Abstract
Verbal fluency tasks are commonly used to explore semantic memory and executive functions. The aim of this study was to gain a better understanding of the cognitive and neural mechanisms underlying verbal fluency impairment in the frontal variant of frontotemporal dementia (fv-FTD) and in semantic dementia (SD). Semantic and phonemic fluency tasks were performed by 36 fv-FTD and SD patients and 18 elderly controls. We also carried out a neuropsychological investigation of semantic memory, working memory and shifting and updating processes. We performed correlative and regression analyses of fluency scores and neuropsychological data. In addition, patients underwent a resting positron emission tomography examination, and statistical parametric mapping was used to establish correlations between resting-state FDG uptake in the whole brain and fluency scores for each patient group. Both patient groups displayed impaired performances on both fluency tasks compared with controls, but with different patterns. While fv-FTD patients scored higher than SD patients on semantic fluency, their performances on the phonemic task did not differ. Correlation and regression analyses clearly demonstrated that the fv-FTD patients’ performances on both fluency tasks depended on their executive abilities, while those of the SD patients were hampered by the impairment of their semantic memory store. Correlations with resting FDG uptake were consistent with the results of the cognitive study. In fv-FTD, both fluency performances were related to the metabolism of the frontal lobes, while we observed significant correlations between performances on both fluency tasks and the left temporal lobe metabolism in SD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The frontal variant of frontotemporal dementia (fv-FTD) produces changes in personality and social behavior [48, 55]. The most common cognitive deficit in fv-FTD is an impairment of executive functions and/or working memory [7, 40]. Patients also present attentional deficits, difficulties in mental set shifting and perseverative tendencies [64]. Deficits in planning, organization and other aspects of executive function become universal as the disease progresses. In accordance with these neuropsychological features, structural and functional neuroimaging studies of fv-FTD have shown that the locus of pathology typically starts in the ventromedial portions of the prefrontal cortex [29, 63] and then extends to the dorsolateral prefrontal cortex. In the advanced stages of the disease, the lesions affect extensive cortical and subcortical regions [19, 66].
Patients with the temporal variant of frontotemporal dementia, referred to as semantic dementia (SD) [27, 65], have degraded knowledge about the meanings of words and objects. This lexical semantic impairment is in singular contrast to a relative preservation of the syntactic and phonological processes of language [28]. Executive functions, visuospatial abilities, perceptual abilities and at least some aspects of episodic memory are relatively preserved. Brain imaging reveals a characteristic pattern of atrophy and hypometabolism involving the anterior portion of the temporal lobes, almost always asymmetrical and frequently predominant in the left lobe [16, 20, 42], but inevitably becoming bilateral as the disease progresses.
Given the respective executive dysfunction and semantic disorders of fv-FTD and SD, a comparison of the verbal fluency performances of these patients can yield particularly interesting data. Tests of verbal fluency consist in generating words belonging to a particular semantic category (e.g., animals) or words beginning with a given letter (e.g., the letter ‘p’) within a specified time limit, such as 2 min. These tasks are amongst the measures most widely used in neuropsychological examinations to explore semantic memory and executive functions. Semantic fluency is regarded rather simplistically as being largely dependent upon the integrity of semantic memory, while according to Perret’s much-cited conclusions [47], phonemic fluency is supposedly more sensitive to executive dysfunction. By administering a dual-task procedure to normal subjects, Martin et al. [39] found that phonemic fluency was dissociated from semantic fluency. Whereas phonemic fluency was more negatively affected by the performance of a concurrent motor task than by that of a concurrent object decision task, the opposite was true for semantic fluency. This dissociation suggests that phonemic fluency is more dependent upon the frontal lobe, whereas semantic fluency depends more on the temporal lobes. However, converging lines of evidence suggest that such a dichotomy is somewhat of an oversimplification and that interpreting verbal fluency deficits is not quite that easy.
In a meta-analytic review, investigating verbal fluency task performances by patients with focal cortical lesions, Henry and Crawford [25] showed that while both types of fluency impose comparable demands on the frontal structures, semantic fluency is relatively more dependent upon the integrity of temporal structures. Executive processes are involved in the initiation and monitoring of both fluency tasks, but semantic fluency is relatively more dependent on the integrity of semantic memory [25, 61]. More specifically, both fluency tasks involve different cognitive processes, including immediate attention in order to initiate the generation of words, an available knowledge base from which to select relevant items, an ability to retrieve items from verbal declarative memory and executive abilities to coordinate these processes, including working memory to monitor performance and avoid breaking rules [62]. Even though some of these cognitive processes are common to both semantic and phonemic fluency tasks, additional processes are differentially involved in the two forms of fluency. Hence, although both fluency tasks involve access to semantic information, different types of strategic semantic retrieval are engaged according to the nature of the task. Whereas the semantic fluency task, based on a categorical criterion, requires a categorization process, efficient phonemic fluency production, limited by an orthographic criterion, involves set shifting processes to a greater extent. Because both measures place comparable demands on frontal structures, although semantic fluency is relatively more dependent on temporal structures, it has been suggested that comparing the relative magnitude of deficits on phonemic and semantic fluency could be used to draw inferences about the prominence of executive dysfunction and semantic memory dysfunction, respectively.
