Abstract
Exosome-encapsulated miRNAs could potentially be sensitive biomarkers of human diseases. Since a lipid bilayer membrane surrounds exosomes, the exosomal miRNA may stably exist in body fluids with diseases as well as biological fluids. Therefore, exosomal miRNA may be helpful for autopsy diagnosis. Assuming cadaver blood would be most useful, we initially examined serum exosome stability with regard to storage temperatures and periods. Characteristic analyses of the exosome revealed that exosomes and the content, miRNA, were stably preserved until at least three days when stored at below 20 °C. Subsequently, exosomal miRNA expression profiling was performed on the serum of acute myocardial infarction (AMI, 4 cases) autopsy bodies and on hemorrhagic shock bodies used as the control (CT, 3 cases). Results showed that significant twofold up- and downregulations of expression of 18 and 16 miRNAs were detectable in AMI as compared to the CT, respectively. miR-126-3p, which has been reported to be increased in serum of AMI patients and a mouse model, was one of the significantly upregulated miRNAs. Furthermore, dysregulation of exosomal miRNAs, such as miR-145-5p, miR-143-3p, and miR-222-3p, which are involved in cardioprotection, may be associated with AMI pathogenesis. These findings provide a novel perspective on the potential role of exosomal miRNA in determining the cause of death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exosomes, which are endogenous nano-sized (40–150 nm) extracellular vesicles (EVs), contain various types of functional molecules, such as messenger RNAs (mRNAs), microRNAs (miRNAs), DNAs, lipids, and proteins [1]. Depending on the tissue’s pathological and physiological states, exosomes transfer these molecules from host cells to recipient cells via biological fluids, such as blood, urine, saliva, bronchoalveolar lavage fluid, and milk. Exosomes function as pivotal mediators of cell-to-cell communication and have been well-documented to contribute to the pathogenesis of many diseases, such as cardiac disease [2,3,4], cancer [5, 6], and neurodegenerative disease [7]. These studies have suggested that exosomes can be used in clinical applications, such as diagnosis and treatment.
miRNAs are small non-coding RNAs that regulate the target gene expression at the post-transcriptional level [8]. The circulating miRNAs are transported to recipient cells after being packed in EVs, such as exosomes, or as protein-miRNA complexes [9]. Recent studies have shown that EV miRNA could potentially be non-invasive biomarkers of various human diseases, including cardiovascular disease [9, 10]. Since exosomal miRNAs encapsulated by a lipid bilayer membrane are protected from degradation by RNases, they should exist in a very stable state. Thus, exosomal miRNAs are likely to provide a more stable and specific miRNA pattern for disease-dependent pathological and physiological changes as compared to whole cell-free miRNAs. In cadavers, as time elapses, postmortem changes, such as blood coagulation and RNA and DNA degradation, progress. If the contents stably exist in body fluids after death, then the postmortem analyses of exosomal miRNAs may provide physiological information involved in the cause of death, as well as in a clinical diagnosis. However, there have yet to be any studies that have demonstrated whether exosomal miRNA analysis is applicable for autopsy diagnosis.
Acute myocardial infarction (AMI), which is one of the most critical complications of coronary artery disease worldwide, can cause high morbidity and mortality [11]. AMI is also one of the most common causes of sudden cardiac death seen in the field of forensic pathology [12]. Exosomal miRNAs are associated with the development and progression of various cardiovascular diseases [13]. After an AMI in humans, there is an acute increase in the number of EVs in the circulating plasma in accordance with the extent of the myocardial injury [14]. It has been reported that after an AMI, EVs are released from damaged endothelial cells that promote splenic monocyte mobilization and activation to the damaged myocardium [14]. Some EV miRNAs specific for AMI have been identified in humans and mice [14]. The investigation of miRNA expression profiling in exosomes may be helpful in exploring the physiological changes observed in a deceased person who died from AMI. If specific exosomal miRNAs could be detected in the body fluids of a cadaver, it would be primarily or supplementarily applicable to an autopsy diagnosis.
