Abstract
We tested the hypothesis that fatigue in MS is related to a dysfunction in cortical areas involved in movement preparation. Thirty-three patients with clinically definite MS (16 with fatigue MS-F, 17 without fatigue MS-NF) and a relapsing-remitting course, matched for disease severity and duration, disability scores and level of depression were enrolled. They underwent a combined assessment with magnetic resonance imaging (MRI) and transcranial magnetic stimulation (TMS) and, for the electrophysiological study, were compared with 12 healthy controls. MRI was used to assess regional and total lesion-load volume (LL) on T1- and T2-weighted sequences and total brain volume on T1-weighted sequences. With TMS we tested central motor conduction time, short intracortical inhibition (SICI) and facilitation (ICF), pre-movement facilitation related to a simple reaction time paradigm and the effect of short trains of 5-Hz repetitive TMS (rTMS). No significant differences were found in total and regional LL between MS-F and MS-NF, except for a significant increase in frontal lobe LL in MS-F. Neurophysiological assessment did not disclose any difference of SICI and ICF among the three groups. The significant increase of MEP size produced by 5 Hz rTMS in controls was absent in both MS-NF and MS-F. MS-F lacked pre-movement facilitation compared with MS-NF and controls. The lack of pre-movement facilitation and the increased frontal lobe lesion load were significantly correlated to the FSS score, suggesting that central fatigue in MS is probably due to a dysfunction of cortical motor areas involved in movement preparation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatigue is a common and disabling symptom of Multiple Sclerosis (MS), presenting in about 60% of patients [1] and interfering with normal activities of daily life. Several pathogenetic mechanisms have been proposed to explain it [9]. Neurophysiological studies have shown that central fatigue in MS is not related to the direct involvement of the corticospinal tract [37] or to the phenomenon of frequency dependent conduction block (FDCB) [37]. Other studies in fatigued MS patients have demonstrated a defect of intracortical inhibitory circuits [23] and a failure of the inhibitory mechanisms engaged after movement termination [22, 27]. Finally, metabolic abnormalities of frontal cortex and basal ganglia, revealed by positron emission tomography (PET) [31], have supported the hypothesis that fatigue might be correlated with a dysfunction of circuitries involved in motor planning [35]. Whereas neurophysiological and functional neuroimaging studies have linked central fatigue with abnormalities in cortical and subcortical structures engaged in motor programming and execution [16], conventional magnetic resonance imaging (MRI) studies have been inconclusive. Indeed, while some studies have reported no correlations between measures of fatigue and lesion load at MRI [38], others have shown a significantly higher T2 lesion load of the entire brain and of selected brain regions [8, 36]. None of these studies have done MRI-neurophysiological concomitant assessment in order to correlate structural with functional abnormalities. In the present paper we tested the hypothesis that central fatigue in MS might be correlated with a dysfunction in the cortical areas upstream of the pyramidal tract involved in motor planning and preparation. To investigate our hypothesis, we chose to combine neurophysiology, through transcranial magnetic stimulation (TMS), and neuroimaging, through conventional MRI, and to correlate them to subjective fatigue measures. We compared MS patients with and without fatigue with low disability scores, matched for disease severity and duration, immunomodulatory treatment, disability scores and level of depression.
Methods
Subjects
Thirty-three patients with clinically definite MS [30] (16 with fatigue, 17 without fatigue) and a relapsing-remitting (RR) course [24], with no clinical relapses for at least 6 months and with mild neurological impairment (Expanded Disability Status Scale, EDSS ≤2.5), underwent MRI and were compared with 12 healthy age-matched subjects in the neurophysiological study. Clinical features are shown in Table 1. Patients underwent electrophysiological assessment and MRI on different days at a maximum interval of 1 week. The order between MRI and neurophysiological sessions was counterbalanced among patients.
Exclusion criteria were a Mini Mental State Examination (MMSE) <24 and concomitant therapy with steroids, amantadine, antidepressants or antipsychotics. Fatigue was assessed using the Fatigue Severity Scale (FSS) [19] completed the day of the first experimental session. According to Krupp et al. [18], a FSS score >4 defined MS patients with fatigue (MS-F), whereas a FSS score <4 defined MS patients without fatigue (MS-NF). The 17-items Hamilton Depression Rating Scale (17-HDRS) [13] evaluated the presence and the degree of depression. The experiment was approved by the local Ethics Committee and all subjects gave their written informed consent for the experiments, according to the declaration of Helsinki.
