Opinion statement
Retinae of patients with multiple sclerosis (MS), as part of the central nervous system (CNS), display inflammatory and neurodegenerative changes. There is increasing evidence suggesting that retinal changes, and in particular neurodegeneration, mirror global CNS alterations in MS. Spectral domain optical coherence tomography (SD-OCT) is an inexpensive, rapid, non-invasive, and reproducible imaging technique that generates high-resolution images of tissues such as the retina. An advantage of SD-OCT over magnetic resonance imaging techniques in the assessment of neurodegeneration may be its sensitivity to capture changes at the individual patient level. Several studies demonstrate that changes within the inner retina (primarily as a reflection of optic neuropathy), as assessed by OCT, correlate with reduced quality of life, visual dysfunction, and global disability in MS. Moreover, longitudinal studies suggest that inner retinal thinning is an early phenomenon in MS and that retinal thinning may occur independent of previous symptomatic episodes of optic neuritis, significantly correlating with inflammatory disease. Preliminary studies suggest that MS disease-modifying therapies may have differential effects on OCT-determined rates of retinal atrophy, supporting a potential utility for OCT to investigate the neuroprotective benefits of disease-modifying therapies in MS, as well as an outcome in trials of putatively neuroprotective strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
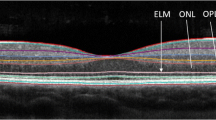
Multiple sclerosis (MS) is regarded as an autoimmune, demyelinating disorder of the central nervous system (CNS) [1]. Neurodegeneration resulting from these processes is recognized as a principal substrate of long-term disability. Optical coherence tomography (OCT) is an inexpensive, rapid, well-tolerated, reproducible, non-invasive imaging technique that uses near-infrared light to generate cross-sectional or three-dimensional images of tissues, such as the retina [2,3,4] (Fig. 1). Research using OCT in MS was initially focused on evaluation of the peripapillary retinal nerve fiber layer (pRNFL). But modern high-definition spectral domain OCT, which renders 3- to 5-μm resolution images [5], has allowed detailed qualitative assessment of individual retinal layers by the application of OCT segmentation techniques able to quantify discrete macular retinal layers, including the macular ganglion cell layer + inner plexiform layer (GCIPL), inner nuclear layer (INL), outer nuclear layer (ONL), and photoreceptors [6,10,•, 7, 8•, 9–11]. The advent of newer spectral domain optical coherence tomography (SD-OCT) devices and improved segmentation techniques allowing reliable GCIPL quantification represents a major advancement since GCIPL thickness measures may have superior reliability, reproducibility, and structure–function relationships as compared to conventional pRNFL thickness measurements [6•, 7]. The histopathology of retinal tissue from MS patients demonstrates a multitude of pathologic changes, including axonal loss, neuronal soma shrinkage, synaptic loss, activation of microglia, and inflammation [12•]. Qualitative and quantitative assessment of all the discrete layers of the retina on SD-OCT allows us to potentially gain insight into these mechanisms in vivo in MS. For instance, GCIPL thinning has been reported to be a marker of neuroaxonal injury [13], INL thickening has been proposed as a marker of inflammation in patients with MS [14•, 15], and transient ONL thickening has been reported during the acute phase of optic neuritis (ON) [16•, 17]. Since 1999, when Parisi et al. reported the earliest description of pRNFL thickness reductions in MS patients as compared to healthy volunteers [18], numerous studies have investigated the role of OCT measurements in order to improve our understanding of MS pathobiology and identify novel biomarkers for monitoring neurodegeneration in MS and therapeutic response.
The different layers of the retina. The different layers of the retina are illustrated in a central vertical scan through the middle of the foveolar. ILM inner limiting membrane, RNFL retinal nerve fiber layer, GCL ganglion cell layer, IPL inner plexiform layer, GCIP ganglion cell and inner plexiform layer, INL inner nuclear layer, BM Bruch membrane, RPE retinal pigment epithelium, ISOS inner and outer segments, ELM external limiting membrane, ONL outer nuclear layer, OPL outer plexiform layer, OPT outer photoreceptor tips.
Retinal changes mirror global central nervous system burden in MS
Magnetic resonance imaging (MRI) is the most widely used imaging method for quantifying neurodegeneration and neuroinflammation in MS. The diagnostic guidelines for MS rely on MRI-derived lesion load for establishing a diagnosis of MS [19]. Moreover, MRI-derived measures of brain atrophy are increasingly used to monitor neurodegeneration [20].
An association between neuroaxonal damage in the retina, as determined by pRNFL thickness, and measures of brain volume was reported almost 10 years ago [21, 22] and subsequently confirmed in numerous studies since [23], including a longitudinal study [24•]. The association between retinal thickness and brain volume in MS raises questions regarding the mechanisms and pathobiology underpinning these associations, as well as the translation of these findings in terms of potential clinical utility.
