Abstract
In recent decades, several methods based on biochemical and molecular changes caused by aging have been proposed to improve the accuracy of forensic age estimation. The present study aimed to measure changes in furosine and pentosidine, two markers of non-enzymatic glycation of proteins (NEGs), in human dentine and clavicle with aging, and to identify possible differences between turnover rates in different mineralized tissues. Furosine and pentosidine were quantified in 32 dentine samples from living donors between 14 and 80 years of age, and in a second group of samples consisting of a tooth and a piece of clavicle collected from the same cadaver (15 individuals aged 18 to 85 years). Furosine concentration was much higher than pentosidine concentration in the same tissue, although they were strongly correlated in both dentine and bone. A close relationship between furosine and/or pentosidine content and chronological age was found in both tissues (r > 0.93). Moreover, age estimation was more accurate when furosine or pentosidine content was determined in dentine, with specificity values for the tests higher than 82% in all age groups. In clavicle, furosine concentration and pentosidine concentration were much lower (2.6-fold and 3.1-fold, respectively) than in dentine from the same individuals. In conclusion, although the results show strong correlations between chronological age and furosine or pentosidine concentrations determined in mineralized tissues, there is still a need for further research with larger data sets, including patients with diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The estimation of chronological age in both living and deceased individuals is a recurrent issue in forensic sciences. The most common methods available for forensic age determination are based on the study of physiological changes that occur in dental and skeletal structures with aging. However, the accuracy of these procedures is significantly reduced in adulthood, and they cannot be applied when morphological information is limited [1]. Therefore, in the last three decades, biochemical and molecular biology studies have been performed in different tissues in order to develop new diagnostic tools to estimate age in unidentified human remains [2,3,4].
After death, human bone and tooth tissues may undergo different stages of modification until complete decomposition or fossilization [5,6,7,8]. The composition of bone and teeth in buried cadavers is the result of changes that occur after death but prior to burial [7, 8], and during the post-burial phase, i.e., diagenesis [7]. Defined as post-mortem biochemical alterations in bones, diagenetic processes are highly heterogeneous due to post-depositional time, taphonomic conditions, and the burial environment [6, 9,10,11]. Despite these challenges, molecular studies of mineralized tissues are of great importance in forensic practice given that bone and teeth are the human tissues most resistant to degradation and putrefaction under harsh conditions [12, 13].
The natural process of aging leads to alterations in tissues and organs over a lifetime. Among the many changes that are detectable with biochemical methods, two fundamental protein alterations are observed during aging: the racemization of aspartic acid and the generation of advanced glycation end-products [2]. Conversion of the L to the D form of aspartic acid with age has been studied in different tissues and shows an almost linear increase with age, dentine being the tissue of choice for estimating age with the aspartic acid racemization method [14].
Human bone and dentine collagen constitute almost 90% of the organic matrix [15, 16]. To stabilize the collagen network, covalent cross-links exist between the collagen molecules. Collagen and cross-links are biochemically important in mineralized tissues, providing plastic, ductile properties, whereas the mineral component confers stiffness [16]. It has been demonstrated that the content of cross-links in human bone, articular cartilage, and dentine tissues changes with age [17,18,19].
Collagen cross-links can also be produced spontaneously by other mechanisms such as non-enzymatic glycation of proteins (NEGs) [20,21,22], the so-called Maillard reaction. This reaction can be subdivided into three main stages. In the early stage, glucose (or other reducing sugars such as fructose, galactose, mannose, ribose, and xylulose) reacts with a free amino group in different molecules, including proteins, nucleic acids, and lipids, to form a stable ketoamide, the Amadori compound [20,21,22]. Under in vivo conditions, the Amadori compound accumulates in both short-lived and long-lived proteins. In the intermediate stage, the Amadori product degrades into a variety of carbonyl compounds (glyoxal, methylglyoxal, and 3-deoxyglucosone). Finally, in the late stage, the carbonyl compounds react with amino acids and the Amadori product undergoes complex rearrangements, cleavage, and covalent binding reactions which lead to the formation of stable adducts and protein cross-links usually called advanced glycation end-products (AGEs), which accumulate on long-lived proteins and cause damage [22]. The formation of AGEs in vitro and in vivo is dependent on the turnover rate of the chemically modified protein target, time, and sugar concentration [20,21,22]. The early stages of the Maillard reaction can be studied by measuring furosine (ε-N-(furoylmethyl)-L-lysine), an amino acid formed during acid hydrolysis of the Amadori compound fructosyl-lysine, produced by the reaction of ε-amino groups in lysine with glucose. Furosine is currently the most specific and important indicator of the early Maillard reaction [22]. In another approach to determining turnover rates, glycoxidation of tissue proteins can be assessed by measuring pentosidine [23]. This protein-bound AGE is produced in the final stage of the Maillard reaction pathway from the reaction of dicarbonyl compounds with lysine and arginine residues in proteins [24].