Our current study examined the application of exosomal miRNA for autopsy diagnosis. In the first step, we examined the stability of the exosomes in serum under different storage conditions found for the cadaver blood. Subsequently, we then performed exosomal miRNA expression profiling on the serum of AMI autopsy bodies (AMI, 4 cases) and on hemorrhagic shock bodies, which were used as a control (CT, 3 cases). We compared the exosomal miRNA differential expression between AMI and CT and evaluated its potential use in the determination of the cause of death.
Materials and methods
Chemicals
Chemicals and reagents used in this study were obtained as follows: anti-CD9 antibody (#sc-59140), anti-CD63 (#sc-5275) antibody, radioimmunoprecipitation (RIPA) buffer containing a protease inhibitor, phenylmethylsulfonyl fluoride (PMSF), and sodium orthovanadate from Santa Cruz Biotechnology (Santa Cruz, CA); polyvinylidene difluoride (PVDF) blocking reagent from Toyobo (Osaka, Japan); ECL™ Prime Western Detection Reagent from Cytiva (Marlborough, MA); horseradish peroxidase (HRP)-tagged anti-mouse immunoglobulin G (IgG) antibody from MBL (Nagoya, Japan); ExoQuick™ from System Biosciences (Palo Alto, CA); Trizol™ reagent, Halt™ Phosphatase Inhibitor Cocktail, Bicinchoninic Acid (BCA) Protein Assay Kit, and TaqMan MicroRNA assays from Thermo Fisher Scientific (Waltham, MA); miRNeasy Micro Kit from Qiagen (Hilden, Germany).
Human serum
The blood was collected for standard clinical tests in vessels containing the serum-separating agent and processed as per the manufacturer’s instructions at Nagoya City University Hospital. Mixtures of the remaining volumes were kept at 4 °C until use up to 2 days.
Exosome isolation and characterization
The serum samples were placed in a 1.5-mL polypropylene tube and stored at 4 °C, 20 °C, or 30 °C for 0, 1, 2, or 3 days. The exosome isolation procedure and characterization analyses were performed as per the details in our previous report [15]. The resulting exosomes fraction was used for RNA isolation and exosome characterization. Portions of the extracted exosome fraction were resuspended in PBS for morphological examination or lysed with RIPA buffer for western blotting. The existence of exosomes was confirmed by morphological observation using transmission electron microscopy (TEM) and by assessing the levels of CD9 and CD63 proteins using western blotting. Size distribution was analyzed by a dynamic light scattering method using a Zeta-potential & Particle Size Analyzer (ELS-Z2, Photo Otsuka Electronics, Osaka, Japan).
Exosomal RNA isolation and miRNA measurement
Total exosomal RNA was isolated and purified using Trizol™ reagent. Finally, the RNA was dissolved in 20 μL RNase-free water. The RNA concentration was quantified using a NanoDrop One spectrophotometer (Thermo Fisher Scientific). We selected hsa-miR-16, which is abundantly contained in serum exosome, as the target miRNA, and ath-miR-159a as the spike-in control. For miRNA detection, RT-qPCR was performed using the TaqMan MicroRNA assays with primers specific for hsa-miR-16 and ath-miR-159a, as per the manufacturer’s instructions. The PCR amplification was performed using a real-time PCR system (QuantStudio 12 K Flex, Thermo Fisher Scientific). Data were analyzed using the SDS 2.4 Real-Time PCR data analysis software.
Autopsy cases
A total of seven cases, four for the subject and three for the control, were selected for exosomal miRNA analysis. All cases were autopsied from 2017 to 2022 at the Department of Forensic Medicine, Nagoya City University Graduate School of Medical Sciences. AMI cases include recurrent myocardial infarction (AMI-1, -3, -4) and apparent new-onset one (AMI-2). Aside from coronary atherosclerosis, the former exhibited extensive fibrosis in the myocardium, whereas the latter had myocardial necrosis and inflammatory cell infiltration.
For the three control cases, all of the victims died from hemorrhagic shock due to stab wounds. Table 1 summarizes the case profiles. The cadavers were preserved for 4–17 h at room temperature (below 25 °C) and then stored at 4 °C (Table 1).