ON occurs frequently in MS, leads to optic nerve damage, and results in retrograde neuroaxonal damage within the retina, which can be detected and quantified by OCT measurements such as pRNFL and GCIPL thicknesses [16•, 17, 25•]. Non-conventional MRI of the optic nerve, such as diffusion tensor imaging (DTI) [26, 27] and magnetization transfer imaging [28], similarly reflects this damage. Optic nerve damage in MS may lead to anterograde trans-synaptic neurodegeneration via the lateral geniculate nucleus within weeks to several months [29•], as insinuated by a decrease of N-acetyl aspartate (a marker of neurodegeneration) in magnetic resonance spectroscopy of the primary visual cortex [30•].
Likewise, optic radiation lesions in MS are associated with visual dysfunction and potentially lead to retrograde trans-synaptic degeneration, as suggested by pRNFL and GCIP thickness reductions in OCT [31,32,33]. The functionally anatomic association of damage in different parts of the visual pathway intervened between the retina and visual cortices in MS is further supported by the relationship between DTI-derived fractional anisotropy (FA) measures (thought to primarily reflect axonal integrity) in the optic radiation and retinal OCT [34].
However, the noted associations between retinal neurodegeneration (estimated by OCT) and global neurodegeneration (as estimated by MRI volumetric assessments primarily of whole brain, gray matter, and white matter volumes) in MS seem to extend beyond OCT and MRI simply reflecting functional anatomic damage isolated to the visual pathways. pRNFL thickness correlates not only with FA of the optic radiation but also with the combined FA of all white matter tracts [34]. Likewise, a relationship between retinal neuroaxonal damage and cortical gray matter, as well as caudate volume, has been reported [35]. This association between MRI and OCT is upheld in eyes without a history of ON, supporting the possibility of an underlying disease activity similarly affecting more global regions of the CNS in MS [35, 36]. This is furthermore supported by a surprisingly strong association between spinal cord damage measured by MRI and retinal atrophy measured by OCT [37]. In this regard, ON may cause disproportionate localized retinal tissue injury, thereby masking retinal–global CNS relationships [38•].
An interesting OCT parameter for reflecting MS disease activity may be INL thickness. Increased INL thickness was reported to be associated with a moderately increased odds ratio to develop new T2 lesions or contrast-enhancing lesions [14•, 15]. A similar connection seems to exist in patients with radiologically isolated syndrome, in which an increased INL thickness was indicative of future MS-related disease activity [39].
Against this background, the clinical utility of OCT to complement MRI measures of disease activity still needs to be further defined. The first large longitudinal study of the relationships between OCT parameters and brain atrophy was recently published. The rates of GCIP and whole-brain atrophy were associated across the cohort (r = 0.45, p < 0.001), with the relationship primarily driven by the association between the rates of GCIP and gray matter (as opposed to white matter) atrophy [24•].
Neuroaxonal injury on OCT correlates with reduced quality of life and visual and global disability in MS
Since the extent of axonal loss increases with disease progression, the greatest retinal atrophy has been described in secondary progressive MS, followed by relapsing–remitting MS and then clinically isolated syndrome [40,41,42, 43•, 44], particularly in eyes previously affected by ON as compared to eyes without a history of clinical ON (non-ON eyes) [40,41,42]. Even amongst patients with purported benign MS, without prior ON [45], and patients with primary progressive MS [41, 46] who typically do not manifest overt episodes of ON, studies using OCT have shown pRNFL thickness reductions in these patient cohorts as compared to healthy volunteers.
Microcystic macular edema (MME) is characterized by cystic areas of hyporeflectivity in the INL on OCT. Even though MME is not specific to MS [47], the presence of MME in MS has been associated with a more severe disease phenotype. In a study of 318 MS patients, 15 patients were found to have MME. MME was particularly frequent in ON eyes, and subjects with MME were generally older, had longer disease duration, and had worse global and visual disability scores as compared to patients without MME [48•]. In a longitudinal study, MME was associated with INL thickening. Moreover, independent of MME, INL thickening at baseline was predictive of clinical relapses, the formation of new T2 lesions and contrast-enhancing lesions on MRI, as well as disability progression during follow-up [14•]. It is worth noting that MME may be a misnomer since the “cystic” areas are actually unlikely to be true cysts. Therefore, macular microcystoid changes might be a more appropriate term to describe the OCT appearances that have been described as MME.