Transcranial magnetic stimulation
Focal TMS was applied through a standard figure-of-eight coil with mean loop diameters of 9 cm connected to a High Power Magstim 200 stimulator (The Magstim Company, Whitland, United Kingdom). For the paired pulse study, TMS was performed with a figure-of-eight coil connected to two Magstim 200 stimulators and a Bistim module. Repetitive TMS (rTMS) was performed with a Magstim Rapid stimulator delivering magnetic stimuli with a biphasic waveform (pulse width of approximately 300 μs). The coil was held tangentially to the skull with the handle pointing backwards and laterally at an angle of 45° to the sagittal plane, thus generating a posterior-anterior current in the brain. The optimal position for activating the contralateral first dorsal interosseous muscle (FDI) and abductor pollicis brevis (APB) (marked with a pen as the “motor hot spot”) was established by moving the coil in 0.5 cm steps around the presumed primary motor hand area. Stimulus intensity was set at a stimulator output that induced motor evoked potentials (MEPs) of about 0.5 mV in the relaxed FDI/APB muscle. MEPs were recorded using a pair of Ag–AgCl surface electrodes placed over the FDI and APB muscle using a belly-tendon montage. Raw signals were amplified and filtered using a time constant of 3 ms and a high pass filter set at 3 kHz (Neurolog System, Digitimer Ltd., Welwyn Garden City, Herts, UK) and digitalized using a CED 1401 laboratory interface (Cambridge Electronic Design Ltd., Cambridge, UK). Data were collected on a personal computer (Signal 3.0, Cambridge Electronic Design Ltd., Cambridge, UK) and analysed off-line. High gain audio-visual EMG monitoring was used to ensure complete muscular relaxation. We tested several parameters of corticospinal excitability: resting and active motor threshold, short-latency intracortical inhibition and facilitation, central motor conduction time, the effect of trains of 10 stimuli of rTMS at 5 Hz (all these parameters recorded from right FDI muscle), pre-movement facilitation associated to a simple reaction time paradigm (recorded from right APB muscle).
Resting motor threshold (RMT) was defined as the minimum intensity that could evoke a peak-to-peak MEP of 50 μV in at least five out of ten consecutive trials in the relaxed muscle [32]. Active motor threshold (AMT) was defined as the lowest stimulus intensity at which an amplitude of ~200 μV was elicited in at least five out of ten consecutive trials in the contracted target muscle [34]. Central motor conduction time (CMCT) was calculated by subtracting the peripheral conduction time from spinal cord to muscles from the latency of responses evoked by cortical stimulation in all patients [12].
For short intracortical inhibition (SICI) and facilitation (ICF) [20] at rest, a conditioning subthreshold stimulus set at 90% of AMT was paired with a second suprathreshold stimulus, which was referred to as the test stimulus at an interstimulus interval (ISI) of 2 ms (SICI) and 10 ms (ICF). The intensity of the test stimulus was adjusted to elicit MEPs of about 0.5 mV peak-to-peak amplitude when given alone. The magnitude of SICI and the strength of ICF were expressed as the ratio of the conditioned MEPs evoked by paired-pulse TMS at ISI of 2 and 10 ms and the MEP test alone. Fifteen trials for each ISI and test pulse alone were performed in a pseudo-randomized order with an interval of 6 s between trials. For rTMS, stimulation intensity was set at 120% of the RMT. Four trains of 10 stimuli were delivered at 5 Hz with a 1-min interval between trains with the subject resting the FDI muscle. We measured the peak to peak amplitude of the MEP evoked after each stimulus [2].
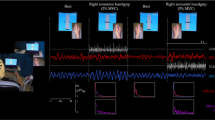
We finally assessed pre-movement facilitation associated to a simple reaction time paradigm [6]. The experimental session consisted of three parts: initially, we assessed for each subject the mean simple reaction time (RT) by recording 20 trials in which the subject briskly abducted the right thumb in response to a visual go light signal in order to produce a single EMG burst, while sitting in front of a computer monitor, with the right hand resting comfortably on a table. Subjects were asked to maintain complete relaxation between trials and all of them were able to perform properly the task after few minutes training. Then, we recorded 10 control TMS trials to assess the mean amplitude of the control MEP. Finally, we recorded a block of 30 RT trials combined with TMS delivered after the go signal and before the calculated mean onset of EMG burst at 50, 100 and 150 ms (ten trials each) (Fig. 1). The order of RT-TMS trials at different time intervals was randomly intermixed. The trials were repeated every 6 s. The amplitude of the MEP at 50, 100 and 150 ms before EMG burst was evaluated and was compared to the control MEP.