Serum collection from the autopsied bodies and exosome isolation
The blood samples were collected without anti-coagulant from 7 cadavers at autopsy and centrifuged at 1500 g for 10 min at 4 °C. After centrifugation, the resulting serum was stored at – 80 °C until use. The exosome extraction was prepared by ultracentrifugation with minor modification [16]. Briefly, an aliquot (0.5 mL) of serum was diluted with 9.5 mL PBS and centrifuged at 10,000 g for 20 min at 4 °C to remove debris followed by filtration through a 0.45-μm pore filter (Millex-HV Filter Unit, Merck Millipore, Darmstadt, Germany). The filtrated serum sample was ultracentrifuged at 100,000 g for 70 min at 4 °C. The pellet exosomes were washed with PBS, followed by ultracentrifugation at 100,000 g for 70 min at 4 °C. After resuspension of pellet exosomes in sterile PBS, the sample was used for the miRNA analysis.
miRNA extraction and analysis
The exosomal miRNA in serum was extracted using a miRNeasy micro kit in accordance with the manufacturer’s instructions. After miRNA extraction, the RNA was eluted in RNase-free water. The analyses of all cadavers were performed using the same amount of RNA (231 ng).
Analysis of miRNA array
The measurements of miRNA expression levels were conducted by GeneChip miRNA 4.0 Array (Thermo Fisher Scientific) in accordance with the manufacturer’s instructions. The array row data (the reads for each miRNA) were normalized, and the microarray analysis quality was checked by signal intensity range using Transcriptome Analyses Console Software version 4.0.1 (TAC, Thermo Fisher Scientific). Differential gene expression analysis with a heatmap was performed using TAC. Differentially expressed miRNAs with ≥ twofold or ≤ 0.5-fold changes in AMI cases were compared to CT cases and then identified.
Statistical analysis
Data are presented as means ± SEM. Statistical analyses were performed by ANOVA followed by Bonferroni’s post hoc analysis. The statistical significance level was set at p ≤ 0.05. For the miRNA expression, statistical analyses were performed by t-test.
Results
Characterization of exosomes
In order to examine the stability of serum exosome, exosome characterization was confirmed by Western blotting, size distribution, and TEM. As shown in Fig. 1A, both CD9 and CD63 proteins, which are common exosome protein markers, were abundantly detected in the lysate of serum-derived exosomes in the control sample without storage. CD63, a glycoprotein, was detected as a smeary 30- to 60-kDa band of glycosylated protein. Both protein levels were unchanged for up to 3 days at 4 °C. However, they were decreased in exosomes at 20 °C for 3 days. At 30 °C, both protein levels were day dependently decreased starting from 2 days onward. Results of the particle size distribution measurement by DLS showed there were a large number of particles with a diameter of 50–100 nm that were mainly present in both the control serum and serum stored at 4 °C (Fig. 1B). The size distributions were not significantly changed by storage temperatures or periods. However, whereas most of the exosome isolated from the serum stored at 4 °C exhibited a size distribution between 50 and 100 nm, there was the tendency for the samples stored at 20 °C and 30 °C to contain an increased number of smaller sized particles that were less than 33.2 nm. TEM analyses showed that exosomes were present up until 3 days at all of the temperatures (Fig. 1C).
Effects of storage temperatures and periods on the stability of serum exosome determined by western blot analyses of CD9 and CD63 levels (A), size distribution (B), and morphology (C). Human serum was stored at 4, 20, and 30 °C for 0 (controls without storage), 1, 2, and 3 days. Human serum-derived exosomes were isolated using a kit. A The lysate of the resulting exosome fraction was resolved by SDS-PAGE and electroblotted onto a PVDF membrane. The blot was probed with anti-CD9- and CD63-antibodies, followed by a corresponding HRP-tagged secondary antibody. Data are representative of three experiments. B Isolated exosomes were suspended in 100 μL PBS. The size of the exosomes was measured by dynamic light scattering. C The morphology of exosomes was examined by TEM. Typical exosomes are indicated by the yellow arrows. Scale bar = 100 nm
Stability of exosomal miRNA
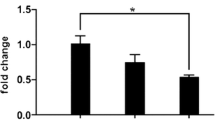
As shown in Fig. 2, the hsa-miR-16 expression level was unchanged in exosomes isolated from serum and stored at 4 °C and 20 °C for 3 days as compared to the control. However, the exosome level in the serum was reduced when stored at 30 °C for 3 days.