Visual dysfunction is a common finding in MS, with 50% of patients presenting with visual disturbance as their first symptom of MS and 80% reporting visual disturbances throughout the course of the disease [49,50,51]. Deficits in vision are strongly correlated to worse quality of life (QOL) in patients with MS [52, 53]. GCIPL thickness is an important structural marker of visual disability in MS [6•, 54, 55]. Low-contrast letter acuity (LCLA) has emerged as the leading outcome measure for visual function in MS clinical trials. LCLA captures visual loss not measured by high-contrast visual acuity (HCVA) [50, 56•]. Even when HCVA has recovered to 20/40 or better, deficits in LCLA and vision-specific quality of life are seen, revealing dysfunction commonly reported by patients, but not measured by standard HCVA testing [57]. Several studies have shown correlations between RNFL and GCIPL thicknesses, HCVA, and, in particular, LCLA scores [9, 54, 58, 59•]. Patients with dyschromatopsia in non-ON eyes have been found to have worse global disability and cognitive scores as well as reduced pRNFL thicknesses and lower normalized brain parenchymal volume. Patients developing incident dyschromatopsia after 1 year of follow-up had greater disability worsening than those who remained with normal color vision [60•]. Visual field (VF) deficits (measured by mean deviation, MD) have been associated with disability and brain and retinal markers of neuroaxonal injury [61]. MS patients with baseline impaired VFs have been shown to have a threefold risk of disability progression during follow-up [61]. Another study also found a linear relationship between pRNFL thickness and MD in ON eyes, but not in non-ON eyes [62].
The 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) is a valid and useful tool in assessing visual dysfunction in MS patients [63, 64]. Complementing the NEI-VFQ-25, the ten-item Neuro-Ophthalmic Supplement (NOS) was developed in MS cohorts and captures patient-reported visual disability [65]. Longitudinal studies have shown that reductions of pRNFL and GCIPL thicknesses, even in the absence of ON, correlate with clinically meaningful reductions in NEI-VFQ-25 and NOS scores [53, 54, 57, 65, 66]. Deficits in LCLA and vision-specific QOL are found many years after ON, even when HCVA has recovered [63]. Patients with MS-related ON report poor QOL even with “good” visual recovery to 20/40 or better at high contrast. Such self-reported deficits may reflect underlying axonal and neuronal loss and visual dysfunction, potentially highlighting an unmet therapeutic need [57].
Several cross-sectional studies have reported correlations between pRNFL thickness reductions and higher (worse) Expanded Disability Status Scale (EDSS) scores [21,68,69,, 22, 67–70]. A recent multicenter study of 879 MS patients showed that pRNFL at baseline thickness is predictive of disability course over time. Subjects with pRNFL thicknesses lower or equal to 87 μm measured by Cirrus SD-OCT or 88 μm measured by Spectralis SD-OCT had double the risk of disability worsening by EDSS progression any time after the first and up to the third year of follow-up (Fig. 2). After the third and up to the fifth year of follow-up, this risk increased nearly fourfold, indicating that measuring pRNFL thickness is useful in determining risk of disability worsening over time in MS [71•]. One of the limitations of EDSS is that it includes mainly physical neurological impairment. For this reason, some authors have specifically investigated the relationship between OCT parameters and cognitive outcomes, but results have been inconclusive since some studies have found correlations between pRNFL thickness and Symbol Digit Modalities Test [67, 72] while others have reported that there is no correlation between these outcomes [73, 74]. Nevertheless, assessments of the relationships between OCT and comprehensive neuropsychological assessments in MS are generally lacking. Finally, Garcia-Martin et al. analyzed the relationship of pRNFL thickness with overall QOL (measured by the MSQOL-54 questionnaire). Reductions in pRNFL thickness were associated with worse QOL and EDSS scores. Questions on the MSQOL-54 relating to the patients’ perception of their physical condition correlated in particular with pRNFL thickness. Baseline pRNFL thickness predicted decreases in QOL [75].
Cumulative percentage of disability worsening in a cohort of patients with multiple sclerosis according to pRNFL thickness in eyes without optic neuritis. Number at risk: number of patients at risk of disability worsening just before the selected time points. The difference in the number of patients between one time point and the next is the sum of the number of events and number of censored patients. HR hazard ratio, pRNFL peripapillary retinal nerve fiber layer. pRNFL thicknesses are lowest (≤87 μm), intermediate (>87–97 μm [Spectralis OCT] and >87–98 μm [Cirrus OCT]), and highest tertiles (>97 μm [Spectralis OCT] and >98 μm [Cirrus OCT]). This figure was reprinted from [71], Copyright (2016), with permission from Elsevier.
Retinal thinning in patients with MS is prominent early in the disease
Understanding the dynamics of neuroaxonal injury throughout the MS course and its relationship with inflammatory activity is crucial in order to define the best therapeutic strategy for MS. If the rate of neuroaxonal injury was fastest early in the disease course and in relation with inflammation, neuroprotection in MS would mean early and active anti-inflammatory treatment. Conversely, if the rate of neuroaxonal injury was greatest later in the MS disease course and therefore less related to inflammatory activity, primary neuroprotective therapies would be necessary.