MR imaging protocol
MR scans were performed with a 1.5-T field strength scanner (Magnetom Impact; Siemens Medical Systems, Erlangen, Germany). Patients were scanned in the same scanner and the same acquisition sequences were used throughout the duration of the study. The scanning procedure included a pre-gadolinium sagittal T1 3D volumetric acquisition scan (TR = 22 ms, TE = 10 ms, 1.3-mm slice thickness 192 × 192 matrix; no gap interslice); pre- and post-gadolinium axial T1-weighted scans (TR = 30 ms, TE = 11 ms, 3-mm slice thickness, and 256 × 256 matrix; no gap interslice); turbo spin echo (TSE) T2-weighted axial scan (TR = 5420 ms, TE = 83 ms, 3-mm slice thickness, and 256 × 256 matrix; no gap interslice); proton density (PD) weighted axial scan (TR = 2590 ms, TE = 12 ms, 3-mm slice thickness, and 256 × 256 matrix; no gap interslice). Gadolinium dose was 0.1 mmol/kg, administered i.v. over 1 min, followed by a post-injection delay of 5 min before scanning.
Images were saved as DICOM-format and evaluated in random order by one investigator (V.D.) expert in neuroradiology, blinded to the clinical and neurophysiological findings. The lesion-load volume (LL) on T1- and T2-weighted sequences and the brain volume (total, grey matter, white matter) on T1-weighted sequences was determined. Brain lesions were defined as sharply demarcated areas of high signal intensity on both intermediate- and T2-weighted images and as sharply demarcated areas of low signal intensity (less then grey matter) on T1-weighted images. The LL measurement was performed using a semi-automated technique (dedicated software: Jim version 3.0) and the brain volume was measured by SIENAX software (FMRIB Software Library). Focal lesions were divided into six types on the basis of location: (a) juxtacortical (JC), lesion touching or extending into the grey matter of the cortex; (b) periventricular (PV), lesion adjacent to ventricles or less than 1 cm from them as measured from the center of the lesion; (c) deep grey matter, basal ganglia lesions (DGM); (d) infratentorial, cerebellum and brainstem lesions (IT); (e) deep white matter lesions (DWM), lesions that did not fulfill one of the previous criteria; (f) Frontal lobes lesions. Frontal lobe borders were defined according to the segmentation method described by Bodke and coworkers [4] which has been shown to have high intra- and inter-rater reliability. Patients with brain enhanced abnormalities were excluded.
Statistical analysis
For MRI data, each measure of lesion load and brain volume was compared between the two groups (F-MS, NF-MS) using the unpaired t tests.
RMT, AMT, CMCT and RT were compared across the three groups (Controls, F-MS, NF-MS) using factorial ANOVA with “Group” as a dependent variable, followed by post-hoc Bonferroni/Dunn tests (p level set at <0.016 because of multiple comparisons). For SICI and ICF, we computed separate repeated measures ANOVA with interstimulus interval (“ISI”) as within-subjects factor and “Group” (controls, F-MS, NF-MS) as between-subjects factor. The effect of 5 Hz rTMS trains on MEP amplitude was tested by repeated measure ANOVA with “Stimulus” (ten levels: stim 1-stim 10) as within-subjects factor and “Group” (three levels: controls, F-MS, NF-MS) as between-subjects factor. For pre-movement facilitation associated to a reaction time paradigm, we performed a repeated-measures ANOVA with “Time” (control TMS, 50, 100, 150 ms) as within factor and Group as between factor. For 5 Hz rTMS and pre-movement facilitation parameters, we computed separate one-way repeated-measure ANOVA for each group, respectively with “Stimulus” and “Time” as within-subject factors, to explore within-group effects. The Greenhouse-Geisser method was used where necessary to correct for non-sphericity. Conditional on a significant F value, post-hoc multiple paired t test with Bonferroni’s correction were performed. For all other statistical tests, significance level was p < 0.05.
Regression analysis was used to correlate CMCT and RT in the MS group. Correlations in the patients group among clinical, MRI and electrophysiological data were tested using Spearman rank-correlation. Data are given as mean ± standard error of the mean (SEM).
Results
Clinical and demographic features
No significant differences were found between MS-F and MS-NF regarding the following variables: age (p = 0 0.14), EDSS (p = 0.39), HDRS-17 (p = 0.98), disease’s duration (p = 0.95). The two groups of patients differed in degree of fatigue as tested with FSS (p < 0.001).