The products of NEGs are generated over a period of weeks, thereby affecting long-lived proteins. Structural components of the connective tissue matrix or basement membrane, such as collagen, are prime targets [20] which undergo cross-link formation. The pathological cross-links induced by NEGs lead to increased stiffness in the protein matrix, hence impeding function as well as increasing resistance to removal by proteolysis, which in turn affects the process of tissue remodeling [25]. These changes occur with advancing age and are accelerated by hyperglycemia, leading to the gradual development of diabetic complications [20,21,22,23,24]. Different authors have found that Maillard products (including furosine or pentosidine) accumulate in collagen from the skin [23, 24], cartilage [26, 27], aorta [28], human lens [29], bone [30,31,32,33,34], dentine [35,36,37], and pericardial fluid [38] with age.
The aim of this study was to measure changes in NEG products in human mineralized tissues with aging. For this purpose, furosine as an indicator of the early Maillard reaction and pentosidine as a representative of glycoxidation of tissue proteins produced in the final stages of the Maillard reaction pathway were quantified in samples of dentine and clavicle from human individuals of different known ages. In addition, these compounds were compared in human tooth and bone—representing different body compartments—from the same individuals to analyze possible differences between mineralized tissue turnover rates in relation to aging. It was hypothesized that measurements of furosine and pentosidine might be useful for studying aging changes, and therefore for age estimation, from dental and bone tissues in forensic cases.
Material and methods
Material
The samples for this study were obtained from two different sources. Group I comprised material from 32 healthy erupted human permanent teeth (1 central incisor, 2 first premolars, 2 s premolars, 5 first molars, 7 s molars, and 15 third molars; Table 1) extracted for valid clinical reasons (periodontal disease, malocclusion, or orthodontic treatment), obtained from the Department of Oral Surgery at Virgen de las Nieves Hospital in Granada (Spain), and from private dental clinics. Samples in group II consisted of a permanent tooth (6 first premolars, 8 s premolars, and 1 first molar; Table 2) and a piece of clavicle (medial third) from the same individual. These samples were collected from a total of 15 cadavers autopsied at the Institute of Forensic Medicine of the University of Copenhagen (Denmark), all with a known time and cause of death (Table 2), and without clinical evidence of a previous history of diabetes. The corpses were kept at 4 °C until the autopsies were performed within a period ranging between 24 and 48 h after death. The protocols to collect samples from human subjects were approved by the corresponding Ethics Committees for Human Research of the University of Granada (Spain) and the University of Copenhagen (Denmark), and the study was conducted in accordance with the ethical standards laid down by the Declaration of Helsinki.
Extraction of dentine and bone proteins
After removal from the body, human tissue samples were frozen and kept at − 40 °C until biochemical analysis. Tissue preparation and protein extraction were done as described previously for human dentine [19] with some modifications. Briefly, each dentine or bone sample was frozen with liquid nitrogen, crushed by pounding with a steel hammer on a cold metallic surface and then weighed. The dentine or bone powder obtained was placed in a 6000 Da cut-off Spectator dialysis tube with 0.6 M HCl solution and proteinase inhibitor. Then, the samples were thoroughly dialyzed and demineralized at 4 °C with constant magnetic stirring against large volumes of 0.6 M HCl solution, changed every 2 days over a total period of 6 days. Finally, the demineralized residue was obtained by centrifugation (20,000g, 50 min, 4 °C) and then frozen at − 20 °C until analysis.
Furosine, pentosidine, and collagen quantification
Furosine was measured with a previously described method [39]. Briefly, 50 mg of the demineralized sample, weighed with analytical accuracy, was hydrolyzed with 1-mL 6.0 M HCl at 110 °C for 23 h in a Pyrex screw-cap vial with PTFE-faced septa. High-purity N2 gas was bubbled through the solution for 2 min. A 0.5-mL portion of the filtrate was applied to a Sep-pack C18 cartridge (Millipore, Billerica, MA, USA) pre-wetted with 5 mL methanol and 10 mL deionized water and was then eluted with 3-L 3 M HCl and evaporated under a vacuum [40]. The dried sample was dissolved in 0.2 mL of a mixture of water, acetonitrile, and formic acid (95:5:0.2) and 50 μL of the resulting solution was injected into a liquid chromatograph (Perkin-Elmer model 250, Norwalk, CT) equipped with an autosampler (Waters 717plus, Milford, MA) and a diode array detector (Perkin-Elmer model 235). Data were collected with a software data system (Perkin-Elmer). The furosine was separated using a Spherisorb ODS2 5 μm column (250 mm × 4.6 mm i.d., Phenomenex, Torrance, CA, USA). The mobile phase consisted of a solution 5 mM sodium heptane sulfonate in 20% acetonitrile and 0.2% formic acid [41]. Elution was done at isocratic temperature and a flow rate of 1.2 mL/min. The UV detector was set at 280 nm. Duplicate samples were analyzed. The external standard method was used for quantification. A standard stock solution containing 1.2 mg/mL furosine (NeoMPS, Strasbourg, France) was used to prepare the working standard solution. The method was calibrated with a furosine standard curve (R2 = 0.9997).