In addition to other imaging modalities such as MRI, SD-OCT may be useful for assessing the dynamics of retinal neuroaxonal injury throughout the course of MS. Most individual axons have 1 μm diameter on average. Even though brain tracts comprise numerous axons, tract diameters are still usually measured in micrometers [76]. Changes in MRI outcomes are quantified in cubic millimeters, while changes in SD-OCT outcomes are measured in micrometers, suggesting SD-OCT may have an advantage over MRI, at least in terms of resolution.
Previous studies have quantified retinal neuroaxonal loss in patients with MS [13,78,79,, 24•, 77–80]. All studies found significant retinal neuroaxonal injury by means of pRNFL and GCIPL thinning during the follow-up, except for the study published by Serbecic et al. which evaluated retinal atrophy in a group of 37 patients followed over nearly 2 years [77]. The rate of pRNFL thinning ranged from −1.49 μm/year [78] to −0.21 μm/year [13], whereas the rate of GCIPL thinning ranged from −0.55 μm/year [80] to −0.30 μm/year [79]. Three out of these six studies specifically addressed whether the rates of retinal atrophy were different between eyes with and without a history of ON and did not find significant difference between these groups [78,79,80]. Ratchford et al. found that the rate of retinal thinning was faster for patients with disease duration lower than 5 years than for those with a longer disease duration. Interestingly, these authors found no differences when they repeated these analyses using 10 years as a cutoff instead of 5 years [13]. Similarly, Balk et al. assessed the rates of retinal neuroaxonal injury and found that the rates of retinal atrophy were fastest in the early period of MS and described a plateau effect for patients with disease duration longer than 20 years [80]. Both studies suggest that the rate of neuroaxonal loss may be highest early in the MS disease course. Finally, Ratchford et al. found that the rates of retinal GCIPL atrophy were faster in patients with relapses (42% faster), patients with new gadolinium-enhancing lesions (54% faster), and patients with new T2 lesions (36% faster) than in patients without these clinico-radiological features [13]. Therefore, the rate of retinal neuroaxonal loss may be partially related to the degree of ongoing inflammatory activity.
OCT measurements as promising markers of therapeutic response in MS
Collectively, the above findings provide ample support for the proposed utility of OCT measures, and in particular GCIPL thickness, as outcomes for objectively assessing and monitoring neurodegeneration and, accordingly, neuroprotection. Yet, there has been a paucity of such investigations to date. The currently available disease-modifying therapies (DMTs) for relapsing–remitting multiple sclerosis (RRMS) modulate or suppress the immune system, reducing the risk of future inflammation and associated neurodegeneration. Accordingly, the effect of DMTs on MRI-derived estimates of brain atrophy has become a common outcome in MS trials. However, unlike MRI volumetry which lacks sensitivity and requires large numbers of patients to detect significant differences, due to being highly reliable, sensitive, and objective outcomes for assessing neuroprotection, OCT measures may be able to detect significant differences between small cohorts of patients.
In the 2016 American Academy of Neurology annual meeting, authors from the University of British Columbia reported an improvement in retinal measurements (pRNFL thickening of over 1.5 μm) over 2 years in a small cohort of 26 RRMS patients who were treated with alemtuzumab. The authors reported a significant inverse correlation between pRNFL global changes and EDSS (r = −0.42, p = 0.047) [81]. Although promising, the findings need validation in future studies as well as further explanation of the mechanisms behind these results. In a recent retrospective and preliminary assessment of the effects of glatiramer acetate (GA, n = 48), natalizumab (NTZ, n = 46), and interferon beta-1a subcutaneously (IFNBSC, n = 35) and intramuscularly (IFNBIM, n = 28) in RRMS patients monitored over a mean follow-up period of 3 years, relative to NTZ-treated patients, IFNBSC- and GA-treated patients exhibited 0.37 μm/year (p < 0.001) and 0.14 μm/year (p = 0.035) faster rates of GCIPL thinning, respectively. Despite the small treatment cohort sizes, the findings were strikingly in accordance with the effects of these DMTs upon brain atrophy observed in large-scale studies. The IFNBSC cohort had a 0.14-μm/year (p = 0.001), 0.11-μm/year (p = 0.011), and 0.12-μm/year (p = 0.023) faster rate of INL thinning compared to the NTZ, GA, and IFNBIM cohorts, respectively. Moreover, the GA, IFNSC, and IFNIM cohorts exhibited 0.18 μm/year (p = 0.014), 0.38 μm/year (p < 0.001), and 0.22 μm/year (p = 0.013) faster rates of ONL thinning relative to NTZ-treated patients, respectively. The rate of GCIPL thinning in the healthy control (HC) cohort was 0.14 μm/year, adjusting for baseline GCIPL thickness, age, and sex. The GA-, IFNBSC-, and IFNBIM-treated patients exhibited 0.16 μm/year (p = 0.010), 0.40 μm/year (p < 0.001), and 0.14 μm/year (p = 0.08) greater rates of GCIPL thinning compared to HCs, respectively. On the other hand, NTZ-treated patients and HCs did not differ in their rates of GCIPL loss (p = 0.720) [82•]. This study, however, did not assess the effects of oral DMTs or monoclonal antibodies other than NTZ on retinal atrophy or include patients with PMS, limitations that need to be addressed in future, larger prospective studies.