Five patients in the MS-NF and 6 patients in the MS-F were depressed according to 17-HDRS [40], having a score >7. All patients, except one, were receiving immunomodulatory (interferon-beta-1, glatiramer acetate) or immunosuppressive therapy (azathioprine).
MRI findings
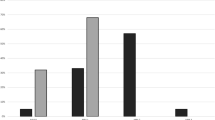
MRI data were available from 33 patients. Total T1 (t = 1.01; p = 0.29) and T2 lesion load was comparable between the two groups (t = 0.74; p = 0.46). Two patients (pt 11 and pt 14) were excluded from the analysis due to a T2 lesion load which exceeded the mean + 2 SD. No difference between MS-F and MS-NF were found in the LL on T1 and T2 sequences for JC, PV, DGM, IT and DWM lesions (Table 2). MS-NF and MS-F showed also similar grey matter (GM), white matter (WM) and total brain volume (Table 3). Frontal GM volume was comparable between MS-NF and MS-F. Frontal lobe T1-LL showed a trend to be higher in MS-F (576.2 ± 212.9) compared to MS-NF (185.9 ± 57.9) (t = 1.89; p = 0.07). Frontal lobe T2-LL was significantly increased in MS patients with fatigue compared to those without fatigue (t = 2.54; p = 0.017) (Fig. 2).
a Increased frontal lesion load in MS patients with central fatigue (MS-F) compared to patients without fatigue (MS-NF) (*p = 0.017). b Representative MRI from a MS patient with fatigue (right) and without fatigue (left). The increased frontal LL is evident in the MS-F patient. Error bars represent standard errors
Electrophysiological measures
All the electrophysiological measures were assessed in 10 patients with MS-NF, 11 patients with MS-F and 12 healthy controls. No differences were found regarding RMT and AMT between controls, MS-F and MS-NF.
CMCT was significantly prolonged (main effect of “Group” at factorial ANOVA: F = 7.2; p = 0.003), both in MS-F (7.3 ± 1. ms; p = 0.006) and MS-NF (7.7 ± 1.5 ms; p = 0.001) compared to controls (5.6 ± 0.9 ms). CMCT did not significantly differed between MS-F and MS-NF (p = 0.5).
The amount of SICI and ICF was comparable between the three groups (MS-NF = 0.47 ± 0.07; MS-F = 0.54 ± 0.1; controls = 0.54 ± 0.06). For SICI, repeated measures ANOVA disclosed a main effect of “ISI” (F = 35.7, p < 0.0001) but it was neither an effect of “Group” (F = 0.7, p = 0.4) nor a “Group” × “ISI” interaction (F = 0.06, p = 0.9). A main effect of “ISI” (F = 23.6, p < 0.0001), but neither an effect of “Group” (F = 0.5, p = 0.6) nor a “Group” × “ISI” interaction (F = 0.2, p = 0.8), was also found for ICF (MS-NF = 1.23 ± 0.1; MS-F = 1.32 ± 0.1; controls = 1.40 ± 0.1).
5 Hz rTMS increased MEP amplitude over the course of the train of stimuli in control subjects, whereas no change in MEP amplitude was achieved in both groups of patients (Fig. 3). This was shown by a significant “Stimulus” × “Group” interaction (F = 2.9, p = 0.001) and a main effect of “Group” (F = 3.4, p = 0.03). In a separate repeated measure ANOVA, a main effect of the factor “Stimulus” was present in healthy subjects (F = 3.7, p = 0.0006) but not in MS-NF (F = 0.9, p = 0.5) and MS-F (F = 1.6, p = 0.1).
For simple RT at baseline, there was a significant main effect of “Group” (F = 10.9, p = 0.003) since RT was increased in patients with MS-F (225.1 ± 12.5 ms) (p = 0.001) and MS-NF (211.6 ± 4.8 ms) (p = 0.002) compared with healthy controls (170.2 ± 5.2 ms). There was no difference in RT onset between MS-F and MS-NF (p = 0.3). Regression analysis showed no correlation between the increase in CMCT and RT onset in the group of MS patients (F = 2.1; p = 0.1).