Effects of storage temperatures and periods on exosomal hsa-miR-16 expression. Human serum was stored at 4, 20, and 30 °C for 0 (control without storage) and 3 days. Total exosomal RNA was isolated from serum exosomes and purified using Trizol™ reagent. RT-qPCR was performed with TaqMan MicroRNA assays with primers specific for hsa-miR-16 (as a target) and ath-miR-159a (as a spike in the control). Data are presented as means ± SEM (N = 3). #p < 0.05, compared to the control group
Exosomal miRNA array analyses
The 34 miRNAs with significant changes in expression ≥ twofold upregulated or ≤ 0.5-fold downregulated in the comparison set (AMI versus CT) are represented in a heatmap (Fig. 3A). The heatmap of CT and AMI shows some similar expression patterns. Tables 2 and 3 list the changes in the 18 and 16 miRNAs that were upregulated ≥ twofold and downregulated ≤ 0.5-fold in the comparison set. Among the upregulated miRNAs, expression of miR-4505 showed the greatest fold change (Table 2). Conversely, miR-145-5p was strongly downregulated to less than one-tenth in the average of AMI versus CT (Table 3).
Exosomal miRNAs exhibiting significantly different changes in expression ≥ twofold or ≤ 0.5-fold when comparing the average of acute myocardial infarction (AMI) to that of the control (CT). A Heatmaps showing exosomal miRNA expression levels with a fold change ≥ 2 or with a fold change ≤ 0.5 in acute myocardial infarction (AMI) compared to control (CT). The intensity log2 plot: relatively higher (red) and lower (blue). B Comparison of ratios of has-miR-126-3p, has-miR-145-5p and has-miR-143-3p identified a significant change in gene expression. #p < 0.05, compared to the control group
Evaluation based on miRNA database and published reports
Among the 34 miRNAs with differential expression, those related to cardiovascular disease and function were evaluated using the miRBase version 22.1 and a search of the PubMed database (Table 4). The comparison of expression levels of exosomal miRNAs, miR-126-3p, miR-145-5p, and miR-143-3p, which are reported to be closely related to MI, are shown in Fig. 3B. The expression levels of miR-126-3p were significantly increased in AMI, while those of miR-145-5p and 143-3p were significantly decreased in AMI.
Discussion
The search for and diagnosis of lesions in forensic autopsy are generally based on gross and histopathological examinations. As the latter takes several days to complete, it can sometimes be challenging to morphologically detect early changes, for example, AMI. In addition to conventional examinations, it would be helpful in autopsy diagnosis investigations if indicators were available and could be quickly and stably detected in body fluids of cadavers. Therefore, in common with clinical diagnosis, the introduction of sensitive and specific biomarkers is desirable for pathological forensic investigations. Recent studies have reported that exosome-encapsulated miRNA is one of the candidates for use as sensitive biomarkers [10]. However, there have yet to be any reports that have examined the exosome stability in cadaver body fluids or the application of the fluid examination during autopsy diagnosis. Although there have been some reports on the stability of EVs in biofluids under various storage conditions [2, 17], most of these studies evaluated and focused on the freezing temperatures and the periods for storage [18].
Only a few studies have examined the stability that occurs at room temperature [2, 19]. In clinical cerebrospinal fluid, EV number and morphology, total RNA, and representative miRNAs have been reported to be well preserved at room temperature for at least up to 7 days [2]. Furthermore, cell-derived EVs only showed minor changes in size, EVs concentration, and protein concentration when stored at 37 °C for 24 h [19]. Our current results are consistent with these previous studies. In our present study, Western blotting (Fig. 1A), morphological analyses (Fig. 1B, C), and quantitative analysis for miRNA (Fig. 2) revealed that exosomes were stably preserved up until at least 3 days when maintained at 4 °C and 20 °C, and 1 day at 30 °C, respectively. These results led to the expectation that an analysis of exosomes in cadavers could potentially provide some information on the internal conditions that lead to the death. Therefore, we selected seven autopsy cases for use in examining the postmortem period and cause of death. The exosomes were expected to be stable in these seven selected cases because the bodies were left at room or ambient temperature for 4–17 h after death and then refrigerated at 4 °C until autopsy (Table 1). Four AMI and three stabbing cases were assigned as the subjects and controls, respectively. These cadavers were used to experimentally investigate whether exosomes in the fluid sample taken at autopsy could be applicable for subsequent pathological analyses.