Conclusions
There is increasing evidence that retinal changes mirror global central nervous system aberrations in MS. Current evidence provides strong support that OCT measures of pRNFL and GCIPL thicknesses reflect global neurodegeneration in MS, with numerous studies finding consistent correlations between brain volumes and retinal thickness measures. Moreover, over time, the rates of GCIPL thinning and brain atrophy have been shown to mirror one another in MS. In addition, studies raise the possibility that INL thickness may have utility in predicting and/or monitoring inflammatory activity in MS. Thinning of axonal and neuronal layers on OCT correlates with reduced quality of life, vision, and disability in MS. Patients with MS display significant retinal thinning during their disease course, regardless of whether or not they have a history of ON. Retinal neuroaxonal loss seems to be greatest during the early phases of the disease and particularly prominent for patients with active MS. While few studies have investigated the role of OCT measurements in assessing the neuroprotective effects of DMTs in MS, emerging preliminary data do strongly support the feasibility of OCT to investigate differences in the neuroprotective benefits of various DMTs.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17.
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81.
Hrynchak P, Simpson T. Optical coherence tomography: an introduction to the technique and its use. Optom Vis Sci. 2000;77:347–56.
Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008;4:664–75.
Warner CV, Syc SB, Stankiewicz AM, Hiremath G, Farrell SK, Crainiceanu CM, et al. The impact of utilizing different optical coherence tomography devices for clinical purposes and in multiple sclerosis trials. PLoS One. 2011;6:e22947.
• Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17:1449–63. This was one of the first studies to demonstrate that GCIP thickness may have superior structure–function relationships with disability and visual function in MS as compared to RNFL thickness.
Saidha S, Syc SB, Ibrahim MA, Eckstein C, Warner CV, Farrell SK, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134:518–33.
• Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521–33. This paper showed how the ganglion cell layer reflects neurodegeneration in acute optic neuritis without confounding swelling and introduced this as a novel parameter to track optic neuritis severity.
Seigo MA, Sotirchos ES, Newsome S, Babiarz A, Eckstein C, Ford E, et al. In vivo assessment of retinal neuronal layers in multiple sclerosis with manual and automated optical coherence tomography segmentation techniques. J Neurol. 2012;259:2119–30.
Lang A, Carass A, Hauser M, Sotirchos ES, Calabresi PA, Ying HS, et al. Retinal layer segmentation of macular OCT images using boundary classification. Biomed Opt Express. 2013;4:1133–52.
Lang A, Carass A, Sotirchos E, Calabresi P, Prince JL. Segmentation of retinal OCT images using a random forest classifier. Proc SPIE Int Soc Opt Eng 2013;8669.
• Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–601. This oculo-histopathology study of MS eyes shows that 79% of MS eyes have a dropout of ganglion cell neurons. Moreover, 40% of MS eyes have a dropout of neurons within the inner nuclear layer. In addition, activated microglia can be seen in the retinas of MS patients.
Ratchford JN, Saidha S, Sotirchos ES, Oh JA, Seigo MA, Eckstein C, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80:47–54.
• Saidha S, Sotirchos ES, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–72. This study provides evidence that changes in the inner nuclear layer thickness/volume are related with inflammatory activity in patients with multiple sclerosis.
Knier B, Schmidt P, Aly L, Buck D, Berthele A, Muhlau M, Zimmer C, Hemmer B, Korn T. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain. 2016 Aug 30. pii: aww219. [Epub ahead of print]
• Gabilondo I, Martinez-Lapiscina EH, Fraga-Pumar E, Ortiz-Perez S, Torres-Torres R, Andorra M, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77:517–28. This paper provides an extensive investigation in retinal changes after optic neuritis.
Al-Louzi OA, Bhargava P, Newsome SD, Balcer LJ, Frohman EM, Crainiceanu C, et al. Outer retinal changes following acute optic neuritis. Mult Scler. 2016;22:362–72.
Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–66.
Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488–94.
Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69:1603–9.
Dorr J, Wernecke KD, Bock M, Gaede G, Wuerfel JT, Pfueller CF, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 2011;6:e18132.
• Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78:801–13. This study provides the strongest evidence to support the association between retinal and brain atrophy in patients with multiple sclerosis.
• Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–9. This study provides the first evidence of a nonlinear association between retinal thickness and clinical outcome: a threshold of RNFL thickness (75 μm), below which RNFL measurements predicted persistent visual dysfunction after acute optic neuritis.
Naismith RT, Xu J, Tutlam NT, Snyder A, Benzinger T, Shimony J, et al. Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology. 2009;72:589–94.
Kolbe SC, Marriott M, Walt A, Fielding J, Klistorner A, Mitchell PJ, et al. Diffusion tensor imaging correlates of visual impairment in multiple sclerosis and chronic optic neuritis. Invest Ophthalmol Vis Sci. 2012;53:825–32.
Wang Y, van der Walt A, Paine M, Klistorner A, Butzkueven H, Egan GF, et al. Optic nerve magnetisation transfer ratio after acute optic neuritis predicts axonal and visual outcomes. PLoS One. 2012;7:e52291.
• Gabilondo I, Martinez-Lapiscina EH, Martinez-Heras E, Fraga-Pumar E, Llufriu S, Ortiz S, et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75:98–107. This study provided evidence to support the potential role of trans-synaptic mechanisms of neurodegeneration in patients with multiple sclerosis.
• Pfueller CF, Brandt AU, Schubert F, Bock M, Walaszek B, Waiczies H, et al. Metabolic changes in the visual cortex are linked to retinal nerve fiber layer thinning in multiple sclerosis. PLoS One. 2011;6:e18019. This study provided evidence to support the potential role of trans-synaptic mechanisms of neurodegeneration in patients with multiple sclerosis.
Reich DS, Smith SA, Gordon-Lipkin EM, Ozturk A, Caffo BS, Balcer LJ, et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol. 2009;66:998–1006.
Klistorner A, Sriram P, Vootakuru N, Wang C, Barnett MH, Garrick R, et al. Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology. 2014;82:2165–72.
Sinnecker T, Oberwahrenbrock T, Metz I, Zimmermann H, Pfueller CF, Harms L, et al. Optic radiation damage in multiple sclerosis is associated with visual dysfunction and retinal thinning—an ultrahigh-field MR pilot study. Eur Radiol. 2015;25:122–31.
Scheel M, Finke C, Oberwahrenbrock T, Freing A, Pech LM, Schlichting J, et al. Retinal nerve fibre layer thickness correlates with brain white matter damage in multiple sclerosis: a combined optical coherence tomography and diffusion tensor imaging study. Mult Scler. 2014;20:1904–7.
Saidha S, Sotirchos ES, Oh J, Syc SB, Seigo MA, Shiee N, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70:34–43.
Young KL, Brandt AU, Petzold A, Reitz LY, Lintze F, Paul F, et al. Loss of retinal nerve fibre layer axons indicates white but not grey matter damage in early multiple sclerosis. Eur J Neurol. 2013;20:803–11.
Oh J, Sotirchos ES, Saidha S, Whetstone A, Chen M, Newsome SD, et al. Relationships between quantitative spinal cord MRI and retinal layers in multiple sclerosis. Neurology. 2015;84:720–8.
• Zimmermann H, Freing A, Kaufhold F, Gaede G, Bohn E, Bock M, et al. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler. 2013;19:443–50. This study demonstrates that the global relationships reflected by retinal measures in MS are skewed/masked by prior optic neuritis possibly due to disproportionate localized retinal tissue injury following optic neuritis.
Knier B, Berthele A, Buck D, Schmidt P, Zimmer C, Muhlau M, et al. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult Scler. 2016;22:893–900.
Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci. 2009;281:74–9.
Costello F, Hodge W, Pan YI, Eggenberger E, Freedman MS. Using retinal architecture to help characterize multiple sclerosis patients. Can J Ophthalmol. 2010;45:520–6.
Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One. 2012;7:e36847.
• Oberwahrenbrock T, Schippling S, Ringelstein M, Kaufhold F, Zimmermann H, Keser N, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305. This large cross-sectional study summarizes time domain OCT findings in different multiple sclerosis subtypes.
Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, Bellmann-Strobl J, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler. 2013;19:1887–95.
Lange AP, Zhu F, Sayao AL, Sadjadi R, Alkabie S, Traboulsee AL, et al. Retinal nerve fiber layer thickness in benign multiple sclerosis. Mult Scler. 2013;19:1275–81.
Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–92.
Burggraaff MC, Trieu J, de Vries-Knoppert WA, Balk L, Petzold A. The clinical spectrum of microcystic macular edema. Invest Ophthalmol Vis Sci. 2014;55:952–61.
• Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135:1786–93. This was the first study to demonstrate macular microcystoid changes in MS and identify an association between the presence of such retinal findings and increased disability in MS.
McDonald WI, Barnes D. The ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual system. J Neurol Neurosurg Psychiatry. 1992;55:747–52.