For pre-movement facilitation associated to the reaction time paradigm, repeated measure ANOVA disclosed a significant effect of the factors “Time” (F = 17.1, p = 0.0001) and “Group” (F = 8.6, p = 0.001) and “Time” דGroup” interaction (F = 3.6, p = 0.003; Fig. 4a). A separate one-way repeated measure ANOVA computed in each group revealed a main effect of the factor “Time” in healthy subjects (F = 7.4, p = 0.0009) and MS-NF (F = 9.9, p = 0.0001) but not in MS-F (F = 1.8, p = 0. 1). This was due to a lack of pre-movement facilitation in MS-F. On the other hand, in healthy subjects and MS-NF there was an increase of MEP amplitude when TMS was delivered at 50 ms (controls: p = 0.0002; MS-NF: p < 0.0001), 100 ms (controls: p = 0.001; MS-NF: p = 0.001), 150 ms (controls: p = 0.01; MS-NF: p = 0.005) before the calculated EMG onset (p level set at <0.016 because of multiple comparisons). Representative trials of pre-movement facilitation from a control subject, an MS-F and an MS-NF patient are shown in Fig. 4b.
Correlations between fatigue severity score and MRI and TMS variables
Frontal lesion load correlated positively with FSS score (Rho = 0.58; p = 0.002) (Fig. 5a).
Correlation analysis between FSS, TMS (pre-movement facilitation) and MRI (frontal lesion load) variables. a FSS score is inversely correlated to the ratio of pre-movement facilitation at 50 ms (Rho corrected for ties = −0.61; tied p value = 0.0056). b Frontal lesion load correlates positively with FSS score (Rho = 0.58; p = 0.002)
FSS inversely correlated with the ratio of pre-movement facilitation at 50 ms (Rho corrected for ties = −0.61; tied p value = 0.0056) (Fig. 5b), 100 ms (Rho corrected for ties = −0.53; tied p value = 0.01) and 150 ms (Rho corrected for ties = −0.59; tied p value = 0.008) (Fig. 5b).
Analysis of MS patients with borderline fatigue
In order to minimize the confounding effect of MS patients with borderline fatigue having FSS scores between 4.1 and 4.9, we performed a supplemental analysis of the significant TMS and MRI measures by splitting the MS sample into three groups: MS-NF (n = 17, FSS <4), borderline fatigue (n = 8; FSS >4 and <5), MS-F (n = 8, FSS >5). This supplementary analysis was performed only in the MS group.
As for MRI variables, borderline fatigue MS (p = 0.03) and MS-F (p = 0.01) have increased T2 frontal LL compared to MS-NF. Pre-movement facilitation was lacking both in the borderline fatigue and in the MS-F group compared to the MS-NF (p = 0.02, “Time” × “Group” interaction, repeated measures ANOVA). Indeed, only in the MS-NF group was there an increase of MEP amplitude when TMS was delivered at 50 ms (p = 0.005), 100 ms (p = 0.008), 150 ms (p = 0.002) before the calculated EMG onset.
Discussion
We have demonstrated that frontal lobe impairment is associated with fatigue in MS, as suggested by a significant increase of the burden of lesion load in frontal areas correlating with the degree of fatigue itself, expressed by the FSS score. This was paralleled by a functional impairment of the motor areas involved in movement planning and preparation in fatigued patients, revealed by a lack of pre-movement facilitation in a simple reaction time paradigm. Again, the lack of pre-movement facilitation was greater with higher degrees of fatigue. In addition, neither the corticospinal tract nor the inhibitory and excitatory neurons modulating its activity seemed to be involved in the pathogenesis of central fatigue in MS.
