Abstract
Thinning of the retinal nerve fiber layer (RNFL) of clinically unaffected eyes is seen in patients with multiple sclerosis (MS). It is uncertain when this thinning occurs, and whether ongoing RNFL loss can be measured over time with optical coherence tomography (OCT). Using time-domain OCT, we studied 34 patients with progressive MS (16 primary progressive MS, 18 secondary progressive; 14 male; 20 female; mean age at study entry 51 years; median EDSS 6; mean disease duration at study entry 12 years) on two occasions with a median interval of 575 (range 411–895) days apart. Eighteen healthy controls (10 male; eight female; mean age at study entry 46 years) were also studied twice, with a median interval of 656 days (range 398–890). Compared to controls, the patients had significant decreases in the RNFL thickness and macular volume of their clinically unaffected eyes at study entry. No significant decrease in RNFL thickness was observed between baseline and follow-up in either patients or controls. Macular volume declined significantly in patients and controls, but there was no difference in this change between the two groups. The study findings suggest that time domain OCT detects little disease-related ongoing loss of retinal axons in progressive forms of MS and has limited use for monitoring potential neuroprotective therapies at this stage of disease. Further studies are needed using higher-resolution OCT systems and in larger groups of patients, to elucidate the timing and mechanism of RNFL loss that is observed in clinically unaffected nerves in MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neuroaxonal loss is thought to be the basis for persistent disability in multiple sclerosis (MS). Axonal loss can be inferred in vivo by measuring brain, spinal cord, or optic nerves atrophy with MRI [12, 16, 23]; however, this may be subject to confounding by myelin loss and other tissue changes such as inflammation or gliosis.
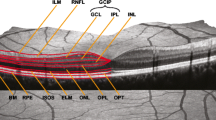
The retinal nerve fiber layer (RNFL) is formed largely of the axons of retinal ganglion cells. These are unmyelinated, and thus measurement of the thickness of this layer is not subject to possible confounding by myelin loss. Investigation of the RNFL thickness in vivo thus provides a means for detecting axonal damage in the anterior visual pathway. RNFL axonal loss has been recognized in MS since Frisén and Hoyt observed slit-like defects in the RNFL using fundoscopy [11] and the development of optical coherence tomography [17] (OCT) as an imaging tool has allowed quantification of these RNFL changes. An early OCT study demonstrated thinning of the RNFL following unilateral optic neuritis [26] compared to the fellow eye. This finding has been confirmed with newer-generation time domain OCT devices [4, 34], and a single event of optic neuritis typically results in a mean loss of about 18–22% of RNFL thickness compared to the fellow eye [5, 21].
A number of studies have also reported thinning of the RNFL in clinically unaffected eyes (i.e., eyes in which there has not been a previous clinical episode of optic neuritis) of people with MS when compared with healthy controls [10, 15, 26, 28]. The reductions are less marked than those seen following optic neuritis, but nonetheless are statistically significant. The degree of RNFL loss following optic neuritis and in MS has been consistently correlated with quantitative measures of visual dysfunction [3, 15, 28, 34], and in some studies [13, 33] but not others [15] it has also been correlated with global measures of disability in MS.
The time course of thinning of the RNFL that occurs following acute optic neuritis has been partly characterized [4, 5]—most of the loss being evident after 3–6 months. However, it is not known when and how the loss of RNFL thickness that is observed in clinically unaffected nerves in MS occurs. Two possible explanations—which are not mutually exclusive—is that there is a relatively sudden (i.e., over months) stepwise loss of the RNFL associated with sub-clinical attacks of inflammatory demyelination in the optic nerve or that there is a gradual and progressive loss of nerve fibers in the anterior visual pathway. The former mechanism would be expected to be most evident in the earlier relapsing and remitting stage of MS and the latter in progressive stages of the disease either primary or secondary.