Pentosidine was determined according to the method described by Takahashi et al. [42] with minor modifications. Sample hydrolysis was performed as described previously for furosine, but for pentosidine, the hydrolysate was not purified with Sep-pack cartridges. A 1-mL portion of the hydrolysate was evaporated under a vacuum and the dried sample was dissolved in 0.2 mL water. Finally, 50 μL of sample was injected into a liquid chromatograph (Jasco LC Pump, model PU-2089, Jasco Corporation, Madrid, Spain) coupled to a fluorescence detector (Jasco, model FP-2020) and a computing integrator connected to a PC. Pentosidine was separated on an ODS-B 5 μm column (Tracer Excel 120 ODS-B 5 μm, 250 mm × 4.0 mm i.d., Tecknokroma, Barcelona, Spain) thermostated at 32 °C. The mobile phase consisted of a mixture of 22% acetonitrile in water with 20 mM heptafluorobutyric acid as a counterion. Elution was isocratic at a flow rate of 1 mL/min. The fluorescence detector was set at λex = 335 nm and λem = 385 nm. Duplicate samples were analyzed. The external standard method was used for quantification. A standard stock solution containing 245 pmol/mL pentosidine (kindly donated by Prof. V. M. Monnier, Case Western Reserve University, Cleveland, OH, USA) was used to prepare the working standard solution. Calibration was done with a pentosidine standard curve (R2 = 0.9994).
Collagen was determined by quantitation of the 4-hydroxyproline released in sample hydrolysates, with the technique used by Jamall et al. [43]. Briefly, a standard curve for hydroxyproline was constructed for concentrations ranging from 0 to 1.6 μg hydroxyproline in 1.2 mL of 50% isopropanol. Then, 50 μL of a 1:80 hydrolysate sample dilution was added to each point. The final volume was adjusted to 1.2 mL with isopropanol/water (50:50), and then, 200 μL of 0.58% Cloramine T solution (in acetate-citrate buffer, pH 6.0) was added. After 10 min, 1-mL Ehrlich reagent was added and the sample was then incubated for 90 min at 50 °C in a water bath. Finally, samples were cooled in water at room temperature for 15 min and absorbances were read at 558 nm using water as the reference. To calculate collagen content, it was assumed that collagen mass was 7.1 times the hydroxyproline mass, as calculated from the composition of human dentine collagen [44].
Statistical analysis
Statistical analyses were done with SPSS software, version 23.0 (IBM Corporation., Armonk, NY, USA). Descriptive statistics were recorded and expressed as the mean ± standard deviation of the mean. To compare values between variables, parametric and nonparametric tests were used. Statistical dependence analysis between variables was done with the Pearson correlation coefficient. Regression analysis was used to obtain linear regression equations to estimate the age. A value of p less than 0.5 was considered statistically significant. The incidence of true and false positives and negatives, and sensitivity and specificity values, were calculated for furosine and pentosidine concentrations in dentine at different ages.
Results
Group I consisted of dentine samples of 32 permanent teeth from 15 males (46.88%) and 17 females (53.13%) ranging in age from 14 to 80 years (44.06 ± 22.08 years) (Table 1). In vivo, furosine concentration in dentine (18.5 ± 11.5 mmol/mol of collagen, range 2.7–40.1) was 85.6 times as high as pentosidine concentration (0.216 ± 0.080 mmol/mol of collagen, range 0.095–0.395) (Table 1). Comparisons of furosine and pentosidine contents in each sex yielded no statistically significant differences. Furosine and pentosine concentrations were also compared between age groups by stratifying the samples (< 25, 25–40, 41–60, and > 60 years) (Table 3).