Electrophysiological evidence for frontal lobe impairment in fatigued MS patients
MS patients with fatigue lack pre-voluntary movement increase in corticospinal excitability in a visual reaction time setting. This parameter reflects motor planning and movement preparation [6]. Whereas in normal subjects corticospinal excitability increases progressively at 150 ms before the onset of simple RT movement to reach the highest level at 50 ms [6, 21, 33], in MS-F patients MEP amplitudes remain unmodified at all the three time intervals tested. On the other side, MS-NF patients show facilitation of MEP responses, even though to a lesser extent than control subjects. The relationship between pre-movement facilitation and fatigue is also supported by the inverse correlation between these two variables: overall, the amount of fatigue, expressed by the FSS score, was inversely correlated to the magnitude of pre-movement facilitation. These data support the hypothesis that one of the factors involved in central fatigue might be an impairment of the motor areas engaged in motor programming [37]. On the other hand, earlier reports have also suggested that fatigue in MS might be correlated to impaired conduction in the demyelinated corticospinal tract, generating a FDCB [25, 39]. According to our results, FDCB in the corticospinal tract is unlikely to generate fatigue in MS for two reasons. First, CMCT was slightly increased both in MS-F and MS-NF without significant differences between the two groups. In addition, to probe the corticospinal tract to a repetitive stimulation, we studied cortical excitability during rTMS of the primary motor area, using a frequency of 5 Hz which is known to produce an increase of MEP amplitude [2, 26]. Whereas in normal subjects trains of stimuli at 5 Hz consistently produced facilitation, both groups of MS patients, regardless of fatigue, showed no increase of MEP amplitudes during the stimulation protocol, thus excluding the involvement of the FDCB as a pathogenetic factor for fatigue, as already demonstrated by Sheean et al. [37]. Furthermore, the lack of pre-movement facilitation does not seem to depend on alterations of cortical excitability, since inhibitory and facilitatory circuits modulating pyramidal tract output were normal in both groups of our MS sample, as shown by comparable values of SICI and ICF. This is in contrast with Leipert’s study, which demonstrated that SICI was reduced in MS-F [23], probably explained by the higher EDSS score (MS-F = 3.1 ± 0.3) compared to our sample of patients with milder neurological impairment (MS-F = 1.8 ± 0.1).
MRI evidence of frontal lobe involvement in MS-F
A novel finding of our study is that T2 lesion load in the frontal lobe was increased in patients with MS-F compared to MS-NF and correlated with the FSS score. Also, T1 lesion load in the frontal lobe showed a trend to be increased in MS-F. Previous MRI studies comparing MS-F and MS-NF patients failed to find correlations between regional lesion load and fatigue [1, 38]. Discrepancies in the results might arise by different patients’ selection criteria. Earlier studies have enrolled patients with a wide range of disability (EDSS: 0–5 in van der Werf’s study [38] versus EDSS ≤2.5 in our study) and with different subtypes of the disease, thus including both relapsing-remitting MS and secondary progressive MS [1]. Our cohort of patients was, indeed, quite homogeneous, the two groups being matched for EDSS, disease duration and total lesion load. Finally, they were also matched for degree of depression by the 17-HDRS score, which often occurs in MS-F [1]. Another study [8] found that non-disabled MS fatigued patients (EDSS ≤1.5) had higher parietal lobe, internal capsule and periventricular trigone lesion loads compared to those without fatigue. However, it should be stressed that in this study a lower FSS score was used as cut-off to define fatigued MS patients, whereas we chose a mean FSS of ≥4, based on previous work on MS patients with fatigue [18]. Similar to our findings, Sepulcre et al. [36], using a voxel-by-voxel lesion probability mapping approach and a cohort of MS patients comparable to ours, recently demonstrated a correlation between fatigue and lesions in the right parieto-temporal and left frontal white matter regions. Moreover, in this study gray matter atrophy in frontal regions also correlated with fatigue score.
Could central fatigue be associated to an impairment of motor areas involved in motor preparation?
Central fatigue in MS recognizes a multifactorial origin, being variously associated to immunomodulatory drugs, depression, and sleep disorders. Impairment of volitional drive to the descending motor pathways has been proposed as a mechanism determining central fatigue by a few functional neuroimaging and electrophysiological studies [22, 31]. Our combined approach using neurophysiology and neuroimaging allowed us to evaluate, in the same patient, both functional and structural abnormalities in cortical areas involved in motor programming and execution.
The increased lesion load in the frontal lobes at MRI associated to the electrophysiological evidence of impaired pre-movement facilitation and their correlations with FSS, support the hypothesis that central fatigue might arise from a disruption of frontal motor areas involved in motor programming and preparation. In accordance with our findings, Roelcke et al. [31] using PET-FDG disclosed hypometabolism bilaterally in the prefrontal cortex, in the premotor and in the right supplementary cortex as well in the basal ganglia in a group of MS patients with fatigue. Moreover, MRI perfusion studies [17] of the deep gray matter (GM) in patients with RR and primary progressive MS demonstrated decreased blood flow in the thalamus, putamen and caudate nuclei compared to controls correlating with the fatigue score. In our study, frontal GM volume was comparable between MS-F and MS-NF, suggesting that fatigue might be related to a functional disconnection of frontal areas from basal ganglia, probably induced by an increased lesion load in those areas, rather than to a structural damage of frontal GM structures. The relative sparing of GM in the pathophysiology of fatigue is also supported by a quantitative study using magnetization transfer and diffusion tensor MRI [7], which demonstrated no differences in GM pathology between MS patients with and without fatigue. The concept that central fatigue is related to a disruption of cortical-subcortical connections involved in motor planning and execution is sustained by a study which assessed changes in EEG related to voluntary movement [22]. In normal subjects, preparation and execution of voluntary movement was associated to a reduction of EEG rhythms in the 10–20 Hz frequency bands in sensori-motor cortex defined as event-related desynchronisation (ERD), whereas after movement execution there was increased expression of the same rhythms, defined as event related synchronization (ERS), which is a correlate of cortical deactivation [28, 29]. Leocani and coworkers [22] demonstrated that ERD correlating with voluntary movement was more widespread in the fatigued MS patients, especially in the midline frontal electrode corresponding to the supplementary motor area (SMA) location [14].