Longitudinal studies are needed to investigate further the temporal evolution of RNFL loss in clinically unaffected eyes of people with MS. To date, only one longitudinal study has been published reporting both MS patient and comparative healthy control data [30], which demonstrated—in a cohort with predominantly relapsing remitting MS or clinically isolated syndromes—a loss of RNFL thickness over time greater than that seen in controls, although it was not stated if this was statistically significant.
We previously reported a cohort of primary and secondary progressive MS patients [15] in whose clinically unaffected eyes there was significant loss of RNFL thickness that was correlated with measures of visual dysfunction. We now report longitudinal OCT findings in a subset of subjects from the same cohort in whom follow-up was possible at a second time point after a median interval of approximately one and a half years. The aim of this follow-up study was to investigate whether increasing loss of the RNFL could be detected in a progressive MS cohort in the absence of clinical episodes of optic neuritis.
Methods
Patients and controls who had participated in a cross-sectional study with OCT analysis in multiple sclerosis were asked to return for review, including one patient who had been excluded from the earlier analysis because of prior bilateral acute optic neuritis. The original cohorts consisted of 50 subjects with progressive MS and 20 controls: of these, 34 with MS and 18 controls returned for follow-up. Sixteen patients (ten male and six female) had primary progressive and 18 patients (four male and 14 female) secondary progressive MS. Of the original cohort, one control moved overseas and one was lost to follow-up; seven patients with secondary progressive MS entered a clinical trial of a potential neuroprotective treatment and two developed disability that precluded returning for the study investigations; one patient with primary progressive MS had moved overseas, and six were lost to follow-up. All patients met the revised McDonald criteria for MS [27] and their disease course was classified according to the criteria of Lublin and Reingold [24]. No subject was receiving disease-modifying therapy.
Of the patients with secondary progressive MS, eight had had unilateral optic neuritis prior to study entry, and one had had separate episodes of optic neuritis in both eyes at different times prior to study entry. No patient or control had any other known ophthalmological disease.
Clinical assessment
A history was taken from patients and controls to determine the presence of ophthalmological and neurological disorders. The patients had their level of disability assessed using the Extended Disability Status Score (EDSS) [22].
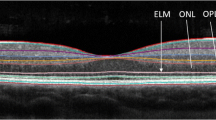
Retinal imaging
OCT images were acquired with a time domain OCT (Stratus OCT Model 3000; Carl Zeiss Meditec, Dublin, CA, USA). All OCT imaging was performed by a single observer (AH). RNFL images were acquired by taking three circular 3.4-mm scans, centered on the optic disc, the mean of which was used to express RNFL thickness (Fast RNFL thickness protocol). The thicknesses of the quadrants of the RNFL were automatically calculated by the OCT device software. Macular thickness maps were acquired by making six radial scans centered on the fovea, and by construction of a map from these scans (Fast macular thickness map scanning protocol). Both OCT protocols were performed twice at both the original and follow-up visit, and the mean value at each visit was used for the purpose of statistical analysis. One patient was not able to fix with one eye, due a large centrocecal scotoma, and measures from this eye were excluded from analysis as accurate RNFL thickness measurements and macular volume maps could not be obtained. OCT images are given a signal strength by the Stratus OCT device, with a maximum of 10. OCT images were rejected if an individual eye was <7, the inter-eye signal strength difference was >2, or if the difference in signal strength between baseline and follow-up scans was >2.
Visual testing
Visual acuity was measured in all subjects with a retro-illuminated Early Treatment Diabetic Retinopathy Study (ETDRS) chart, and was recorded as the 4-m logarithm of the minimum angle of resolution (logMAR acuity). Sloan 25, 5, and 1.25% contrast ETDRS charts were used at 4 m to calculate the low-contrast visual acuity. If subjects were unable to perceive any letters at 4 m, they were retested at 1 m, and a correction made for the change in distance as proposed by Ferris et al. [9]. Subjects used their usual distance refractive correction when tested.
