participants in this study or that, in a population sample including those not actively seeking specialist care, sex differences are not apparent. Whilst this sample had a lower proportion of males than these other neuromuscular population studies, the sample did have a higher proportion of males than the NZ norms potentially affecting the findings. However, as sex was not linked to ratings of QoL in this sample the risk is low. Our findings also contrast with previous research as we did not find links between cognitive functioning and QoL [27]. These contrasting findings may reflect differences in the conditions being studied, particularly noting that our sample had relatively low levels of cognitive impairments. A strength of this study was that participants were recruited via a national prevalence study enabling larger numbers of people to be studied for conditions that have low prevalence and comparisons to be made on certain characteristics with those who did not take part to explore representativeness of the findings.
This study also highlighted newly identified factors influencing improved QoL including being in employment, being in a relationship, fewer comorbidities, higher social support and lower emotional and behavioural dyscontrol. Whilst the prevalence of emotional and behavioural dyscontrol, anxiety and depression were low in this sample, the links with QoL outcomes indicates that when low mood, high anxiety or emotional difficulties are present they are likely to affect a person’s perceived QoL. Depression, in particular, was an independent predictor on all QoL outcomes. Screening for depression may be helpful to support early identification and treatment where required to improve QoL.
One of the challenges in exploring QoL in genetic muscle disorders is the large number of neuromuscular diseases being studied [28]. Despite the wide range in symptom presentation, age of onset and prognosis between conditions, there were no notable differences in QoL between participants with different conditions. This concurs with previous research into muscular dystrophies, where no significant differences in overall QoL were found between the different muscular dystrophies [6]. However, combining such a diverse set of conditions may miss or overestimate disease-specific relationships between predictive variables and QoL. For example, the results may have affected by the large number of participants with myotonic dystrophy. Whilst there were no differences in the age, sex or ethnicity between those who completed the impact assessments and those who did not, we were not able to determine if there were differences in other variables that may affect outcome such as socioeconomic status, education and access to healthcare.
A limitation of this study is that the assessments relied on participant self-report. Participants often commented to our research team that they found it difficult to determine whether some of their difficulties or symptoms were due to their genetic muscle disorders, associated conditions or to other unrelated comorbidities when answering the questions. For the purposes of this study participants were asked to answer the questions in relation to their genetic muscle disorder, consequently the overall impact of the genetic muscle disorders on their lives may have been underestimated. Due to the exploratory nature of this study, assessments were broad and comprehensive to identify the range of impacts and influences on the lived experience of these conditions. However, it is acknowledged that as a result the assessment took a long time and that not all assessments used were fully validated for all modes of assessment delivery. Strategies to prevent any negative impact on data quality were put in place, e.g. conducting the assessment over several sessions, ordering of the measures by importance, providing a flexible mode of administration. Many participants did complete the assessments over several sessions. It is acknowledged that the flexible mode of administration to facilitate participation for those with complex needs, often excluded from research, may have affected data quality.
One advantage using the WHOQOL-BREF to assess QoL was that a normative sample was available for the NZ population to enable comparison. However, the measure does not take into account concern about the impact of their condition on others which has been found to be an important consideration for people living with neuromuscular disorders [29]. Additionally, further work is needed to validate the WHOQOL in these populations [30]. This impact should be considered in future research. It is also acknowledged that not all measures used in this study have been validated specifically for this population. The cross-sectional study design has enabled us to identify factors linked with QoL. Critically, future research could be undertaken in a longitudinal context to determine how the factors identified here and their prevalence and influence change over time and over the course of the disorder.
Data availability
In respect of permissions for data sharing from participants, anonymized study data are only available for participants who agreed for their data to be shared outside of the study team and upon reasonable request to the corresponding author, subject to agreement by the study scientific committee.
Code availability
Not applicable.
References
Cardamone, M., Darras, B. T., & Ryan, M. M. (2011). Inherited myopathies and muscular dystrophies. Seminars in Neurology, 28(2), 250–259.
Graham, C. D., Rose, M. R., Grunfeld, E. A., Kyle, S. D., & Weinman, J. (2011). A systematic review of quality of life in adults with muscle disease. Journal of Neurology, 258(9), 1581–1592.