Several human studies performed with PET suggests that the SMA, the premotor cortex (PMC), the primary motor cortex, the parietal cortex and the basal ganglia are the structures involved during movement planning, in a reaction time setting [10, 11, 15]. Therefore, it can be postulated that demyelination and/or axonal loss within the cortico-subcortical dorsolateral prefrontal-premotor circuits might be responsible for the lack of pre-movement facilitation observed in fatigued MS patients. It is likely that these alterations of motor planning may account for the subjective lack of physical and/or mental energy that is perceived by fatigued MS patients even at rest. It is worthy to note that pre-movement facilitation is also reduced in Parkinson’s disease patients [5], where the alteration of motor planning is a core feature of the disease [3].
Limitations of the study
We acknowledge our study presents a few limitations. First, executive functions were not tested in our sample of MS patients. Though cognitively impaired subjects were excluded with the MMSE score, we are not aware whether MS-F had an impairment of executive functions which might parallel the MRI and TMS findings, thus supporting our hypothesis of frontal lobe dysfunction in the pathogenesis of fatigue.
Second, although SICI and ICF were normal in MS patients, we cannot rule out the involvement of inhibitory intracortical circuits since we did not assess ISIs other than 2 and 10 ms, nor did we test long intracortical inhibition (elicited at ISI of 50–200 ms), which might be related to lack of pre-movement facilitation. Third, we evaluated pre-movement facilitation for only one type of movement (index abduction) which was triggered by an external cue. Future studies are needed to ascertain whether an impairment in movement preparation in MS patients with fatigue will be found also for more complex motor tasks. Finally, sample size was small and our data need to be replicated on a larger group of MS patients using very strict selection criteria in order to rule out potentially confounding factors, such as co-morbid depression and progression of disease.
In conclusion, our results suggest that fatigue in MS is related to a functional impairment of the motor system at the cortical level, upstream from the corticospinal tracts, evident even during the performance of a simple reaction time task. Diffusion tensor tractography could be used in the future to better define the structural involvement of the cortico-subcortical dorsolateral prefrontal-premotor loop in MS patients with fatigue.
References
Bakshi R, Shaikh ZA, Miletich RS, Czarnecki D, Dmochowski J, Henschel K, Janardhan V, Dubey N, Kinkel PR (2000) Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult. Scler 6:181–185
Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, Modugno N, Manfredi M (1998) Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res 122:79–84
Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124:2131–2146
Bokde AL, Teipel SJ, Schwarz R, Leinsinger G, Buerger K, Moeller T, Moller HJ, Hampel H (2005) Reliable manual segmentation of the frontal, parietal, temporal, and occipital lobes on magnetic resonance images of healthy subjects. Brain Res Brain Res Protoc 14:135–145
Chen R, Kumar S, Garg RR, Lang AE (2001) Impairment of motor cortex activation and deactivation in Parkinson’s disease. Clin Neurophysiol 112:600–607
Chen R, Yaseen Z, Cohen LG, Hallett M (1998) Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol 44:317–325
Codella M, Rocca MA, Colombo B, Martinelli-Boneschi F, Comi G, Filippi M (2002) Cerebral grey matter pathology and fatigue in patients with multiple sclerosis: a preliminary study. J Neurol Sci 194:71–74
Colombo B, Martinelli BF, Rossi P, Rovaris M, Maderna L, Filippi M, Comi G (2000) MRI and motor evoked potential findings in nondisabled multiple sclerosis patients with and without symptoms of fatigue. J Neurol 247:506–509
Comi G, Leocani L, Rossi P, Colombo B (2001) Physiopathology and treatment of fatigue in multiple sclerosis. J Neurol 248:174–179
Deiber MP, Ibanez V, Sadato N, Hallett M (1996) Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol 75:233–247
Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M (1997) Frontal and parietal networks for conditional motor learning: a positron emission tomography study. J Neurophysiol 78:977–991
Di Lazzaro V, Oliviero A, Profice P, Ferrara L, Saturno E, Pilato F, Tonali P (1999) The diagnostic value of motor evoked potentials. Clin Neurophysiol 110:1297–1307