Statistical analysis
To calculate annualized rates of change, the difference between the first and second sets of measures was divided by the number of days that elapsed between the measures, and then this number was multiplied by 365.25. Within-group changes and between-group comparisons of change were estimated from a single regression model with annualized change as response variable and subject group indicators. When entered into the model, age and sex did not influence the results, and the coefficients shown do not include these variables in their calculation. Relationships between EDSS and retinal measures were investigated using Spearman rank correlation. Changes in EDSS were analyzed for significance with the Wilcoxon sign-rank test, and differences in the change in EDSS by the Wilcoxon rank sum test.
All statistical tests were performed using Stata 9 software (StataCorp; College Station, TX), and p < 0.05 was considered the level of significance.
Results
The demographic details of the patients are summarized in Table 1. When the followed-up MS cohort (n = 34) was compared to the larger group that had been studied at baseline (n = 50), there were no significant differences in age, gender, disease duration, or EDSS; thus the followed-up cohort was thought to be representative of the original study population. None of the patients had an MS relapse or episode of optic neuritis between the first and second study.
OCT measures from clinically unaffected eyes: baseline values
As with the original cohort [15], the follow-up MS group, when compared to the controls, had significantly reduced RNFL thickness and macular volume at baseline, which in the SPMS group was evident for the whole and temporal quadrant RNFL and macular volume, and in the PPMS group for the temporal quadrant RNFL (Tables 2, 3).
OCT measures in clinically unaffected eyes: change compared with baseline values
Whole RNFL None of the subject groups had a significant decrease in mean RNFL thickness when compared to baseline, although there was a non-significant trend in primary progressive MS patients (annual change in mean RNFL thickness −0.99 μm, p = 0.075, 95% CI −2.09, 0.10) (Table 4).
Quadrants There was a significant reduction in inferior quadrant RNFL thickness in primary progressive MS patients (annual change −2.99 μm, p = 0.008, 95% CI −5.15, −0.83). Aside from this, none of the individual quadrants of the RNFL had a significant change in any subject group (see Table 2).
Both controls and progressive MS groups had a significant and similar reduction in macular volume (annual change for controls −0.038 mm3, p = 0.013, 95% CI −0.067, −0.008; progressive MS −0.031 mm3, p = 0.005, CI −0.052, −0.009). The macular volume decrease was also significant in the primary progressive subgroup (p = 0.03) with a borderline significant trend in secondary progressive MS (p = 0.059).
Longitudinal OCT measures from clinically unaffected eyes: comparisons of groups
There were no significant differences between the annual change in any of the OCT measures (whole and quadrant RNFL thickness and macular volumes) in the progressive MS versus control group and in either MS clinical subgroup versus controls or each other (Table 5).
OCT measures from eyes with a prior history of optic neuritis
At baseline, eyes that had previously been affected by an episode of optic neuritis had significantly lower RNFL thickness (mean 76.3 μm SD 15.5 vs. 86.1 μm SD 9.4; p = 0.012 by paired t test) and macular volume (mean 6.27 mm3 SD 0.40 vs. 6.48 mm3 SD 0.41; p = 0.005 by paired t test) compared with clinically unaffected fellow eyes. The follow-up RNFL thickness and macular volume of the affected eyes was not significantly decreased compared with the baseline measure (mean change in RNFL in eyes previously affected by optic neuritis −0.75 μm, p = 0.926 95% CI −17.9, 16.4; mean change in macular volume 0.122 mm3, p = 0.577, 95% CI −0.340, 0.586; both p values by paired t test).
When serial changes in OCT measures were compared in the eyes with a history of optic neuritis versus the fellow eye from the same patient, there were no significant differences between the changes in RNFL thickness (ON affected eye–fellow eye difference 0.05 μm, p = 0.975 95% CI: −3.80, 3.91), macular volume (ON affected eye–fellow eye difference 0.071 mm3, p = 0. 0.313 95% CI: −0.087, 0.228), or any individual quadrant of the RNFL (temporal quadrant ON affected eye–fellow eye difference −2.00 μm, p = 0.565 95% CI: −9.83, 5.83; superior quadrant ON affected eye–fellow eye difference −1.69 μm, p = 0.773 95% CI: −14.97, 11.60; nasal quadrant ON affected eye–fellow eye difference 2.93 μm, p = 0.510, 95% CI: −7.06, 12.97; inferior quadrant ON affected eye–fellow eye difference 1.44 μm, p = 0.726 95% CI: −7.89, 10.76).