Fv-FTD patients score more poorly than controls on both semantic and phonemic tasks, but display the same disproportionate results as the latter, i.e., like the controls, they perform better on the semantic fluency task than on the phonemic one [29, 44]. This profile is regarded as reflecting an inefficient search process (e.g., difficulty in generating search strategies, or switching to a new subset when previous ones are exhausted). SD patients, on the other hand, perform equally poorly on both fluency tasks [36, 59]. These performances are thought to stem from the disruption of semantic knowledge caused by SD. Although a few studies have directly compared the verbal fluency performances of fv-FTD and SD patients [59], none of them has studied the underlying cognitive determinants of these patients’ performances. Furthermore, even though frontal and temporal dysfunctions are thought to be responsible for verbal fluency impairment in fv-FTD and SD, respectively, the precise location of such dysfunction has yet to be properly established.
The aim of this study was to provide a direct comparison of performances by fv-FTD and SD patients on semantic and phonemic fluency tasks, thus holding out the hope of improving the differential diagnosis between fv-FTD and SD patients. We also sought to investigate the processes and the main cortical regions involved in both semantic and phonemic fluency tasks in fv-FTD and SD. We used positron emission tomography (PET) and statistical parametric mapping (SPM) to establish the correlations between resting-state FDG uptake in the whole brain and the fluency scores of the fv-FTD and SD patient groups, separately. The sites of significant correlation can be considered as reflecting the structures indispensable to the cognitive process being assessed [68], while activation mapping essentially highlights the cerebral structures implicated in, but not necessarily required by the task [52]. Voxel-based mapping of the correlations between cognitive performances and resting-state FDG uptake is a sensitive approach to delineating the neural substrates of cognitive impairment (for a detailed discussion, see [17]). This method has successfully been used in Alzheimer’s disease [14, 15, 18, 26, 34, 57], but only rarely in fv-FTD [51] and never, to the best of our knowledge, in SD.
Materials and methods
Subjects
We studied a group of 36 patients suffering from fv-FTD (n = 18) and SD (n = 18) selected according to the criteria established by Neary et al. [43]. For each patient, the selection was made according to a codified procedure, by senior neurologists (VDLS and SB) whose main activity is the diagnosis and follow-up of patients suffering from neurodegenerative disorders. To perform reliable differential diagnosis, all patients underwent a neurological examination, an extensive neuropsychological assessment including a non-verbal episodic memory assessment and information concerning patients’ past and present behaviors was obtained with a caregiver. Diagnoses were also supported by structural and/or functional imaging (SPECT). Some of these patients have already been described in previous investigations [16, 40, 51]. We also studied a group of 18 healthy elderly subjects, selected to match the fv-FTD and SD patients in terms of age, sex and education level. The demographic data of the patient and control groups are presented in Table 1.
All participants were French native speakers and had a minimum level of education equivalent to the ‘certificat d’études primaires,’ a diploma generally obtained at ~14 years, after 8 years of primary education. None of the participants had a history of alcoholism, head trauma or neurological or psychiatric illness. Duration of illness was the length of time between the testing session and the appearance of the first symptoms of the disease according to the patients and/or their caregivers. All subjects gave their written informed consent to the study, which was approved by the local ethics committee.
Verbal fluency tasks
Both semantic and phonemic verbal fluency tasks were administered using the standard procedure developed by a reflection group on executive functions evaluation (GREFEX) widely used by French-speaking clinicians and researchers. Instructions insist on the variety of items to produce by giving an example with another category, but no indication on subcategories to explore is given. Such a procedure does not provide clues to the patients and does not suggest strategy to improve their performance or to overcome their difficulties.
In the semantic task, participants were instructed to generate as many different names of animals as possible in the space of 2 min. In the phonemic task, they had to produce as many words as possible beginning with the letter ‘p’ in 2 min. They were told that proper names, repetitions or words with different suffixes would not count. For all participants, the semantic fluency task was proposed first. The total score was the total number of words generated, excluding perseverative and intrusive errors.
Neuropsychological assessment
In addition, all the patients and controls underwent the same neuropsychological assessment. Verbal fluency impairment appears to be due to a semantic memory deficit and/or to the poor initiation or inflexibility of search and retrieval processes [67]. We therefore investigated semantic memory, working memory and a number of executive functions (updating and set shifting processes).
Semantic memory assessment
In a semantic knowledge task [21], three components (naming of drawings, categorical knowledge and concept attribute knowledge) were assessed. The subjects had to name 30 drawings corresponding to 30 concepts. Correct naming was scored 2 points. If the subject failed, a recognition task of the noun was carried out and was scored 1 point. Then, the subject had to answer ‘yes’ or ‘no’ to a series of questions about each of the 30-item concepts: superordinate category (“Does it occur naturally or is it manmade?”), category membership (“Is it an animal, a plant, an object or a body part?”), subcategory (“Is it a domestic or a wild animal?”) and specific attribute—either functional (“Is it edible?”) or perceptual (“Does it have a mane?”). The score was the total number of correct answers, the maximum being 236. The percentage of correct responses was calculated.
Working memory and executive function assessment
The working memory assessment comprised four tasks used to explore the three components of Baddeley’s theoretical framework [4]. The phonological loop was tested by a forward digit span (FD-Span) and the visuospatial sketchpad by a forward visuospatial span (FVS-Span). To assess the central executive, we used a backward digit span (BD-Span) and backward visuospatial span (BVS-Span).