Balcer LJ, Baier ML, Cohen JA, Kooijmans MF, Sandrock AW, Nano-Schiavi ML, et al. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology. 2003;61:1367–73.
Newman N. Multiple sclerosis and related demyelinating diseases. In: Miller NR, Newman NJ, editors. Walsh and Hoyt’s clinical neuro-ophthalmology. 5th ed. Baltimore: Williams & Wilkins; 1998;5539-5676
Rudick RA, Miller D, Clough JD, Gragg LA, Farmer RG. Quality of life in multiple sclerosis. Comparison with inflammatory bowel disease and rheumatoid arthritis. Arch Neurol. 1992;49:1237–42.
Ma SL, Shea JA, Galetta SL, Jacobs DA, Markowitz CE, Maguire MG, et al. Self-reported visual dysfunction in multiple sclerosis: new data from the VFQ-25 and development of an MS-specific vision questionnaire. Am J Ophthalmol. 2002;133:686–92.
Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119:1250–7.
Lampert EJ, Andorra M, Torres-Torres R, Ortiz-Perez S, Llufriu S, Sepulveda M, et al. Color vision impairment in multiple sclerosis points to retinal ganglion cell damage. J Neurol. 2015;262:2491–7.
• Baier ML, Cutter GR, Rudick RA, Miller D, Cohen JA, Weinstock-Guttman B, et al. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology. 2005;64:992–5. This article described the important role of low-contrast letter acuity test to capture visual dysfunction in patients with multiple sclerosis.
Sabadia SB, Nolan RC, Galetta KM, Narayana KM, Wilson JA, Calabresi PA, et al. 20/40 or better visual acuity after optic neuritis: not as good as we once thought? J Neuroophthalmol. 2016;36:369–76.
Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32.
• Talman LS, Bisker ER, Sackel DJ, Long Jr DA, Galetta KM, Ratchford JN, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–60. In this study, the authors found that MS patients with increasing reduction in retinal nerve fiber layer thickness had increasing loss of visual function over time.
• Martinez-Lapiscina EH, Ortiz-Perez S, Fraga-Pumar E, Martinez-Heras E, Gabilondo I, Llufriu S, et al. Colour vision impairment is associated with disease severity in multiple sclerosis. Mult Scler. 2014;20:1207–16. This study demonstrates that MS patients developing impairment of color vision over time have more severe MS.
Ortiz-Perez S, Andorra M, Sanchez-Dalmau B, Torres-Torres R, Calbet D, Lampert EJ, et al. Visual field impairment captures disease burden in multiple sclerosis. J Neurol. 2016;263:695–702.
Cheng H, Laron M, Schiffman JS, Tang RA, Frishman LJ. The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Invest Ophthalmol Vis Sci. 2007;48:5798–805.
Cole SR, Beck RW, Moke PS, Gal RL, Long DT. The National Eye Institute Visual Function Questionnaire: experience of the ONTT. Optic Neuritis Treatment Trial. Investig Ophthalmol Vis Sci. 2000;41:1017–21.
Noble J, Forooghian F, Sproule M, Westall C, O’Connor P. Utility of the National Eye Institute VFQ-25 questionnaire in a heterogeneous group of multiple sclerosis patients. Am J Ophthalmol. 2006;142:464–8.
Raphael BA, Galetta KM, Jacobs DA, Markowitz CE, Liu GT, Nano-Schiavi ML, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142:1026–35.
Mowry EM, Loguidice MJ, Daniels AB, Jacobs DA, Markowitz CE, Galetta SL, et al. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry. 2009;80:767–72.
Toledo J, Sepulcre J, Salinas-Alaman A, Garcia-Layana A, Murie-Fernandez M, Bejarano B, et al. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler. 2008;14:906–12.
Siepman TA, Bettink-Remeijer MW, Hintzen RQ. Retinal nerve fiber layer thickness in subgroups of multiple sclerosis, measured by optical coherence tomography and scanning laser polarimetry. J Neurol. 2010;257:1654–60.
Abalo-Lojo JM, Limeres CC, Gomez MA, Baleato-Gonzalez S, Cadarso-Suarez C, Capeans-Tome C, et al. Retinal nerve fiber layer thickness, brain atrophy, and disability in multiple sclerosis patients. J Neuroophthalmol. 2014;34:23–8.
Behbehani R, Al-Hassan AA, Al-Khars A, Sriraman D, Alroughani R. Retinal nerve fiber layer thickness and neurologic disability in relapsing-remitting multiple sclerosis. J Neurol Sci. 2015;359:305–8.
• Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15:574–84. This study provides the strongest evidence so far to support the role of OCT as a predictor of disability worsening in patients with multiple sclerosis.