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Homan RW, Herman J, Purdy P (1987) Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiol 66:376–382
Horwitz B, Deiber MP, Ibanez V, Sadato N, Hallett M (2000) Correlations between reaction time and cerebral blood flow during motor preparation. Neuroimage 12:434–441
Inglese M, Liu S, Babb JS, Mannon LJ, Grossman RI, Gonen O (2004) Three-dimensional proton spectroscopy of deep gray matter nuclei in relapsing-remitting MS. Neurology 63:170–172
Inglese M, Park SJ, Johnson G, Babb JS, Miles L, Jaggi H, Herbert J, Grossman RI (2007) Deep gray matter perfusion in multiple sclerosis: dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch Neurol 64:196–202
Krupp LB, Coyle PK, Doscher C, Miller A, Cross AH, Jandorf L, Halper J, Johnson B, Morgante L, Grimson R (1995) Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology 45:1956–1961
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale, Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46:1121–1123
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M (2000) Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123 (Pt 6):1161–1173
Leocani L, Colombo B, Magnani G, Martinelli-Boneschi F, Cursi M, Rossi P, Martinelli V, Comi G (2001) Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement–EEG evidence. Neuroimage 13:1186–1192
Liepert J, Mingers D, Heesen C, Baumer T, Weiller C (2005) Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult Scler 11:316–321
Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis results of an international survey, National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 46:907–911
McDonald WI, Sears TA (1970) The effects of experimental demyelination on conduction in the central nervous system. Brain 93:583–598
Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117 (Pt 4):847–858
Perretti A, Balbi P, Orefice G, Trojano L, Marcantonio L, Brescia-Morra V, Ascione S, Manganelli F, Conte G, Santoro L (2004) Post-exercise facilitation and depression of motor evoked potentials to transcranial magnetic stimulation: a study in multiple sclerosis. Clin Neurophysiol 115:2128–2133
Pfurtscheller G (1992) Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol 83:62–69
Pfurtscheller G, Berghold A (1989) Patterns of cortical activation during planning of voluntary movement. Electroencephalogr. Clin. Neurophysiol 72:250–258
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13:227–231
Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W, Plohmann A, Dellas S, Maguire RP, Missimer J, Radu EW, Steck A, Leenders KL (1997) Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 48:1566–1571
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al (1994) Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee, Electroencephalogr Clin Neurophysiol 91:79–92
Rossini PM, Zarola F, Stalberg E, Caramia M (1988) Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res 458:20–30
Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W (1999) Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52:97–103
Sandroni P, Walker C, Starr A (1992) ‘Fatigue’ in patients with multiple sclerosis. Motor pathway conduction and event-related potentials. Arch Neurol 49:517–524
Sepulcre J, Masdeu J, Goni J, Arrondo G, Velez de Mendizabal N, Bejarano B, Villoslada P (2009) Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler 15:337–344
Sheean GL, Murray NM, Rothwell JC, Miller DH, Thompson AJ (1997). An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain 120 (Pt 2):299–315
van der Werf SP, Jongen PJ, Nijeholt GJ, Barkhof F, Hommes OR, Bleijenberg G (1998) Fatigue in multiple sclerosis: interrelations between fatigue complaints, cerebral MRI abnormalities and neurological disability. J Neurol Sci 160:164–170
Waxman SG (1981) Clinicopathological correlations in multiple sclerosis and related diseases. Adv Neurol 31:169–182
Zimmerman M, Chelminski I, Posternak M (2004) A review of studies of the Hamilton depression rating scale in healthy controls: implications for the definition of remission in treatment studies of depression. J Nerv Ment Dis 192:595–601
Acknowledgments
This study was supported by FISM (Fondazione Italiana Sclerosi Multipla): grant 2004/R/10 awarded to Prof. Paolo Girlanda.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morgante, F., Dattola, V., Crupi, D. et al. Is central fatigue in multiple sclerosis a disorder of movement preparation?. J Neurol 258, 263–272 (2011). https://doi.org/10.1007/s00415-010-5742-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-010-5742-x