Visual measures: change compared with baseline values
Controls did not have a significant change in either LogMAR or low contrast visual acuity over the study period. The whole progressive MS cohort had small but significant reductions in LogMAR visual acuity (mean 0.03 95% CI 0.00, 0.07 p = 0.003) and Sloan 25% contrast acuity (mean 0.04 95% CI 0.01, 0.07 p = 0.020). This change was also significant in the secondary progressive MS subgroup but not in the primary progressive MS group. There was no significant change in Sloan 5% contrast and Sloan 1.25% contrast acuity in any patient group (Tables 6, 7).
Compared with controls (Table 7), there was a significant deterioration in LogMAR acuity and Sloan 25% contrast acuity for the whole progressive MS group and for secondary progressive patients, but not for primary progressive patients (although there was a trend for deterioration in the LogMAR acuity, p = 0.079).
Relationship of change in visual measures and change in OCT measures
With the exception of macular volume and Sloan 25% contrast acuity in secondary progressive MS patients, (which does not survive adjustment for multiple comparisons and is likely to be a chance finding), there were no statistically significant relationships between change in retinal measures and change in visual acuity or low contrast acuity measures.
Change in disability and relationship with change in OCT measures
There was no change in EDSS in either MS group over the study follow-up period (median EDSS change overall 0, range −0.5 to 1, p = 0.129 for all patients, p = 0.610 for primary progressive MS patients and p = 0.129 for secondary progressive MS patients by Wilcoxon signed rank test), and there was no difference in the change in EDSS between secondary progressive MS (median EDSS change 0, range −0.5 to 1) and primary progressive MS patients (median EDSS change 0, range −0.5 to 1; p = 0.370 by Wilcoxon rank-sum test). Mean (SD) EDSS for all patients was 5.75 (1.18) at baseline and 5.93 (0.18) at the second visit, but this increase was not significant (p = 0.529 by t-test).
After adjustment for multiple comparisons, there were no significant relationships between change in any of the retinal measures and change in EDSS. When the group of patients whose EDSS increased by 0.5 or more (n = 10) was compared to the remainder of the patients, there was no significant difference in annualized change in RNFL thickness (p = 0.469) nor macular volume (p = 0.546). This was also true when the group of patients whose EDSS increased by 1.0 or more (n = 5) were compared to the remainder of the patients (p = 0.356 for RNFL thickness, p = 0.246 for macular volume).
Discussion
In this cohort of progressive MS patients who did not experience any relapses and who had a significant (mean −8.7 μm) RNFL loss compared to healthy controls at study entry, we did not observe significant longitudinal decrease in RNFL thickness after an average follow-up of over 18 months when compared with an age-matched control population (mean annualized loss was −0.2 μm greater in patients, p = 0.75). Although significant decrease over time in macular volume was seen in patients and controls its extent was very similar in both groups. These study findings are further discussed in the context of current knowledge of retinal abnormalities using time-domain OCT to study both optic neuritis and MS, in order to better understand possible mechanisms for RNFL loss and to consider the implications for using time-domain OCT as a tool to monitor potential experimental neuroprotective therapies in MS.
RNFL changes on OCT following optic neuritis
Substantial reductions seen in both RNFL thickness and macular volume have been consistently reported following a clinical episode of acute optic neuritis [4, 21, 26, 34]. Typical average reductions compared to the fellow unaffected eye are of the order of 18–22%, although the range in individual eyes is large. Serial and single time point studies at variable intervals following the episode indicate that most of this RNFL loss is evident 3–6 months following the onset of visual dysfunction.