We used the trail making test [58] (TMT) to assess ‘shifting’ and the running span test [54] to assess ‘updating.’ The time taken to process part A of the TMT gives a measurement of processing speed and that taken to process part B a measurement of the ability to flexibly shift the course of an ongoing activity [2]. In the running span task, 16 strings of consonants of an undisclosed length (4, 6, 8 or 10) are given orally, and the patients are required to recall the four most recent consonants in the right order. An ‘updating’ score is calculated on the basis of the 12 trials with a sequence of more than four consonants.
PET methodology
All 18 fv-FTD patients and only 15 SD patients (because of medical contraindications) underwent a resting PET study using [18F]fluoro-2-deoxy-d-glucose. Data were collected using the high-resolution ECAT Exact HR + PET device, with an isotropic resolution of 4.6 mm × 4.2 mm × 4.2 mm (FOV = 158 mm). [18F]Fluoro-2-deoxy-d-glucose uptake was measured in the resting condition, with eyes closed, in a quiet and dark environment. A catheter was introduced in a vein of the arm for radiotracer administration. Following 68 Ga transmission scans, 3–5 mCi of [18F]fluoro-2-deoxy-d-glucose were injected as a bolus at time 0, and a 10 min PET data acquisition was begun at 50 min post-injection. Sixty-three planes were acquired with septa out (volume acquisition), using a voxel size of 2.2 mm × 2.2 mm × 2.43 mm (x, y, z). During PET data acquisition, head motion was monitored continuously with laser beams.
In addition, patients underwent a T1-weighted volume MRI scan (1.5-T Signa Advantage EchoSpeed; General Electric) in order to perform the partial volume effect (PVE) correction, using the optimal voxel-by-voxel method, described in detail in Quarantelli et al. [53], and already used in our laboratory [10, 16]. All image processing steps were carried out using ‘PVE-lab’ software. Using statistical parametric mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK), the PVE-corrected PET data were then subjected to coregistration onto their respective MRIs, to spatial normalization using parameters obtained from the normalization of the corresponding MRI data into a customized MRI template obtained from our patient sample, and to a reslicing with a voxel size of 2 mm × 2 mm × 2 mm. The spatially normalized sets were then smoothed with a 14-mm isotropic Gaussian filter to blur individual variations in gyral anatomy and to increase the signal-to-noise ratio. The “proportional scaling” routine was applied to the PVE-corrected PET data to control for individual variations in overall FDG uptake. In order to minimize “edge effects” without excluding hypometabolic tissue in our subjects, only those voxels with values >40% of the mean for the whole brain were selected for the statistical analysis.
Statistical analysis
The demographic and clinical data, naming scores, total semantic score, working memory scores and updating scores were all subjected to one-way analyses of variance (ANOVA), with group as the between-subjects factor. Two Group × Test ANOVAs were carried out on TMT and fluency performances, with, respectively, TMT part A versus TMT part B performances and total semantic versus phonemic fluency scores as between-subjects variables. These ANOVAs were followed by post-hoc comparisons using Tukey’s honestly significant difference (HSD) tests to compare group means. Age at onset and duration of illness were compared between the patient groups using the paired Student’s t test. Correlations between both fluency scores in each group were studied by means of the Pearson correlation coefficient. Non-parametric equivalent to parametric analyses done were also conduced (data not shown). Results and conclusions were quite the same.
To investigate the relationships between the fluency scores and the neuropsychological data, we used the Pearson’s correlation coefficient, and to determine the predictive value of the neuropsychological scores for fluency scores, we conducted regression analyses. All hypotheses were tested at alpha = 0.05.
Correlations between the verbal fluency scores and resting FDG uptake in the whole brain were performed using SPM2. Considering the within group variability for age and the influence of this factor on cerebral metabolism [31], it was set as a confounding variable in a linear regression. Only correlations in the neurobiologically expected direction were conducted, i.e., positive correlations. We used a statistical threshold of P < 0.005 (uncorrected for multiple tests) for the voxels and a cutoff of k (corresponding to the number of voxels in a particular cluster) >150 to limit the attendant risk of false positives. Anatomical localization was based on Talairach’s atlas, using Brett’s set of linear transformations (see http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html) and the AAL toolbox [70].
Results
Demographic and general clinical data
There were no differences between the patient and control groups for age [F(2,51) = 0.61; P = 0.54] or educational level [F(2,51) = 2.25; P = 0.12]. There was a significant group effect for the MMSE score [F(2,51) = 26.4; P < 0.001], with significantly lower scores for both patient groups compared with controls, and no significant difference between the scores of the SD and fv-FTD patients. There was no difference between the patient groups regarding either age at onset of illness [t(34) = 1.08; P = 0.29] or duration of illness [t(34) = 1.14 P = 0.26] (Table 1).
Fluency task performances
Mean total scores (i.e., number of words produced without rule-breaking errors or perseverations) are shown in Fig. 1. A two-way ANOVA: 3 groups (fv-FTD, SD and controls) × 2 fluency tasks (semantic and phonemic) indicated a significant group effect [F(2,102) = 56.51; P < 0.0001] and a significant fluency task effect [F(1,102) = 15.22; p < 0.0002], but no significant interaction [F(2,102) = 2.36; P = 0.099]. Post-hoc analyses indicated that the performances of both patient groups were poorer than those of the controls on the semantic and phonemic fluency tasks. Fv-FTD patients produced more words in the semantic fluency task than SD patients, whereas there was no difference between the two patient groups on the phonemic task. In addition, like the control group, the fv-FTD patients performed better on the semantic fluency task, whereas there was no significant difference between the two tasks for the SD group.