Our study performed differential miRNA expression analysis of the serum exosome between the AMI and CT cases. Significant twofold up- and downregulations of the expression of 18 and 16 miRNAs were detectable in AMI compared to the CT, respectively. As shown in Table 4, some of the detected exosomal miRNAs are involved in cardiac diseases. Intriguingly, among the detected upregulated miRNAs, exosomal miR-126-3p has been reported to be closely associated with AMI [14, 20, 21]. miR-126-3p and -5p in plasma EVs derived from endothelial cells were highly regulated in human plasma after AMI [14]. Duan et al. reported that levels of exosomal miR-126-3p, a crucial regulator of angiogenesis that is necessary to improve myocardial function after MI, were increased in serum of AMI patients within 12 h of the onset of chest pain [20]. Furthermore, Ling et al. reported that serum levels of exosomal miR-126-3p were increased within 2 h after AMI and that the levels positively correlated with the severity of coronary artery stenosis [21]. The serum exosomal miR-126 and phosphatase and tensin homolog (PTEN) protein levels could potentially be a new diagnostic biomarker for AMI [21]. Since miR-126-3p levels increase rapidly after AMI, serum exosomal miR-126-3p may not only be a diagnostic biomarker, but also a useful indicator in autopsy diagnosis of AMI.
In contrast, among the downregulated miRNAs, it is noteworthy that levels of miR-145-5p and miR-143-3p were significantly reduced in AMI as compared to the CT. Both miR-145 and its co-transcribed cluster member miR-143 are vitally important for proper function of smooth muscle cells (SMCs). Atheroprotective stimuli induce communication between endothelial cells and SMCs via EV-enriched miR-143/145 clusters to combat atherosclerosis [22]. Furthermore, endothelial-derived miR-143/145 clusters are more preferentially released and stabilized by EVs than other miRNAs [22]. Taken together, miR-145 and miR-143 could be good exosomal miRNA candidates for predicting ischemic heart disease. One of the downregulated miRNAs, miR-222, has also been reported to regulate essential physiological vascular processes, such as angiogenesis and vessel wound healing [23]. In a mouse model and embryonic rat heart-derived cells, miR-222 and its co-transcribed cluster member miR-221 have been shown to mediate the release of exosomes from adipose-derived stem cells (ADSCs) as a form of cardioprotection against myocardial ischemia–reperfusion injury [24]. Furthermore, circulating miRNAs, such as miR-145, miR-143, miR-222-3p, and miR-101a-5p, but not limited to those in EVs, reportedly have cardioprotective effects via angiogenesis [25] and inhibition of autophagy [26]. Thus, reductions in these miRNAs may exacerbate MI, which could subsequently trigger sudden death.
Results from miRNA profiling have demonstrated that disease-specific miRNAs can be found in vitreous humor exosomes as well as serum exosomes [27]. Therefore, analyses of various kinds of body fluids obtained at autopsy, such as urine and vitreous humor, could also potentially be helpful for an autopsy diagnosis and may reveal new specific indicators for the cause of death.
A limitation of the present study is that the stability of exosomes was examined in an in vitro experiment. The serum of actual cadavers may be affected by various factors, such as hemolysis and coagulation of the blood. Thus, further studies are needed to apply exosomal miRNA for autopsy diagnosis. In addition, since only seven autopsy cases were used in this experiment, more cases are required to be observed in the future in order to determine disease-specific miRNAs. Nonetheless, the present results demonstrated that some of the exosomal miRNAs with significant up- and downregulation in AMI cases are closely involved in cardiac diseases and suggested that exploiting the exosomal miRNAs could be feasible in determining the cause of death.