RNFL changes on OCT in clinically unaffected eyes in MS
Single time point cross-sectional studies of MS cohorts that compared clinically unaffected eyes (i.e., no previous episode of optic neuritis) with healthy controls have revealed smaller but significant reductions in RNFL thickness. A reduction of RNFL thickness between 5 and 8.6% has been reported, and has been evident in all of the major clinical subgroups of MS: relapsing remitting, primary and secondary progressive [10, 15, 28]. However, the temporal evolution of this RNFL loss is unclear: only one previous serial OCT study has reported findings from clinically unaffected eyes in comparison to a healthy control group. Sepulcre et al. [30] followed a predominantly relapsing cohort of 61 patients with MS or clinically isolated syndromes and 29 healthy controls, and found mean RNFL loss of 2.4 μm per year in patients and 1.1 μm per year in controls. These changes were significant for both patient and control groups when compared to baseline values, although it was not reported whether the difference in longitudinal change between the two groups was significant. A more recent study [6] reported no significant change in RNFL over 2 years in 39 RRMS and seven SPMS patients; however, the study did not report comparative data from healthy controls. In the present study, we found no significant change in RNFL thickness in either progressive MS patients (annualized mean decrease of −0.7 μm/year) or healthy controls (mean −0.5 μm/year).
Sepulcre et al. [30] also found that lower values of temporal quadrant RNFL at baseline were associated with increased relapse number over the two-year follow-up period, suggesting that clinically silent RNFL loss in RRMS is associated with a tendency to experience relapses. In our cohort of progressive MS patients, we observed a trend for more RNFL loss in the SPMS cohort when compared with PPMS [15] (Table 1). Unlike PPMS, SPMS patients have experienced previous relapses. In addition, MRI brain lesion load and activity—which are both indicators of focal inflammatory demyelination—are greater in SPMS than PPMS [31, 32]. Taking all of these observations together, one might build a hypothesis that RNFL loss in clinically unaffected eyes in MS occurs more so in patients who have relapsing disease, particularly in the relapsing remitting phase, as a result of the occurrence of sub-clinical acute inflammatory demyelinating lesions in the optic nerve. On the other hand, our study does not provide evidence for ongoing retinal axonal loss in the progressive and non-relapsing phase of MS. It is also noteworthy that although eyes of the SPMS patients that had previously affected by optic neuritis had more severe RNFL loss at study entry, they did not exhibit a further decrease in RNFL thickness at follow-up.
The lack of evidence of increasing RNFL loss in this progressive MS cohort is consistent with the clinical observation that progressive visual loss is an uncommon manifestation in MS compared with the much more commonly observed progressive myelopathy or ataxia [25, 29]. We did, however, observe a decline in the quantitative measure visual acuity in progressive MS patients, over and above that seen in controls. It is possible that it derives from changes in the posterior visual pathways, or in ocular media, which would not be expected to be detected in OCT measurements that predominantly reflect change in the anterior visual pathway (although acquired posterior visual pathway lesions may cause reductions in the RNFL, putatively through trans-synaptic degeneration [19]. Alternatively, there may have been a greater sensitivity of the visual function than the OCT measures used in this study to detect a subtle increase in axonal damage and dysfunction in the anterior visual pathway. There are non-neural tissue components in the RNFL, and their presence may obscure small degrees of axonal loss.
Macular volume findings
The significant reduction over time of macular volume in healthy controls confirms previous study findings [2, 8, 20]. Using visual field perimetry findings to infer the loss of retinal ganglion cells, Harwerth et al. [14] reported that age-related RNFL layer loss in healthy subjects as determined by OCT is smaller than ganglion cell layer loss. As the macula contains a proportionately greater component of ganglion cells, the macular volume measure may be a more sensitive measure of age-related retinal changes, as the findings in our healthy control cohort suggests. The decline that we observed in macular volume in healthy controls was not accompanied by measurable changes in visual acuity or color vision. The almost identical mean macular volume change observed in progressive MS patients suggests that it was related to normal ageing rather than disease effects and emphasizes the importance of including healthy control data for comparison in longitudinal studies that investigate the macular volume.