Walklet, E., Muse, K., Meyrick, J., & Moss, T. (2016). Do Psychosocial Interventions Improve Quality of Life and Wellbeing in Adults with Neuromuscular Disorders? A Systematic Review and Narrative Synthesis. Journal of Neuromuscular Disorders, 3(3), 347–362.
Haas, B. K. (1999). A multidisciplinary concept analysis of quality of life. Western Journal of Nursing Research, 21(6), 728–742.
World Health Organisation Quality of Life [https://www.who.int/tools/whoqol]
Jacques, M. F., Stockley, R. C., Onambele-Pearson, G. L., Reeves, N. D., Stebbings, G. K., Dawson, E. A., Groves, L., & Morse, C. I. (2019). Quality of life in adults with muscular dystrophy. Health and Quality of Life Outcomes. https://doi.org/10.1186/s12955-019-1177-y
Bos, I., Wynia, K., Almansa, J., Drost, G., Kremer, B., & Kuks, J. (2019). The prevalence and severity of disease-related disabilities and their impact on quality of life in neuromuscular diseases. Disability and Rehabilitation, 41(14), 1676–1681.
Theadom, A., Rodrigues, M., Poke, G., O’Grady, G., Love, D., Hammond-Tooke, G., Parmar, P., Baker, R., Feigin, V., Jones, K. . Te., Ao, B., Ranta, A., Roxburgh, R., On behalf of the MDPrev Research Group. (2019). A nationwide, population-based prevalence study of genetic muscle disorders. Neuroepidemiology, 52(3–4), 128–135.
Norwood, F. L. M., Harling, C., Chinnery, P. F., Eagle, M., Bushby, K., & Straub, V. (2009). Prevalence of genetic muscle disease in Northern England: In-depth analysis of a muscle clinic population. Brain, 132(11), 3175–3186.
Bowling, A. (2005). Mode of questionnaire administration can have serious effects on data quality. Journal of Public Health, 27(3), 281–291.
Jones, K. M., O’Grady, G., Rodrigues, M. J., Ranta, A., Roxburgh, R. H., Love, D. R., Theadom, A., On behalf of the MDPrev Research Group. (2018). Impacts for children living with genetic muscle disorders and their parents—findings from a population-based study. Journal of Neuromuscular Disorders, 5(3), 341–352.
WHOQOLGroup. (1998). Development of the world health organisation WHOQOL-BREF quality of life assessment. Psychological Medicine, 28, 551–558.
World Health Organisation. (1996). WHOQOl-BREF Introduction, administration, scoring and generic version of the assessment. World Health Organisation.
Krägeloh, C. U., Billington, R., Hsu, P. H., Feng, X. J., Medvedev, O. N., Kersten, P., Landon, J. J., & Siegert, R. (2016). Ordinal-to-interval scale conversion tables and national items for the New Zealand version of the WHOQOL-BREF. PLoS ONE. https://doi.org/10.1371/journal.pone.0166065
Vandervelde, L., Van den Bergh, P. Y., Goemans, N., & Thonnard, J. L. (2009). Activity limitations in patients with neuromuscular disorders: A responsiveness study of the ACTIVLIM questionnaire. Neuromuscular Disorders, 19(2), 99–103.
[http://www.rehab-scales.org/activlim-rasch-analysis-neuromuscular-disorders.html]
Krupp, L. B., LaRocca, N. G., Muir-Nash, J., & Steinberg, A. D. (1989). The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology, 46(10), 1121–1123.
Measuring Quality of Life in Neurological Disorders, Final Report of the NeuroQOL Study [http://www.neuroqol.org/Resources/Resources%20documents/NeuroQOL-Final%20report-2013.pdf]
Cella, D., Lai, J. S., Nowinski, C. J., Victorson, D., Peterman, A., Miller, D., Bethoux, F., Heinemann, A., Rubin, S., Cavazos, J. E., Reder, A. T., Sufit, A. T., Simuni, T., Holmes, G. L., Siderowf, A., Wojna, V., Bode, R., McKinney, N., Podrabsky, T., … Moy, C. (2012). Brief measures of health-related quality of life for clinical research in neurology. Neurology, 78(23), 1860–1867.