In conclusion, exosomes and the enclosed miRNA in serum are stably preserved up until at least 3 days at 4 °C and 20 °C. The application of actual autopsy samples made it possible to detect differentially expressed miRNAs with significant twofold up- and downregulations for 18 and 16 miRNAs in cases of AMI compared to the CT, respectively. miR-126-3p, the expression of which changes rapidly in response to AMI, was also detected as one of the highly upregulated exosomal miRNAs in AMI compared to the CT. Furthermore, among the remarkably downregulated miRNAs, dysregulation of exosomal miRNAs, such as miR-145-5p, miR-143-3p, and miR-222-3p, which are involved in cardioprotection, may be associated with the pathogenesis of AMI. Although further studies are required, our current study findings provide insight into the potential use of exosomal miRNAs not only for a clinical diagnosis but also when trying to determine the cause of death at autopsy.
References
Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75:193–208. https://doi.org/10.1007/s00018-017-2595-9
Akers JC, Ramakrishnan V, Yang I, Hua W, Mao Y, Carter BS, Chen CC (2016) Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark 17:125–132. https://doi.org/10.3233/CBM-160609
Iaconetti C, Sorrentino S, De Rosa S, Indolfi C (2016) Exosomal miRNAs in heart disease. Physiol (Bethesda) 31:16–24. https://doi.org/10.1152/physiol.00029.2015
Barile L, Moccetti T, Marban E, Vassalli G (2017) Roles of exosomes in cardioprotection. Eur Heart J 38:1372–1379. https://doi.org/10.1093/eurheartj/ehw304
Kinoshita T, Yip KW, Spence T, Liu FF (2017) MicroRNAs in extracellular vesicles: potential cancer biomarkers. J Hum Genet 62:67–74. https://doi.org/10.1038/jhg.2016.87
Jaiswal R, Sedger LM (2019) Intercellular vesicular transfer by exosomes, microparticles and oncosomes - implications for cancer biology and treatments. Front Oncol 9:125. https://doi.org/10.3389/fonc.2019.00125
Russo I, Bubacco L, Greggio E (2012) Exosomes-associated neurodegeneration and progression of Parkinson’s disease. Am J Neurodegener Dis 1:217–225
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355. https://doi.org/10.1038/nature02871
Mori MA, Ludwig RG, Garcia-Martin R, Brandao BB, Kahn CR (2019) Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab 30:656–673. https://doi.org/10.1016/j.cmet.2019.07.011
Liu T, Zhang Q, Zhang J, Li C, Miao YR, Lei Q, Li Q, Guo AY (2019) EVmiRNA: a database of miRNA profiling in extracellular vesicles. Nucleic Acids Res 47:D89–D93. https://doi.org/10.1093/nar/gky985
Nabel EG, Braunwald E (2012) A tale of coronary artery disease and myocardial infarction. N Engl J Med 366:54–63. https://doi.org/10.1056/NEJMra1112570
Markwerth P, Bajanowski T, Tzimas I, Dettmeyer R (2021) Sudden cardiac death-update. Int J Legal Med 135:483–495. https://doi.org/10.1007/s00414-020-02481-z
Zheng D, Huo M, Li B, Wang W, Piao H, Wang Y, Zhu Z, Li D, Wang T, Liu K (2020) The role of exosomes and exosomal microRNA in cardiovascular disease. Front Cell Dev Biol 8:616161. https://doi.org/10.3389/fcell.2020.616161
Akbar N, Digby JE, Cahill TJ, Tavare AN, Corbin AL, Saluja S, Dawkins S, Edgar L, Rawlings N, Ziberna K, McNeill E, Oxford Acute Myocardial Infarction S, Johnson E, Aljabali AA, Dragovic RA, Rohling M, Belgard TG, Udalova IA, Greaves DR, Channon KM, Riley PR, Anthony DC, Choudhury RP (2017) Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight 2 https://doi.org/10.1172/jci.insight.93344
Kanno S, Hirano S, Sakamoto T, Furuyama A, Takase H, Kato H, Fukuta M, Aoki Y (2020) Scavenger receptor MARCO contributes to cellular internalization of exosomes by dynamin-dependent endocytosis and macropinocytosis. Sci Rep 10:21795. https://doi.org/10.1038/s41598-020-78464-2