Neuropsychological background tests
Regarding the total score on the semantic memory test, a one-way ANOVA revealed a group effect [F(2,51) = 18.07; P < 0.001]. Post-hoc analyses indicated that, unlike the fv-FTD patients, the SD patients scored more poorly than the controls. One-way ANOVAs indicated a group effect for both the FD-Span [F(2,51) = 7.1; P = 0.002] and FVS-Span [F(2,51) = 9.2; P < 0.001]. The SD patients’ performances did not differ from those of the controls, whereas the fv-FTD patients performed significantly worse than the controls and SD patients on the FD-Span. In the backward conditions, one-way ANOVAs indicated significant group effects for the BD-Span [F(2,51) = 12.9; P < 0.001] and the BVS-Span [F(2,51) = 9.7; P < 0.001]. In each condition, the patient groups’ performances, while no different from each other, were significantly poorer than those of the controls. For the updating score, a one-way ANOVA revealed a group effect [F(2,51) = 25.66; P < 0.001]. There was no difference between the fv-FTD and SD groups, and both differed from the controls, as indicated by the post-hoc analyses. Regarding the TMT, a two-way ANOVA: group (controls, fv-FTD and SD) × part (A and B) indicated a group effect [F(2,102) = 14.97; P < 0.001], a part effect [F(1,102) = 54.16; P < 0.001] and a marginal interaction effect [F(2,102) = 2.80; P < 0.066]. Post-hoc analyses did not indicate any significant differences among the three groups of subjects for part A. For part B, however, both patient groups performed more slowly than the controls, but in spite of a longer processing time for fv-FTD patients compared with SD patients, the difference between the two groups was not significant.
Correlation analyses
Interestingly, we observed significant correlations between the semantic and phonemic fluency scores within each patient group (r = 0.72; P > 0.001 for fv-FTD and r = 0.59; P = 0.01 for the SD), whereas there were no significant correlations for the controls (r = 0.30; p = 0.23). Table 2 shows the correlations for each patient group among the semantic and phonemic fluency scores and the semantic memory, working memory and updating scores and the processing times for the TMT parts A and B. Broadly speaking, significant correlations were observed between the two fluency scores and the semantic memory score in the SD group and mainly between the two fluency scores and the working memory and executives process scores in the fv-FTD group.
Regression analyses
Stepwise regression analyses were performed with the TMT part B processing time, semantic memory, FD-Span, BD-Span, BVS-Span and updating scores (i.e., scores correlated with the fluency performances of the fv-FTD and/or SD patients) in order to gauge their effectiveness as predictors of each fluency score in each patient group. In SD patients, the best predictor of both fluency scores was the semantic memory score, while the TMT part B processing time and working memory scores were the best predictors in fv-FTD. The full set of results is presented in Table 3.
Correlations between fluency scores and FDG uptake
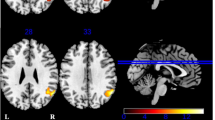
There were significant positive correlations among the fv-FTD group’s semantic fluency score and the normalized resting-state FDG uptake of the left and right frontal cortices, and, to a lesser degree, the right angular and inferior parietal gyri and the posterior part of the right insular cortex. For the phonemic fluency score, significant correlations were restricted to the right frontal inferior gyrus, extending to the posterior part of the insular cortex (Table 4; Fig. 2).
In the SD group, significant correlations concerning the semantic fluency score involved the left and posterior part of the inferior temporal and fusiform gyri, the posterior portion of the superior fontal gyrus and the supplementary motor area. As far as the phonemic fluency score was concerned, there was once again a significant correlation between the SD group’s total score and the normalized resting-state FDG uptake of the left temporal lobe, but this time its anterior part. One cluster involved the middle and superior temporal gyri and another the inferior temporal and fusiform gyri and the amygdala. Significant correlations also involved the left thalamus and caudate and left cerebellum (Table 4; Fig. 2).
Discussion
Cognitive study
We studied the semantic and phonemic fluency of 36 patients suffering from the frontal or temporal variants of frontotemporal dementia. The patient and control groups were rigorously matched for sex, age and educational level. We reported impaired performances for both groups of patients on both fluency tasks. The interaction between groups and types of fluency task was not significant, which may be due to the sample sizes and to the important variability of data. However, we observed differences between performances for both semantic and phonemic tasks for the two groups of patients. While patients suffering from fv-FTD performed better than SD patients on the semantic fluency task, we failed to observe any difference between the two groups in the phonemic fluency task. Previous studies have already reported verbal fluency impairment in fv-FTD [44, 56] and SD patients [36] compared with Alzheimer’s disease patients. However, although one or two studies have directly compared verbal fluency in fv-FTD and SD patients [59], none has investigated the underlying cognitive mechanisms of reported impairments.
The fv-FTD patients in our study presented considerably impaired performances on semantic fluency, whereas they performed normally on the semantic memory test assessing 30 items in different kinds of tasks (picture naming, categorization and specific questions). SD patients presented a similar impairment in phonemic fluency, but displayed a less severe executive impairment. These results confirm the multi-component nature of these tasks [62], suggesting that executive dysfunction contributes to low semantic fluency scores and that phonemic fluency also depends on the integrity of the lexical semantic store. The correlation and regression analyses we carried out unravelled the underlying mechanisms of verbal fluency impairment in fv-FTD and SD.