Zimet, G. D., Dahlem, N. W., Zimet, S. G., & Farley, G. K. (1988). The multidimensional scale of perceived social support. Journal of Personality Assessment, 52, 30–41.
Chen, G., Gully, S. M., & Eden, D. (2001). Validation of a new general self-efficacy scale. Organizational Research Methods. https://doi.org/10.1177/109442810141004
Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370.
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213.
Hopkins, W.G. (2002). A scale of magnitudes for effect statistics. In: A New View of Statistics. www.sportsci.org/resource/stats/effectmag.html
Merluzzi, T. V., Pustejovsky, J. E., Philip, E. J., Sohl, S. J., Berendsen, M., & Salsmanc, J. M. (2019). Interventions to enhance self-efficacy in cancer patients: A meta-analysis of randomized controlled trials. Psycho-Oncology, 28(9), 1781–1790.
Dong, D., Chong, M. K., & Wu, Y. (2020). Gender differences in quality of life among patients with myasthenia gravis in China. Health and Quality of Life Outcomes. https://doi.org/10.1186/s12955-020-01549-z
Orsini, M., Carolina, A., Ferreira, A. F., de Assis, A., Magalhães, T., Teixeira, S., Bastos, V. H., Marinho, V., Oliveira, T., Fiorelli, R., Oliverira, A. B., & de Freitas, M. R. G. (2018). Cognitive impairment in neuromuscular diseases: A systematic review. Neurology international, 10(2), 7473.
Burns, T. M., Graham, C. D., Rose, M. R., & Simmons, Z. (2012). Quality of life and measures of quality of life in patients with neuromuscular disorders. Muscle and Nerve, 46, 9–25. https://doi.org/10.1002/mus.23245
Bann, C. M., Abresch, R. T., Biesecker, B., Conway, K. C., Heatwole, C., Peay, H., Scal, P., Strober, J., Uzark, K., Wolff, J., Margolis, M., Blackwell, A., Street, N., Montesanti, A., & Bolen, J. (2015). Measuring quality of life in muscular dystrophy. Neurology, 84(10), 1034–1042.
Powell, P. A., Carlton, J., Buckley-Woods, H., & Mazzone, P. (2020). Measuring quality of life in Duchenne muscular dystrophy: A systematic review of the content and structural validity of commonly used instruments. Health and Quality of Life Outcomes. https://doi.org/10.1186/s12955-020-01511-z
Acknowledgements
MDPrev Research Group Members: Alice Theadom (Chair and Principal Investigator), Kelly Jones, Braden Te Ao, Kerry Walker, Miriam Rodrigues, Richard Roxburgh, Gina O’Grady, Priya Parmar, Chris Higgins, Valery Feigin, Annemarei Ranta, Rita Krishnamurthi, Alain Vandal, Paul Brown, Donald Love, Jenny Stewart, Gemma Poke, Graeme Hammond-Tooke. We would also like to thank Varsha Parag who provided further support with statistical analysis. The material in this publication is the result of use of the NZ WHOQOL-BREF and the assistance of the AUT University and the World Health Organization is acknowledged.
Funding
This study was funded by the Health Research Council of New Zealand (HRC14/399) and the Brendel Trust.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
A Theadom, A Ranta, G Poke, M Rodrigues, D Love, K Jones, B Te Ao, G Hammond-Tooke, P Parmar, G O’Grady, R Roxburgh report no conflicts of interest.
Ethical approval
The study was approved by the Northern Y Health and Disability Ethics Committee (Ref: 14/NTB/118) and the Auckland University of Technology Ethics Committee (Ref: 14/296).
Consent to participate
All participants gave their informed written consent prior to their inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Theadom, A., Rodrigues, M., Ranta, A. et al. Impact and predictors of quality of life in adults diagnosed with a genetic muscle disorder: a nationwide population-based study. Qual Life Res 31, 1657–1666 (2022). https://doi.org/10.1007/s11136-021-03046-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-021-03046-2