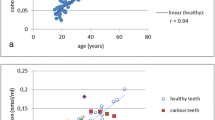
To estimate age from the observed variables, linear regression equations were derived for age and different furosine or pentosidine contents, and estimated ages and real errors were then calculated (Table 1). The correlation between the variables and age showed a strong dependence, as shown by the linear correlation coefficients (r), standard errors (SE), and mean absolute errors (MAE) presented in Table 1. The errors of the estimates did not differ significantly among types of tooth. Although all regressions produced high correlation coefficients, the errors showed that the age estimates from furosine and pentosidine concentrations were most accurate with the equation: age (years) = − 3.843 + 0.892 * furosine concentration + 144.852 * pentosidine concentration (r = 0.962, SE = 6.21, MAE = 4.68). In addition, there was a strong correlation between furosine and pentosidine concentrations (r = 0.903, p < 0.001). The linear regression for this correlation is illustrated in Fig. 1.
Group II consisted of a sample of dentine and a sample of the medial third of the clavicle obtained from the same cadaver (15 cases). The age range of the population was 18 to 85 years (46.80 ± 17.72 years) and 33.3% of the samples were from females (Table 2). Furosine concentration in dentine was 20.6 ± 9.6 mmol/mol of collagen (range 4.5–41.2) and pentosidine concentration was 0.228 ± 0.069 mmol/mol of collagen (range 0.113–0.365). These values yielded a furosine/pentosidine ratio of 90.3. No significant differences were found in furosine or pentosidine concentrations in dentine between cadaveric and living donor teeth. In bone, furosine concentration was 7.9 ± 3.4 mmol/mol of collagen (range 2.8–14.3) and pentosidine concentration was 0.072 ± 0.22 mmol/mol of collagen (range 0.040–0.118). The corresponding furosine/pentosidine ratio was 109.7. There were statistically significant differences in furosine content between bone and dentine (p < 0.001) and in pentosidine content between bone and dentine (p < 0.001). Other significant differences were found between furosine and pentosidine content in bone (p < 0.001) and between furosine and pentosidine content in dentine (p < 0.001). Moreover, there was a very strong linear correlation between furosine and pentosidine content in both tissues, as shown by the high correlation coefficients of r = 0.882 (p < 0.001) in bone and r = 0.970 (p < 0.001) in dentine (Fig. 2). In addition, there was a strong correlation between furosine in dentine and bone (r = 0.954, p < 0.001) and pentosidine in dentine and bone (r = 0.930, p < 0.001).
Scatterplot of the association between furosine and pentosidine concentrations in samples of human dentine and bone (clavicle) from 15 individuals in group II. Solid line: linear regression for dentine samples (y = 0.08 + 0.007x, r = 0.970). Dotted line: linear regression for bone samples (y = 0.03 + 0.00577x, r = 0.882)
On the other hand, no statistically significant differences between sexes were observed for furosine or pentosidine in dentine or bone.
There was a strong linear dependence between age and each of the other variables (furosine and pentosidine content in dentine and bone samples). Moreover, significant differences were found between younger and older age groups for both products and in both tissues (< 25 vs. 41–60 years, p < 0.05; < 25 vs. > 60 years, p < 0.01; 25–40 vs. > 60 years, p < 0.01 for dentine, and p < 0.05 for bone). As shown in Table 3, the regressions with the smallest errors were found with furosine or pentosidine contents measured in dentine as the independent variables. As in group I, no statistically significant differences in the errors of the estimates were found between types of tooth. In addition, the most accurate age estimates were obtained from the observed concentration of furosine and pentosidine in dentine (age (years) = 1.103 + 1.109 * furosine concentration + 100.350 * pentosidine concentration, r = 0.989, SE = 2.79, MAE = 1.85) and in bone samples (age (years) = − 3.434 + 2.413 * furosine concentration + 434.876 * pentosidine concentration, r = 0.978, SE = 4.03, MAE = 3.18).
Using values for all four variables (furosine and pentosidine concentrations in dentine and bone), we derived a multiple regression model to explain age. According to backward and forward stepwise selection, the best model was age (years) = − 0.733 + 1.080 * furosine concentration in dentine + 50.776 * pentosidine concentration in dentine − 0.262 * furosine concentration in bone + 220.835 * pentosidine concentration in bone (r = 0.995, SE = 2.16, MAE = 1.22).
The best cut-off values for furosine and pentosidine concentration in dentine, which yielded maximal sensitivity and specificity in different age groups, are shown in Table 4. The incidence of true and false positive and negative values was obtained considering all dentine samples from group I and group II together (n = 47), and sensitivities and specificities were calculated for furosine and pentosidine concentrations as possible discriminatory criteria to classify an individual in a given age group.