In the fv-FTD group, regression analyses for semantic fluency performances highlighted the extensive involvement of executive processes, especially set shifting and verbal working memory. Significant correlations were also found with the updating score, reflecting the fact that, given the limited capacity of working memory, the content of the phonological store had to be updated while the task was being performed. In the same vein, phonemic fluency performances were mainly related to set shifting and to verbal and visuospatial working memory storage capacities, witness the results of the regression and correlation analyses. One strategy for efficiently performing the phonemic fluency task consists in exploring the different words that can be produced on the basis of an initial phoneme. In addition to the high demand on the updating process, this also requires the subject to shift to another phoneme, once the one that has been selected no longer allows words to be produced. This process clearly involves the phonological loop, as reflected by the significant correlation between the phonemic fluency score and the FD-Span.
In the SD group, regression analyses clearly showed that performances depended mainly on the integrity of semantic memory, whichever fluency task was being performed. Correlation analyses also indicated links with additional cognitive functions. For the semantic fluency task, the significant correlations involved the BVS-Span and TMT scores, which may reflect the strategies implemented to perform the task. In addition to semantic memory, searching for various exemplars could involve visual imaging, especially for concepts defined primarily by their visual, form-related features, such as animals. Moreover, in order to produce a large number of animal names, subjects have to switch between the different subcategories of animals. Regarding phonemic fluency performance, in addition to the semantic memory abilities highlighted by the regression analysis, executive processes were also involved, as revealed by the significant correlations with the updating and BVS-Span scores.
Thus, the results of the cognitive study clearly indicate that, regardless of the nature of the fluency task, the fv-FTD patients’ performances were essentially related to their executive dysfunction, particularly set shifting abilities, whereas the SD patients’ performances depended mainly on their semantic memory abilities. This dichotomy between the underlying mechanisms of verbal fluency impairment in fv-FTD and SD could also be observed in the relative magnitude of deficits on the phonemic and semantic fluency tasks. The highly restricted semantic store of SD patients gives rise to extremely limited production whatever task is being performed. In fv-FTD patients, however, difficulties in retrieving words affect both fluency tasks to an equal degree, with the result that they maintain the performance profile observed in healthy subjects (i.e., with higher scores on semantic fluency than on phonemic fluency). As already highlighted in the meta-analytic review conducted by Henry and Crawford for focal cortical lesions [25], this result challenges the classic view that, compared with semantic fluency, phonemic fluency is more sensitive to executive dysfunction [47].
Correlation between fluency scores and FDG uptake
The difference between the more or less executive or semantic origins of verbal fluency impairment in fv-FTD and SD was also borne out by the metabolic study. In the fv-FTD group, our results indicated that semantic fluency performance was correlated with the metabolism of much of the frontal lobes, including the superior and middle gyri, bilaterally and the right inferior frontal gyrus. Consistent with the semantic nature of this task, the involvement of the left frontal lobe in semantic fluency has already been reported in Alzheimer’s disease, using the correlative method [14]. It has also been observed in activation studies of healthy subjects during semantic memory retrieval, for a review see [8]. The correlation we observed with the frontal lobe could also result from the involvement of executive processes in strategic retrieval from semantic memory. As demonstrated by our cognitive study, semantic fluency mainly involved set shifting in our group of fv-FTD patients (see above). This is consistent with the results of a recent study, which reported that performances on a nonverbal fluency task were specifically related to the volume of the left and right frontal lobes in fv-FTD patients [33]. The authors concluded that both frontal lobes play an important role in set shifting. Consistent with this idea, several functional imaging studies have also reported left-sided dorsolateral and medial frontal activity during various set shifting tasks, including the TMT [41, 71, 73], while other studies have reported a contribution from both frontal lobes [3]. In addition, the involvement of the frontal lobes that we have reported here is consistent with the involvement of the updating and verbal working memory capacities that we reported in the cognitive section of the study. The updating process specifically involves the left frontopolar cortex and the right inferior and superior frontal sulcus [11]. The prefrontal cortex in general has frequently been assigned a major role in working memory [23, 50]. Imaging studies have also demonstrated that bilateral parietal regions are engaged when verbal information has to be retrieved from short-term memory [13, 35]. It has been claimed that the left parietal cortex is the verbal short-term store [30, 45], whereas the right parietal cortex has been found to be involved in task switching [6, 74]. Correlations with the fv-FTD patients’ semantic fluency performances also involved the right frontal cortex. When they tested semantic fluency in normal subjects, Cardebat et al. [9] reported right dorsolateral and medial frontal activation, and assumed that it reflected the implementation of a cognitive strategy. Our findings suggest that this strategy consists in shifting between subcategories in order to conduct an in-depth exploration of the category in question.
Contrasting with the activation of an extensive frontal bilateral network observed for the semantic fluency task, the correlation analysis for the phonemic fluency task mainly highlighted the involvement of the right inferior frontal gyrus. Aron et al. [3] have suggested that this region may be especially involved in the inhibitory processes subtending switching, particularly the suppression of irrelevant responses. This idea is consistent with the involvement of these processes in phonemic fluency, which essentially requires the selection of words respecting the letter criterion from all the words that spring to mind on the basis of phonemic similarities.