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285:17442–17452. https://doi.org/10.1074/jbc.M110.107821
Park SJ, Jeon H, Yoo SM, Lee MS (2018) The effect of storage temperature on the biological activity of extracellular vesicles for the complement system. In Vitro Cell Dev Biol Anim 54:423–429. https://doi.org/10.1007/s11626-018-0261-7
Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z (2014) miRNA in plasma exosome is stable under different storage conditions. Molecules 19:1568–1575. https://doi.org/10.3390/molecules19021568
Schulz E, Karagianni A, Koch M, Fuhrmann G (2020) Hot EVs - How temperature affects extracellular vesicles. Eur J Pharm Biopharm 146:55–63. https://doi.org/10.1016/j.ejpb.2019.11.010
Duan S, Wang C, Xu X, Zhang X, Su G, Li Y, Fu S, Sun P, Tian J (2022) Peripheral serum exosomes isolated from patients with acute myocardial infarction promote endothelial cell angiogenesis via the miR-126-3p/TSC1/mTORC1/HIF-1α pathway. Int J Nanomed 17:1577–1592. https://doi.org/10.2147/ijn.S338937
Ling H, Guo Z, Shi Y, Zhang L, Song C (2020) Serum exosomal microRNA-21, microRNA-126, and PTEN are novel biomarkers for diagnosis of acute coronary syndrome. Front Physiol 11:654. https://doi.org/10.3389/fphys.2020.00654
Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S (2012) Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14:249–256. https://doi.org/10.1038/ncb2441
Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV (2015) Human miR-221/222 in physiological and atherosclerotic vascular remodeling. Biomed Res Int 2015:354517. https://doi.org/10.1155/2015/354517
Lai TC, Lee TL, Chang YC, Chen YC, Lin SR, Lin SW, Pu CM, Tsai JS, Chen YL (2020) MicroRNA-221/222 mediates ADSC-exosome-induced cardioprotection against ischemia/reperfusion by targeting PUMA and ETS-1. Front Cell Dev Biol 8:569150. https://doi.org/10.3389/fcell.2020.569150
Pang J, Ye L, Chen Q, Wang J, Yang X, He W, Hao L (2020) The effect of microRNA-101 on angiogenesis of human umbilical vein endothelial cells during hypoxia and in mice with myocardial infarction. Biomed Res Int 2020:5426971. https://doi.org/10.1155/2020/5426971
Higashi K, Yamada Y, Minatoguchi S, Baba S, Iwasa M, Kanamori H, Kawasaki M, Nishigaki K, Takemura G, Kumazaki M, Akao Y, Minatoguchi S (2015) MicroRNA-145 repairs infarcted myocardium by accelerating cardiomyocyte autophagy. Am J Physiol Heart Circ Physiol 309:H1813–H1826. https://doi.org/10.1152/ajpheart.00709.2014
Ragusa M, Barbagallo C, Statello L, Caltabiano R, Russo A, Puzzo L, Avitabile T, Longo A, Toro MD, Barbagallo D, Valadi H, Di Pietro C, Purrello M, Reibaldi M (2015) miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol Ther 16:1387–1396. https://doi.org/10.1080/15384047.2015.1046021
Zhao Z, Liu G, Zhang H, Ruan P, Ge J, Liu Q (2021) BIRC5, GAJ5, and lncRNA NPHP3-AS1 are correlated with the development of atrial fibrillation-valvular heart disease. Int Heart J 62:153–161. https://doi.org/10.1536/ihj.20-238
Hou J, Wang J, Lin C, Fu J, Ren J, Li L, Guo H, Han X, Liu J (2014) Circulating microRNA profiles differ between Qi-stagnation and Qi-deficiency in coronary heart disease patients with blood stasis syndrome. Evid Based Complement Alternat Med 2014:926962. https://doi.org/10.1155/2014/926962
Gryshkova V, Lushbough I, Palmer J, Burrier R, Delaunois A, Donley E, Valentin JP (2022) MicroRNAs signatures as potential biomarkers of structural cardiotoxicity in human-induced pluripotent stem-cell derived cardiomyocytes. Arch Toxicol https://doi.org/10.1007/s00204-022-03280-8
Jordan NP, Tingle SJ, Shuttleworth VG, Cooke K, Redgrave RE, Singh E, Glover EK, Ahmad Tajuddin HB, Kirby JA, Arthur HM, Ward C, Sheerin NS, Ali S (2021) MiR-126-3p Is dynamically regulated in endothelial-to-mesenchymal transition during fibrosis. Int J Mol Sci 22 https://doi.org/10.3390/ijms22168629