Discussion
In the present study, furosine and pentosidine concentrations were determined in dentine and clavicle samples from living and deceased donors. Furosine content in dentine was much lower than the values found by other authors. Kleter et al. [45] reported concentrations ranging from 220 to 240 mmol/mol of collagen in samples of carious dentine (n = 2). However, apart from the presence of caries, these authors used OPA as a reagent and used fluorescence to quantify other amino acids in addition to furosine in the sample, which might have affected the accuracy of their method.
In clavicle samples, mean furosine concentration was 2.6-fold lower compared to the concentration found in dentine samples from the same individuals, probably due to the slower renewal rate of collagen in dentine [46]. As noted above, protein turnover is considered an important determinant of the level of AGE accumulation. In this connection, Ohtani [47] also reported a much lower rate of aspartic acid racemization in bone compared to dentine. To our knowledge, there are no previous data on furosine concentration levels in human bone.
Pentosidine determinations in teeth have been reported somewhat more frequently in the literature. Kleter et al. also determined pentosidine in the aforementioned study [45] and found concentrations of 1.8 ± 0.7 mmol/mol of collagen (n = 7) in healthy dentine and 3.3 ± 0.5 mmol/mol of collagen (n = 7) in carious dentine. The higher values in carious sections of dentine can be explained by demineralization during caries formation, which facilitates the entry of precursors in the exposed tooth [45]. More recently, Greis et al. [37] reported concentrations of pentosidine ranging from 0.02 to 0.2 nmol/mL of collagen, measured in 63 healthy extracted third molars from individuals 15 to 65 years of age. These authors found an approximately 10-fold increase in pentosidine concentration between the youngest and the oldest individual, whereas in the present study, this increase was only 3.6-fold, even though we considered a broader age range.
In clavicle samples, mean pentosidine concentration was 3.1-fold lower than in dentine from the same individuals, which may be explained by the faster removal of AGEs due to the high turnover rate in bone [34, 46]. Sroga et al. [48] found in vivo concentrations of pentosidine ranging from 2.1 to 13.2 μmol/mmol of collagen in cortical bone and between 2.9 and 13.8 μmol/mmol of collagen in cancellous bone from human tibias of donors between 20 and 90 years old; these levels are much lower than the values obtained in the present study. It should be noted that we considered clavicle samples as a whole, without distinction between cortical and cancellous bone.
The greater amount of furosine in relation to pentosidine in both dental and bone tissues may be explained by the fact that furosine is an indicator of the Amadori product, a compound formed in the early stage of the Maillard reaction, whereas pentosidine is a product of the late phase, and consequently accumulates to a lesser extent. In addition, no statistically significant differences were found in furosine and pentosidine accumulation between men and women, in line with similar studies in mineralized tissues [32, 33].
In the present study, we found that furosine and pentosidine concentrations in both dentine and bone samples increased with aging. In addition, differences were seen between the youngest and oldest age groups. Furosine increased with age at almost the same rate as observed for pentosidine (Figs. 1 and 2). Thus, the strong correlation between furosine and pentosidine concentrations in both tissues could be used to estimate the content of one product from the content of the other. Specifically, furosine concentration could be used as a reliable indicator of the content of pentosidine, a less abundant AGE that is also closely associated with age.
In humans, increasing levels of furosine were reported in the aorta and tendon with age, especially after 20 years [28, 49], as well as a slight increase in skin from 20 to 85 years of age [23]. In their analysis of teeth, Miura et al. [35] investigated the accumulation of N-carboxymethyl-lysine, one of the many types of AGE, in dentine and found an AGE-positive immune reaction in aged dentine. In addition, Greis et al. [37] observed a close relationship between pentosidine concentration in healthy dentine and chronological age (r = 0.94) and reported a 95% prediction interval of ± 9.4 years for age estimation. The dependence of AGE accumulation on age has also been demonstrated in human bone. In particular, the accumulation of pentosidine with increasing age has been observed in cortical bone [30,31,32, 34] but not in cancellous bone [32, 33], possibly as a result of high turnover rates in this latter type of bone tissue [32].
In the present study, although all regressions yielded correlation coefficients higher than 0.93, the regressions with the smallest errors were those for furosine and pentosidine concentrations measured in dentine. This tissue may therefore be more suitable than bone for use in age estimation. Moreover, the sensitivities of the tests (furosine or pentosidine content in dentine) in order to assign individuals to a specific age group ranged from 100 to 53.8% for furosine and from 100 to 69.2% for pentosidine across the age subgroups we used (Table 4). Therefore, age group in our sample was accurately assigned in all individuals younger than 25 years (n = 11). The specificity of the tests was > 82.4% for furosine and > 91.2% for pentosidine in all age subgroups (Table 4); in other words, the likelihood of misclassification on the basis of furosine or pentosidine concentration determined in dentine was very low. A comparison of the errors for multiple vs. simple regression suggests that the multiple regression model is more suitable as long as both furosine and pentosidine content are known. In addition, the potential influence of the type of tooth in age estimation was studied, since different teeth develop at different times. Our results showed that the errors of the estimates were not significantly affected by the type of tooth analyzed.