Study limitations
It is possible that very small but progressive changes in the RNFL occurred that were below the limits of resolution and measurement precision of our time domain OCT device, or that we did not study a large enough group to detect such changes. The patient population studied, relatively older and later in the course of the disease, with relatively stable disease, may not be representative of the progressive MS population. Newer-generation spectral or Fourier domain OCT devices, which have better resolution and measurement precision, and with the capacity for accurate image registration, may allow detection of smaller differences in retinal measures [7]. The sample sizes in this study may be too small to allow detection of small changes in RNFL thickness and macular volume, and was limited by the loss to follow-up of 16/50 of the original baseline cohort.
Notwithstanding, it is noteworthy that compared to controls, the progressive MS patient cohort had a substantial mean reduction in RNFL at study entry of 8.7 μm and on the other hand exhibited a mean annualized reduction in RNFL of 0.2 μm compared to healthy controls at follow-up; if this rate of change is linearly extrapolated backwards it would take more than 40 years for the difference seen at baseline to have developed. An alternative explanation, which we prefer, is that RNFL loss develops in a non-linear, non uniform temporal pattern, e.g., there is more rapid RNFL loss in earlier RRMS when subclinical inflammatory demyelination is common and may involve the optic nerve. Inflammatory MRI brain lesions are also not uncommon at an early clinical stage in primary progressive MS [18].
Additional serial OCT studies are warranted to elucidate the timing and mechanisms of RNFL loss in clinically unaffected eyes in MS. Specifically, information would be of interest on serial measurements of RNFL thickness and macular volume in earlier, relapsing remitting MS and matched controls. In such a MS cohort sub-clinical inflammatory demyelinating episodes are more likely, and concurrent observation using visual evoked potentials and MRI of the optic nerve could provide corroborative evidence for sub-clinical demyelination. More frequent time-point sampling than was the case in the present study would also be informative. There is also a small group of MS patients who have progressive visual loss [25] (none were included in our study), and longitudinal OCT in these subjects could usefully investigate whether they exhibit progressive thinning of the RNFL.
Implications for use of OCT in MS trials of neuroprotection
While acknowledging the foregoing caveats, our overall study findings, when considered along with previous OCT studies, suggest that the time-domain OCT technology used in this study has only limited ability to detect longitudinal axonal changes in the anterior visual pathway in progressive forms of MS. In considering whether serial OCT with time-domain technology might be used to monitor potential neuroprotective therapies in MS, the present study findings suggest that it would be insensitive and unsuitable in progressive forms of MS. Studies that investigate and report serial OCT findings in relapsing remitting MS will be welcome. Further longitudinal studies in progressive forms MS would also be of interest using the new, high-resolution spectral or Fourier domain OCT systems, and/or focusing on the small group of MS patients who experience progressive visual loss. Apart from its unquestionable sensitivity and pathobiological plausibility as a tool to monitor experimental trials of neuroprotection in acute optic neuritis [1], a role for OCT in neuroprotection studies in MS remains unproven.