El Ayoubi NK, Ghassan S, Said M, Allam J, Darwish H, Khoury SJ. Retinal measures correlate with cognitive and physical disability in early multiple sclerosis. J Neurol. 2016;263:2287–95.
Anhoque CF, Biccas-Neto L, Domingues SC, Teixeira AL, Domingues RB. Cognitive impairment and optic nerve axonal loss in patients with clinically isolated syndrome. Clin Neurol Neurosurg. 2013;115:1032–5.
Wieder L, Gade G, Pech LM, Zimmermann H, Wernecke KD, Dorr JM, et al. Low contrast visual acuity testing is associated with cognitive performance in multiple sclerosis: a cross-sectional pilot study. BMC Neurol. 2013;13:167.
Garcia-Martin E, Rodriguez-Mena D, Herrero R, Almarcegui C, Dolz I, Martin J, et al. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurology. 2013;81:76–83.
Edgar JM, Griffiths IR. White matter structure—chapter 5: a microscopist’s view. Elsevier 2009; 74-103.
Serbecic N, Aboul-Enein F, Beutelspacher SC, Vass C, Kristoferitsch W, Lassmann H, et al. High resolution spectral domain optical coherence tomography (SD-OCT) in multiple sclerosis: the first follow up study over two years. PLoS One. 2011;6:e19843.
Narayanan D, Cheng H, Bonem KN, Saenz R, Tang RA, Frishman LJ. Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult Scler. 2014;20:1331–41.
Graham EC, You Y, Yiannikas C, Garrick R, Parratt J, Barnett MH, et al. Progressive loss of retinal ganglion cells and axons in nonoptic neuritis eyes in multiple sclerosis: a longitudinal optical coherence tomography study. Invest Ophthalmol Vis Sci. 2016;57:2311–7.
Balk LJ, Cruz-Herranz A, Albrecht P, Arnow S, Gelfand JM, Tewarie P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263:1323–31.
Nguyen A-L, Lam J, White R, Carruthers R, Traboulsee A. Prospective study of retinal nerve fibre layer thickness in alemtuzumab treated multiple sclerosis patients. Neurology 2016.
• Button J, Al-Louzi O, Lang A, Bhargava P, Newsome SD, Frohman T, et al. Disease-modifying therapies modulate retinal atrophy in multiple sclerosis: a retrospective study. Neurology. 2017;88(6):525–32. This study highlights the potential role of OCT in monitoring response to MS therapies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Alexander U. Brandt received funding from the German Federal Ministry for Economic Affairs and Energy (BMWi Exist 03EFEBE079).
Elena H. Martinez-Lapiscina received funding from the Instituto de Salud Carlos III, Spain, and Fondo Europeo de Desarrollo Regional (FEDER; JR16/00006), Grant for MS Innovation and Marató TV3 Charitable Foundation.
Rachel Nolan declares no funding.
Shiv Saidha received funding from the Race to Erase MS and Genentech Corporation.
Conflict of Interest
Alexander U. Brandt has received travel reimbursement from Bayer, Biogen, Teva, and Novartis and consulting or speaker honoraria from Biogen, Teva, Heidelberg Engineering, Motognosis, and Nexus. He is a member of the working committee of International Multiple Sclerosis Visual System (IMSVISUAL) Consortium.
Elena H. Martinez-Lapiscina is a researcher in the OCTIMS Study, an observational study (which involves no specific drugs) to validate SD-OCT as a biomarker for multiple sclerosis, sponsored by Novartis. She has received speaking honoraria from Biogen and Genzyme and travel reimbursement from Genzyme, Roche, for international and national meetings over the last 3 years. She is a member of the working committee of the International Multiple Sclerosis Visual System (IMSVISUAL) Consortium.
Rachel Nolan is a member of the working committee of the International Multiple Sclerosis Visual System (IMSVISUAL) Consortium.
Shiv Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology, consulting fees from Axon Advisors LLC, speaking honoraria from the National Association of Managed Care Physicians, Family Medicine Foundation of West Virginia, and Advanced Studies in Medicine, and served on scientific advisory boards for Biogen-Idec, Genzyme, Genentech Corporation, and Novartis. He received research support from the Race to Erase MS and Genentech Corporation. He is a member of the working committee of the International Multiple Sclerosis Visual System (IMSVISUAL) Consortium.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Multiple Sclerosis and Related Disorders
Alexander U. Brandt, Elena H. Martinez-Lapiscina, Rachel Nolan and Shiv Saidha contributed equally to this work.
Rights and permissions
About this article
Cite this article
Brandt, A.U., Martinez-Lapiscina, E.H., Nolan, R. et al. Monitoring the Course of MS With Optical Coherence Tomography. Curr Treat Options Neurol 19, 15 (2017). https://doi.org/10.1007/s11940-017-0452-7
Published:
DOI: https://doi.org/10.1007/s11940-017-0452-7