The formation of AGEs is accelerated by hyperglycemia, oxidative stress, advanced renal disease, inflammation and hypertension, as well as by exogenous sources of precursors and AGEs ingested through food or tobacco [50]. Therefore, it should be taken into consideration that the influence of these variables can lead to age overestimations. Unfortunately, we had very limited information regarding the diabetes status of the sample donors; a more detailed study including a group of samples from donors with a verified history of diabetes should be performed in order to evaluate the influence of these factors on AGE levels. This limitation can be considered a major drawback for the applicability of the accumulation of NEGs to forensic age estimation. In addition, the older the person, the higher the exposure to these potentially confounding factors. In the present study, however, this trend was not evident: the highest percentage of overestimation was not found in the oldest age group, as shown in Tables 1 and 3. A further consideration is that heat accelerates AGE formation, so the method tested here may result in age overestimation if used with samples from burnt bodies [37].
Finally, it is important to note that in cases of unknown or very long postmortem intervals, degradation processes may alter the integrity of biomolecules and thus affect the reliability of molecular methods applied to degraded materials. A more precise knowledge of the molecular alterations human mineralized tissues undergo during the postmortem interval, including the degradation of collagen proteins, is thus necessary in studies of molecular markers for age estimation. Nevertheless, some studies have reported collagen proteins to be remarkably stable within relevant forensic postmortem intervals [12, 13] and therefore potentially useful for age estimation.
The results reported here show strong correlations between chronological age and furosine or pentosidine concentrations determined in mineralized tissues. Although the standard and mean absolute errors of the estimates were low enough to be applied for forensic purposes, our results must be interpreted with caution because of our relatively small sample size. Therefore, to further assess the accuracy of the models presented here to predict age, our results should be complemented with studies of larger numbers of cases. This limitation notwithstanding, we conclude that the method reported here is a potentially useful new contribution to age estimation from human skeletal remains, especially when morphological analysis is not possible.
References
Cunha E, Baccino E, Martrille L, Ramsthaler F, Prieto J, Schuliar Y, Lynnerup N, Cattaneo C (2009) The problem of aging human remains and living individuals: a review. Forensic Sci Int 193:1–13. https://doi.org/10.1016/j.forsciint.2009.09.008
Meissner C, Ritz-Timme S (2010) Molecular pathology and age estimation. Forensic Sci Int 203:34–43. https://doi.org/10.1016/j.forsciint.2010.07.010
Bekaert B, Kamalandua A, Zapico SC, van de Voorde W, Decorte R (2015) Improved age determination of blood and teeth samples using a selected set of DNA methylation markers. Epigenetics 10:922–930. https://doi.org/10.1080/15592294.2015.1080413
Márquez-Ruiz AB, González-Herrera L, Valenzuela A (2018) Usefulness of telomere length in DNA from human teeth for age estimation. Int J Legal Med 132:353–359. https://doi.org/10.1007/s00414-017-1595-5
Collins MJ, Nielsen-Marsh CM, Hiller J, Smith CI, Roberts JP, Prigodich RV, Wess TJ, Csapo J, Millard AR, Turner-Walker G (2002) The survival of organic matter in bone: a review. Archaeometry 44:383–394. https://doi.org/10.1111/1475-4754.t01-1-00071
Hedges REM (2002) Bone diagenesis: an overview of processes. Archaeometry 44:319–328. https://doi.org/10.1111/1475-4754.00064
Nicholson RA (2001) Taphonomic investigations. In: Brothwell DR, Pollard AM (eds) Handbook of archaeological science. Wiley, Chichester, pp 179–190
Kontopoulos I, Nystrom P, White L (2016) Experimental taphonomy: post-mortem microstructural modifications in Sus scrofa domesticus bone. Forensic Sci Int 266:320–328. https://doi.org/10.1016/j.forsciint.2016.06.024
Maurer AF, Person A, Tütken T, Amblard-Pison S, Ségalen L (2014) Bone diagenesis in arid environments: an intra-skeletal approach. Palaeogeogr Palaeoclimatol Palaeoecol 416:17–29. https://doi.org/10.1016/j.palaeo.2014.08.020