Lastly, in both fluency tasks, we observed significant correlations with the metabolism of the right insula. These results may reflect the role of this region in aspects of phonological processing, such as rhyming and phonological verbal short-term memory; for a review, see [5].
Concerning the SD group, we found significant correlations between the scores on both fluency tasks and the left temporal lobe (Fig. 2), which is thought to subtend semantic representations [37]; for a review, see [22]. Interestingly, the anterior part of the inferior and superior temporal gyri and the posterior part of the inferior temporal and fusiform gyri would appear to be differentially activated in phonemic and semantic tasks. We believe that this distinction stems from the nature of the semantic representations activated in the different fluency tasks. All kinds of semantic representations may be used in a phonemic fluency task, whereas in the semantic fluency task, they are restricted to a specific category (animals in our study). Interestingly, the anterior temporal lobe is thought to ensure the modality-neutral representation of concepts [60, 69, 72], whereas the left inferior temporal gyrus is specifically involved in the semantic representation of living things [38]. We also found that the fusiform gyrus was associated with the visual features of semantic representations [12]. This result fits in with the sensory/functional theory that visually based categories activate ventral temporal regions that are typically allocated to visual object recognition.
The correlation analysis also indicated a link between semantic fluency performance and the left supplementary motor area and superior frontal area. This could reflect the involvement of working memory processes, as suggested by findings in normal subjects, e.g., [24]. We also observed significant correlations between phonemic fluency performance and the metabolism of the left thalamus and caudate, consistent with previous results in healthy subjects [46], which may be due to their connection with the prefrontal cortex, via the frontostriatal-thalamic circuits described by Alexander et al. [1]. Lastly, correlations with the cerebellum are consistent with the activation of this structure during word-generation tasks [32, 49].
Conclusion
In this study, we observed semantic and phonemic fluency impairment in fv-FTD and SD patients. Our results indicate that executive dysfunctions and semantic memory deficits may impact on both fluency tasks, but differentially so, according to the type of task and the nature of the pathology. They clearly demonstrate that the fv-FTD patients’ performances were primarily linked to executive functions and to the bilateral or right-sided metabolism of the frontal lobe, whereas the SD patients’ impaired performances on both fluency tasks are attributable mainly to a semantic memory disorder and to the hypometabolism of the left temporal lobe. Thus, semantic fluency impairment may be due either to executive dysfunction, especially the ability to shift between subcategories in fv-FTD, or to the deterioration of the semantic store in SD. Similarly, although the phonemic fluency task is thought mainly to involve executive and working memory functions, a semantic memory deficit drastically affects phonemic fluency performances. More specifically, we suggest that the inhibitory aspect of set shifting and the updating function are essential for carrying out phonemic fluency tasks. It is important to note that the relative magnitude of performances on semantic and phonemic fluency tasks could be used to draw inferences about the nature of the underlying deficit in standard examinations.
Abbreviations
- ANOVA:
-
Analysis of variance
- BD-Span:
-
Backward digit span
- BVS-Span:
-
Backward visuospatial span
- FD-Span:
-
Forward digit span
- FVS-Span:
-
Forward visuospatial span
- fv-FTD:
-
Frontal variant of frontotemporal dementia
- MMSE:
-
Mini-mental state examination
- PET:
-
Positron emission tomography
- PVE:
-
Partial volume effect
- SD:
-
Semantic dementia
- TMT:
-
Trail making test
References
Alexander MP, Benson DF, Stuss DT (1989) Frontal lobes and language. Brain Lang 37:656–691
Arbuthnott K, Frank J (2000) Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol 22:518–528
Aron AR, Monsell S, Sahakian BJ, Robbins TW (2004) A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain 127:1561–1573
Baddeley AD (1996) The fractionation of working memory. Proc Natl Acad Sci USA 93:13468–13472
Bamiou DE, Musiek FE, Luxon LM (2003) The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Brain Res Rev 42:143–154
Behrmann M, Geng JJ, Shomstein S (2004) Parietal cortex and attention. Curr Opin Neurobiol 14:212–217
Boxer AL, Miller BL (2005) Clinical features of frontotemporal dementia. Alzheimer Dis Assoc Disord 19(Suppl 1):S3–S6
Cabeza R, Nyberg L (2000) Neural bases of learning and memory: functional neuroimaging evidence. Curr Opin Neurol 13:415–421
Cardebat D, Demonet JF, Viallard G, Faure S, Puel M, Celsis P (1996) Brain functional profiles in formal and semantic fluency tasks: a SPECT study in normals. Brain Lang 52:305–313
Chételat G, Desgranges B, de la Sayette V, Viader F, Berkouk K, Landeau B, Lalevée C, Le Doze F, Dupuy B, Hannequin D, Baron JC, Eustache F (2003) Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain 126:1955–1967
Collette F, Hogge M, Salmon E, Van der Linden M (2006) Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience 139:209–221
D’Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippet LJ, Farah MJ (1997) A functional MRI study of mental image generation. Neuropsychologia 35:725–730