References
Barkhof F, Calabresi PA, Miller DH, Reingold SC (2009) Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 5:256–266
Budenz DL, Anderson DR, Varma R, Schuman J, Cantor L, Savell J, Greenfield DS, Patella VM, Quigley HA, Tielsch J (2007) Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology 114:1046–1052
Cheng H, Laron M, Schiffman JS, Tang RA, Frishman LJ (2007) The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Invest Ophthalmol Vis Sci 48:5798–5805
Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, Freedman MS, Zackon DH, Kardon RH (2006) Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 59:963–969
Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH (2008) Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler 14:893–905
Costello F, Hodge W, Pan YI, Freedman M, Demeulemeester C (2009) Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci 281:74–79
Drexler W, Fujimoto JG (2008) State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res 27:45–88
Eriksson U, Alm A (2008) Macular thickness decreases with age in normal eyes. A study on the macular thickness map protocol in the Stratus OCT. Br J Ophthalmol 93:1448–1452
Ferris FL III, Kassoff A, Bresnick GH, Bailey I (1982) New visual acuity charts for clinical research. Am J Ophthalmol 94:91–96
Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ (2006) Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 113:324–332
Frisén L, Hoyt WF (1974) Insidious atrophy of retinal nerve fibers in multiple sclerosis. Funduscopic identification in patients with and without visual complaints. Arch Ophthalmol 92:91–97
Fu L, Matthews PM, De Stefano N, Worsley KJ, Narayanan S, Francis GS, Antel JP, Wolfson C, Arnold DL (1998) Imaging axonal damage of normal-appearing white matter in multiple sclerosis. Brain 121(Pt 1):103–113
Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, Frohman EM, Cutter G, Calabresi PA (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69:1603–1609
Harwerth RS, Wheat JL, Rangaswamy NV (2008) Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci 49:4437–4443
Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, Miller DH (2008) An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 131:277–287
Hickman SJ, Toosy AT, Miszkiel KA, Jones SJ, Altmann DR, MacManus DG, Plant GT, Thompson AJ, Miller DH (2004) Visual recovery following acute optic neuritis—a clinical, electrophysiological and magnetic resonance imaging study. J Neurol 251:996–1005
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA et al (1991) Optical coherence tomography. Science 254:1178–1181
Ingle GT, Sastre-Garriga J, Miller DH, Thompson AJ (2005) Is inflammation important in early PPMS? A longitudinal MRI study. J Neurol Neurosurg Psychiatry 76:1255–1258
Jindahra P, Petrie A, Plant GT (2009) Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 132:628–634
Kanamori A, Escano MF, Eno A, Nakamura M, Maeda H, Seya R, Ishibashi K, Negi A (2003) Evaluation of the effect of aging on retinal nerve fiber layer thickness measured by optical coherence tomography. Ophthalmologica 217:273–278
Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, O’Day J, Grigg J, Billson F, Yiannikas C (2008) Axonal loss and myelin in early ON loss in postacute optic neuritis. Ann Neurol 64:325–331
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Losseff NA, Webb SL, O’Riordan JI, Page R, Wang L, Barker GJ, Tofts PS, McDonald WI, Miller DH, Thompson AJ (1996) Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 119(Pt 3):701–708
Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 46:907–911
Ormerod IE, McDonald WI (1984) Multiple sclerosis presenting with progressive visual failure. J Neurol Neurosurg Psychiatry 47:943–946
Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, Bucci MG, Pierelli F (1999) Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 40:2520–2527
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58:840–846
Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA (2007) Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 69:2085–2092
Riise T, Gronning M, Fernandez O, Lauer K, Midgard R, Minderhoud JM, Nyland H, Palffy G, Poser S, Aarli JA (1992) Early prognostic factors for disability in multiple sclerosis, a European multicenter study. Acta Neurol Scand 85:212–218
Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P (2007) Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 68:1488–1494
Thompson AJ, Kermode AG, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, McDonald WI (1990) Patterns of disease activity in multiple sclerosis: clinical and magnetic resonance imaging study. BMJ 300:631–634
Thompson AJ, Kermode AG, Wicks D, MacManus DG, Kendall BE, Kingsley DP, McDonald WI (1991) Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol 29:53–62
Toledo J, Sepulcre J, Salinas-Alaman A, Garcia-Layana A, Murie-Fernandez M, Bejarano B, Villoslada P (2008) Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler 14:906–912
Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, Plant GT, Miller DH (2005) Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 58:383–391
Acknowledgments
We thank all the subjects who took part in this study. We also thank Prof. Paul Matthews for enabling the acquisition of the OCT machine; the Multiple Sclerosis Society of Great Britain and Northern Ireland (grant support to NMR Research Unit); GlaxoSmithKline (unrestricted grant for OCT machine) and Department of Health’s Comprehensive Biomedical Research Centre at University College Hospitals Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henderson, A.P.D., Trip, S.A., Schlottmann, P.G. et al. A preliminary longitudinal study of the retinal nerve fiber layer in progressive multiple sclerosis. J Neurol 257, 1083–1091 (2010). https://doi.org/10.1007/s00415-010-5467-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-010-5467-x