Nielsen-Marsh CM, Smith CI, Jans MME, Nord A, Kars H, Collins MJ (2007) Bone diagenesis in the European Holocene II: taphonomic and environmental considerations. J Archaeol Sci 34:1523–1531. https://doi.org/10.1016/J.JAS.2006.11.012
Stathopoulou ET, Psycharis V, Chryssikos GD, Gionis V, Theodorou G (2008) Bone diagenesis: new data from infrared spectroscopy and X-ray diffraction. Palaeogeogr Palaeoclimatol Palaeoecol 266:168–174. https://doi.org/10.1016/j.palaeo.2008.03.022
Ritz-Timme S, Cattaneo C, Collins MJ, Waite ER, Schütz HW, Kaatsch HJ, Borrman HIM (2000) Age estimation: the state of the art in relation to the specific demands of forensic practise. Int J Legal Med 113:129–136
Dobberstein RC, Huppertz J, von Wurmb-Schwark N, Ritz-Timme S (2008) Degradation of biomolecules in artificially and naturally aged teeth: implications for age estimation based on aspartic acid racemization and DNA analysis. Forensic Sci Int 179:181–191. https://doi.org/10.1016/j.forsciint.2008.05.017
Klumb K, Matzenauer C, Reckert A, Lehmann K, Ritz-Timme S (2016) Age estimation based on aspartic acid racemization in human sclera. Int J Legal Med 130:207–211. https://doi.org/10.1007/s00414-015-1255-6
Butler WT (1992) Dentin extracellular matrix and dentinogenesis. Oper Dent Suppl 5:18–23
Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17:319–336. https://doi.org/10.1007/s00198-005-2035-9
Walters C, Eyre DR (1983) Collagen crosslinks in human dentin: increasing content of hydroxypyridinium residues with age. Calcif Tissue Int 35:401–405. https://doi.org/10.1007/BF02405067
Eyre DR, Dickson IR, Van Ness K (1988) Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J 252:495–500
Martin-de las Heras S, Valenzuela A, Villanueva E (1999) Deoxypyridinoline crosslinks in human dentin and estimation of age. Int J Legal Med 112:222–226
Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycation end-products: a review. Diabetologia 44:129–146. https://doi.org/10.1007/s001250051591
Thorpe SR, Baynes JW (2003) Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids 25:275–281. https://doi.org/10.1007/s00726-003-0017-9
Tessier FJ (2010) The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol Biol 58:214–219. https://doi.org/10.1016/j.patbio.2009.09.014
Dyer DG, Dunn JA, Thorpe SR et al (1992) Accumulation of Maillard reaction products in skin collagen in diabetes and aging. Ann N Y Acad Sci 663:421–422
Sell DR (1997) Ageing promotes the increase of early glycation Amadori product as assessed by ε-N-(2-furoylmethyl)-L-lysine (furosine) levels in rodent skin collagen. The relationship to dietary restriction and glycoxidation. Mech Ageing Dev 95:81–99. https://doi.org/10.1016/S0047-6374(97)01863-0
Panwar P, Butler GS, Jamroz A, Azizi P, Overall CM, Brömme D (2018) Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol 65:30–44. https://doi.org/10.1016/j.matbio.2017.06.004
Verzijl N, DeGroot J, Oldehinkel E, et al (2000) Age-related accumulation of Maillard reaction products in human articular cartilage collagen. 350 Pt 2:381–387
Pilin A, Pudil FF, Bencko VV (2007) Changes in colour of different human tissues as a marker of age. Int J Legal Med 121:158–162. https://doi.org/10.1007/s00414-006-0136-4
Oimomi M, Igaki N, Hata F, Kitamura Y, Nishimoto S, Baba S, Maeda S (1989) Age- and diabetes-accelerated glycation in the human aorta. Arch Gerontol Geriatr 8:123–127. https://doi.org/10.1016/0167-4943(89)90056-3
Frye EB, Degenhardt TP, Thorpe SR, Baynes JW (1998) Role of the Maillard reaction in aging of tissue proteins: advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem 273:18714–18719. https://doi.org/10.1074/jbc.273.30.18714
Saito M, Marumo K, Fujii K, Ishioka N (1997) Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem 253:26–32. https://doi.org/10.1006/abio.1997.2350
Wang X, Shen X, Li X, Mauli Agrawal C (2002) Age-related changes in the collagen network and toughness of bone. Bone 31:1–7. https://doi.org/10.1016/S8756-3282(01)00697-4
Odetti P, Rossi S, Monacelli F et al (2005) Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci 1043:710–717. https://doi.org/10.1196/annals.1333.082
Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM (2005) Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 37:825–832. https://doi.org/10.1016/j.bone.2005.07.019