Davachi L, Maril A, Wagner AD (2001) When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci 13:1059–1070
Desgranges B, Baron JC, de la Sayette V, Petit-Taboué MC, Benali K, Landeau B, Lechevalier B, Eustache F (1998) The neural substrates of memory systems impairment in Alzheimer’s disease. A PET study of resting brain glucose utilization. Brain 121:611–631
Desgranges B, Baron JC, Giffard B, Chételat G, Lalevée C, Viader F, de la Sayette V, Eustache F (2002) The neural basis of intrusions in free recall and cued recall: a PET study in Alzheimer’s disease. NeuroImage 17:1658–1664
Desgranges B, Matuszewski V, Piolino P, Chételat G, Mézenge F, Landeau B, de la Sayette V, Belliard S, Eustache F (2007) Anatomical and functional alterations in semantic dementia: A voxel-based MRI and PET study. Neurobiol Aging 28:1904–1913
Eustache F, Desgranges B, Aupée AM, Guillery B, Baron JC (2000) Functional neuroanatomy of amnesia: positron emission tomography studies. Microsc Res Tech 51:94–100
Eustache F, Piolino P, Giffard B, Viader F, de la Sayette V, Baron JC, Desgranges B (2004) ‘In the course of time’: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain 127:1549–1560
Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR, DeCarli CS, Turner RS, Koeppe RA, Higdon R, Minoshima S (2007) FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 130:2616–2635
Galton CJ, Patterson K, Graham KS, Lambon-Ralph MA, Williams G, Antoun N, Sahakian BJ, Hodges JR (2001) Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology 57:216–225
Giffard B, Desgranges B, Nore-Mary F, Lalevée C, de la Sayette V, Pasquier F, Eustache F (2001) The nature of semantic memory deficits in Alzheimer’s disease: new insights from hyperpriming effects. Brain 124:1522–1532
Giffard B, Laisney M, Mézenge F, de la Sayette V, Eustache F, Desgranges B (2008) The neural substrates of semantic memory deficits in early Alzheimer’s disease: clues from semantic priming effects and FDG-PET. Neuropsychologia 46:1657–1666
Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RS, Dolan RJ (1993) Functional mapping of brain areas implicated in auditory–verbal memory function. Brain 116:1–20
Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR (2002) Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: an fMRI study with clinical implications. Brain 125:1024–1038
Henry JD, Crawford JR (2004) A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology 18:284–295
Hirono N, Mori E, Ishii K, Imamura T, Tanimukai S, Kazui H, Hashimoto M, Takatsuki Y, Kitagaki H, Sasaki M (2001) Neuronal substrates for semantic memory: a positron emission tomography study in Alzheimer’s disease. Dement Geriatr Cogn Disord 12:15–21
Hodges JR, Patterson K, Oxbury S, Funnell E (1992) Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain 115:1783–1806
Hodges JR, Patterson K, Tyler LK (1994) Loss of semantic memory: implications for the modularity of of mind. Cogn Neuropsychol 11:505–542
Hodges JR, Patterson K, Ward R, Garrard P, Bak T, Perry RJ, Gregory CA (1999) The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: a comparative neuropsychological study. Neuropsychology 13:31–40
Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR (1998) The role of parietal cortex in verbal working memory. J Neurosci 18:5026–5034
Kalpouzos G, Chételat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, Desgranges B (2009) Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging 30:112–124
Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC (1995) The neural substrates underlying word generation: a bilingual functional-imaging study. Proc Natl Acad Sci USA 92:2899–2903
Kramer JH, Quitania L, Dean D, Neuhaus J, Rosen HJ, Halabi C, Weiner MW, Magnotta VA, Delis DC, Miller BL (2007) Magnetic resonance imaging correlates of set shifting. J Int Neuropsychol Soc 13:386–392
Lekeu F, Van der Linden M, Chicherio C, Collette F, Degueldre C, Franck G, Moonen G, Salmon E (2003) Brain correlates of performance in a free/cued recall task with semantic encoding in Alzheimer disease. Alzheimer Dis Assoc Disord 17:35–45
Majerus S, Laureys S, Collette F, Del Fiore G, Degueldre C, Luxen A, Van der Linden M, Maquet P, Metz-Lutz MN (2003) Phonological short-term memory networks following recovery from Landau and Kleffner syndrome. Hum Brain Mapp 19:133–144
Marczinski CA, Kertesz A (2006) Category and letter fluency in semantic dementia, primary progressive aphasia, and Alzheimer’s disease. Brain Lang 97:258–265
Martin A (2001) Functional neuroimaging of semantic memory. In: Cabeza R, Kingstone A (eds) Handbook of functional neuroimaging cognition. MIT Press, Cambridge, pp 153–186
Martin A, Chao LL (2001) Semantic memory and the brain: structure and processes. Curr Opin Neurobiol 11:194–201
Martin A, Wiggs CL, Lalonde F, Mack C (1994) Word retrieval to letter and semantic cues: a double dissociation in normal subjects using interference tasks. Neuropsychologia 32:1487–1494
Matuszewski V, Piolino P, de la Sayette V, Lalevée C, Pélerin A, Dupuy B, Viader F, Eustache F, Desgranges B (2006) Retrieval mechanisms for autobiographical memories: insights from the frontal variant of frontotemporal dementia. Neuropsychologia 44:2386–2397
Moll J, de Oliveira-Souza R, Moll FT, Bramati IE, Andreiuolo PA (2002) The cerebral correlates of set-shifting: an fMRI study of the trail making test. Arq Neuropsiquiatr 60:900–905
Mummery CJ, Patterson K, Wise RJ, Vandenbergh R, Price CJ, Hodges JR (1999) Disrupted temporal lobe connections in semantic dementia. Brain 122:61–73