Xue S, Liu D, Zhu W, Su Z, Zhang L, Zhou C, Li P (2019) Circulating MiR-17-5p, MiR-126-5p and MiR-145-3p are novel biomarkers for diagnosis of acute myocardial infarction. Front Physiol 10:123. https://doi.org/10.3389/fphys.2019.00123
Li M, Ren C, Wu C, Li X, Li X, Mao W (2020) Bioinformatics analysis reveals diagnostic markers and vital pathways involved in acute coronary syndrome. Cardiol Res Pract 2020:3162581. https://doi.org/10.1155/2020/3162581
Xu C, Jia Z, Cao X, Wang S, Wang J, An L (2022) Hsa_circ_0007059 promotes apoptosis and inflammation in cardiomyocytes during ischemia by targeting microRNA-378 and microRNA-383. Cell Cycle 21:1003–1019. https://doi.org/10.1080/15384101.2022.2040122
Eyyupkoca F, Ercan K, Kiziltunc E, Ugurlu IB, Kocak A, Eyerci N (2022) Determination of microRNAs associated with adverse left ventricular remodeling after myocardial infarction. Mol Cell Biochem 477:781–791. https://doi.org/10.1007/s11010-021-04330-y
Su Y, Sun Y, Tang Y, Li H, Wang X, Pan X, Liu W, Zhang X, Zhang F, Xu Y, Yan C, Ong SB, Xu D (2021) Circulating miR-19b-3p as a novel prognostic biomarker for acute heart failure. J Am Heart Assoc 10:e022304. https://doi.org/10.1161/jaha.121.022304
Corsten MF, Heggermont W, Papageorgiou AP, Deckx S, Tijsma A, Verhesen W, van Leeuwen R, Carai P, Thibaut HJ, Custers K, Summer G, Hazebroek M, Verheyen F, Neyts J, Schroen B, Heymans S (2015) The microRNA-221/-222 cluster balances the antiviral and inflammatory response in viral myocarditis. Eur Heart J 36:2909–2919. https://doi.org/10.1093/eurheartj/ehv321
Zhang Q, Kandic I, Kutryk MJ (2011) Dysregulation of angiogenesis-related microRNAs in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun 405:42–46. https://doi.org/10.1016/j.bbrc.2010.12.119
Verjans R, Peters T, Beaumont FJ, van Leeuwen R, van Herwaarden T, Verhesen W, Munts C, Bijnen M, Henkens M, Diez J, de Windt LJ, van Nieuwenhoven FA, van Bilsen M, Goumans MJ, Heymans S, González A, Schroen B (2018) MicroRNA-221/222 family counteracts myocardial fibrosis in pressure overload-induced heart failure. Hypertension 71:280–288. https://doi.org/10.1161/hypertensionaha.117.10094
Acknowledgements
We acknowledge the assistance of the Research Equipment Sharing Center at Nagoya City University. We would like to thank Mr. Hiroshi Takase of the Research Equipment Sharing Center of Nagoya City University for his assistance with the transmission electron microscopic observations, and Dr. Seishiro Hirano of the National Institute for Environmental Studies for his help with the dynamic light scattering analysis and suggestions.
Funding
JSPS KAKENHI grant (number 21K10526).
Author information
Authors and Affiliations
Contributions
S. K. and T. S.: conceived and designed the experiments; S. K. and T. S.: performed the experiments and data analyses; M. F., H. K., and Y. A.: performed the autopsies and examinations; S. K. wrote the manuscript with contributions from co-authors; Y. A.: project administration.
Corresponding author
Ethics declarations
Ethical approval
This study in use of human serum and autopsy samples was approved by the Institutional Review Board for the Protection of Human Subjects in Research at Nagoya City University (approval number: 60–18-0151 and 60–21-0144), respectively.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Data availability
All data have been reported in the manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanno, S., Sakamoto, T., Fukuta, M. et al. Stability of exosomes in the postmortem serum and preliminary study on exosomal miRNA expression profiling in serum from myocardial infarction cadavers. Int J Legal Med 137, 825–834 (2023). https://doi.org/10.1007/s00414-022-02913-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-022-02913-y