Dong XN, Qin A, Xu J, Wang X (2011) In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone 49:174–183. https://doi.org/10.1016/j.bone.2011.04.009
Miura J, Nishikawa K, Kubo M, Fukushima S, Hashimoto M, Takeshige F, Araki T (2014) Accumulation of advanced glycation end-products in human dentine. Arch Oral Biol 59:119–124. https://doi.org/10.1016/j.archoralbio.2013.10.012
Shinno Y, Ishimoto T, Saito M, Uemura R, Arino M, Marumo K, Nakano T, Hayashi M (2016) Comprehensive analyses of how tubule occlusion and advanced glycation end-products diminish strength of aged dentin. Sci Rep 6:19849. https://doi.org/10.1038/srep19849
Greis F, Reckert A, Fischer K, Ritz-Timme S (2018) Analysis of advanced glycation end products (AGEs) in dentine: useful for age estimation? Int J Legal Med 132:799–805. https://doi.org/10.1007/s00414-017-1671-x
Simm A, Wagner J, Gursinsky T, Nass N, Friedrich I, Schinzel R, Czeslik E, Silber RE, Scheubel RJ (2007) Advanced glycation endproducts: a biomarker for age as an outcome predictor after cardiac surgery? Exp Gerontol 42:668–675. https://doi.org/10.1016/j.exger.2007.03.006
Guerra-Hernandez E, Corzo N (1996) Furosine determination in baby cereals by ion-pair reversed-phase liquid chromatography. Cereal Chem 73:729–731
Resmini P, Pellegrino L (1991) Analysis of food heat damage by direct HPLC of furosine. Int Cromatography Lab 6:7–11
Delgado T, Corzo N, Santa-María G, Jimeno ML, Olano A (1992) Determination of furosine in milk samples by ion-pair reversed phase liquid chromatography. Chromatographia 33:374–376. https://doi.org/10.1007/BF02275921
Takahashi M, Hoshino H, Kushida K, Inoue T (1995) Direct measurement of crosslinks, pyridinoline, deoxypyridinoline, and pentosidine, in the hydrolysate of tissues using high-performance liquid chromatography. Anal Biochem 232:158–162. https://doi.org/10.1006/abio.1995.0002
Jamall IS, Finelli VN, Que Hee SS (1981) A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem 112:70–75. https://doi.org/10.1016/0003-2697(81)90261-X
Nkhumeleni FS, Raubenheimer EJ, Dauth J, van Heerden WFP, Smith PD, Pitout MJ (1992) Amino acid composition of dentine in permanent human teeth. Arch Oral Biol 37:157–158. https://doi.org/10.1016/0003-9969(92)90012-W
Kleter GA, Damen JJM, Buijs MJ, Ten Cate JM (1998) Modification of amino acid residues in carious dentin matrix. J Dent Res 77:488–495. https://doi.org/10.1177/00220345980770030801
Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JWJ, Lafeber FPJG, Baynes JW, TeKoppele JM (2000) Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 275:39027–39031. https://doi.org/10.1074/jbc.M006700200
Ohtani S (1998) Rate of aspartic acid racemization in bone. Am J Forensic Med Pathol 19:284–287. https://doi.org/10.1097/00000433-199809000-00017
Sroga GE, Siddula A, Vashishth D (2015) Glycation of human cortical and cancellous bone captures differences in the formation of Maillard reaction products between glucose and ribose. PLoS One 10:e0117240. https://doi.org/10.1371/journal.pone.0117240
Schleicher E, Wieland OH (1986) Kinetic analysis of glycation as a tool for assessing the half-life of proteins. BBA-Gen. Subjects 884:199–205. https://doi.org/10.1016/0304-4165(86)90244-8
Senatus LM, Schmidt AM (2017) The AGE-RAGE Axis: implications for age-associated arterial diseases. Front Genet 8:187. https://doi.org/10.3389/fgene.2017.00187
Acknowledgments
This research was funded by Project PM97-0182 from the Ministry of Education of Spain. The authors acknowledge Dr. Paula Rodríguez-Bouzas of the Department of Statistics, University of Granada, Spain, for her assistance with statistical studies, and K. Shashok for improving the use of English in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocols to collect samples from human subjects were approved by the corresponding Ethics Committees for Human Research of the University of Granada (Spain) and the University of Copenhagen (Denmark), and the study was conducted in accordance with the ethical standards laid down by the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Valenzuela, A., Guerra-Hernández, E., Rufián-Henares, J.Á. et al. Differences in non-enzymatic glycation products in human dentine and clavicle: changes with aging. Int J Legal Med 132, 1749–1758 (2018). https://doi.org/10.1007/s00414-018-1908-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-018-1908-3