Neary D, Snowden JS, Gustafson L, Passant U, Stuss DT, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Pasquier F, Lebert F, Grymonprez L, Petit H (1995) Verbal fluency in dementia of frontal lobe type and dementia of Alzheimer type. J Neurol Neurosurg Psychiatry 58:81–84
Paulesu E, Frith CD, Frackowiak RS (1993) The neural correlates of the verbal component of working memory. Nature 362:342–345
Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, Perani D, Fazio F (1997) Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport 8:2011–2017
Perret E (1974) The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia 12:323–330
Perry RJ, Hodges JR (2000) Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology 54:2277–2284
Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988) Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 331:585–589
Petrides M, Alivisatos B, Meyer E, Evans AC (1993) Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA 90:878–882
Piolino P, Chételat G, Matuszewski V, Landeau B, Mézenge F, Viader F, de la Sayette V, Eustache F, Desgranges B (2007) In search of autobiographical memories: A PET study in the frontal variant of frontotemporal dementia. Neuropsychologia 45:2730–2743
Price CJ, Mummery CJ, Moore CJ, Frakowiak RS, Friston KJ (1999) Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J Cogn Neurosci 11:371–382
Quarantelli M, Berkouk K, Prinster A, Landeau B, Svarer C, Balkay L, Alfano B, Brunetti A, Baron JC, Salvatore M (2004) Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J Nucl Med 45:192–201
Quinette P, Guillery B, Desgranges B, de la Sayette V, Viader F, Eustache F (2003) Working memory and executive functions in transient global amnesia. Brain 126:1917–1934
Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW (1999) Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain 122:1469–1493
Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D (2007) Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology 21:20–30
Rauchs G, Piolino P, Mézenge F, Landeau B, Lalevée C, Pélerin A, Viader F, de la Sayette V, Eustache F, Desgranges B (2007) Autonoetic consciousness in Alzheimer’s disease: neuropsychological and PET findings using an episodic learning and recognition task. Neurobiol Aging 28:1410–1420
Reitan RM (1958) Validity of the Trail Making test as an indicator of organic brain damage. Percept Mot Skills 8:271–276
Rogers TT, Ivanoiu A, Patterson K, Hodges JR (2006) Semantic memory in Alzheimer’s disease and the frontotemporal dementias: a longitudinal study of 236 patients. Neuropsychology 20:319–335
Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K (2004) Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev 111:205–235
Rosser AE, Hodges JR (1994) The Dementia Rating Scale in Alzheimer’s disease, Huntington’s disease and progressive supranuclear palsy. J Neurol 241:531–536
Ruff RM, Light RH, Parker SB, Levin HS (1997) The psychological construct of word fluency. Brain Lang 57:394–405
Salmon E, Garraux G, Delbeuck X, Collette F, Kalbe E, Zuendorf G, Perani D, Fazio F, Herholz K (2003) Predominant ventromedial frontopolar metabolic impairment in frontotemporal dementia. NeuroImage 20:435–440
Snowden JS, Bathgate D, Varma AR, Blackshaw A, Gibbons ZC, Neary D (2001) Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 70:323–332
Snowden JS, Goulding PJ, Neary D (1989) Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol 2:167–182
Tomaszewski-Farias S, Jagust W (2004) Neuroimaging in non-Alzheimer’s dementias. Clin Neurosci Res 3:383–395
Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss DT (1998) Clustering and switching on verbal fluency: the effects of focal frontal- and temporal-lobe lesions. Neuropsychologia 36:499–504
Tulving E, Habib R, Nyberg L, Lepage M, McIntosh AR (1999) Positron emission tomography correlations in and beyond medial temporal lobes. Hippocampus 9:71–82
Tyler LK, Bright P, Dick E, Tavares P, Pilgrim L, Fletcher P, Greer MJ, Moss HE (2003) Do semantic categories activate distinct cortical regions? Evidence for a distributed neural semantic system. Cogn Neuropsychol 20:541–559
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289
Wager TD, Jonides J, Reading S (2004) Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage 22:1679–1693
Williams GB, Nestor PJ, Hodges JR (2005) Neural correlates of semantic and behavioural deficits in frontotemporal dementia. NeuroImage 24:1042–1051
Zakzanis KK, Mraz R, Graham SJ (2005) An fMRI study of the Trail making test. Neuropsychologia 43:1878–1886
Zurowski B, Gostomzyk J, Gron G, Weller R, Schirrmeister H, Neumeier B, Spitzer M, Reske SN, Walter H (2002) Dissociating a common working memory network from different neural substrates of phonological and spatial stimulus processing. NeuroImage 15:45–57
Acknowledgments
First, we are indebted to the patients, their families and to the control subjects for their willingness to devote such time and effort to this experiment. We also wish to thank Prof. F. Viader, Prof. D. Hannequin, Dr F. Ledoze, L. Bon, E. Bliaux, C. Descat, C. Giry, C. Lalevée, J. Lambert, N. Loisel, and A. Pélerin for their contributions to this study. M.L.’s research was funded by the Conseil Régional de Basse-Normandie, Eisai, Lundbeck, Novartis and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laisney, M., Matuszewski, V., Mézenge, F. et al. The underlying mechanisms of verbal fluency deficit in frontotemporal dementia and semantic dementia. J Neurol 256, 1083–1094 (2009). https://doi.org/10.1007/s00415-009-5073-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5073-y