Abstract
Simultaneous assessment of a panel of protein markers is becoming essential in order to enhance biomarker research and improve diagnostics. Specifically, postmortem diagnostics of early myocardial ischemia in sudden cardiac death cases could benefit from a multiplex marker assessment in the same tissue section. Current analytical antibody-based techniques (immunohistochemistry and immunofluorescence) limit multiplex analysis usually to not more than three antibodies. In this study, mass spectrometry-immunohistochemistry (MS-IHC) was performed by combining laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) with rare-metal-isotope-tagged antibodies as a technique for multiplex analysis of human postmortem myocardial tissue samples. Tissue sections with myocardial infarction were simultaneously analyzed for seven primary, rare-metal-isotope-tagged antibodies (troponin T, myoglobin, fibronectin, C5b-9, unphosphorylated connexin 43, VEGF-B, and JunB). Comparison between the MS-IHC approach and chromogenic IHC showed similar patterns in ionic and optical images. In addition, absolute quantification was performed by MS-IHC, providing a proportional relationship between the signal intensity and the local marker concentration in tissue sections. These data demonstrated that LA-ICP-MS combined with rare-metal-isotope-tagged antibodies is an efficient strategy for simultaneous testing of multiple markers and allows not only visualization of molecules within the tissue but also quantification of the signal. Such imaging approach has a great potential in both diagnostics and pathology-related research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Diagnostics of several pathological conditions would benefit from concurrent assessment of a panel of markers but this requires development of analytical techniques capable of detecting several biomarkers simultaneously (multiplexing), at good spatial resolution, and with high sensitivity. In particular, a panel of markers (instead of just one single marker) has been recently proposed for pathological diagnostics of early myocardial ischemia (EMI) [1, 2]. This acute condition represents the most frequent cause of sudden cardiac death. EMI occurs during the initial 4 to 6 h after occlusion of coronary flow to myocardium and, in cases where it is not suddenly fatal, EMI can lead to the development of myocardial infarction (MI) [1, 2]. Because EMI lacks gross anatomical changes and specific tissue morphology at histological analysis, much effort was made over the past several decades to improve recognition of EMI pathology by identifying pathological pathways and developing new diagnostic tools (molecular targets, strategies, methodologies, and instrumentation) [1, 3]. Currently, efforts are being made to develop multiplex approaches in order to both accelerate the discovery process and integrate multiplexing into routine work for improving diagnostics.
Despite progress in this field, the prevalent methods for tissue pathology diagnosis still lack high-throughput capabilities and allow only semi-quantitative analysis (e.g., immunoassays, immunohistochemistry (IHC), immunofluorescent microscopy (IFM), Western blotting, and antibody-based in situ hybridization). The most frequent and feasible color combination with IHC or IFM is three markers on a single-tissue section [4]. Aspects that impede concurrent screening of more molecules on a single-tissue section include spectral and spatial overlap of reporters, cross-reactivity between the detection reagents (in cases where indirect detection methods are used), and tissue autofluorescence (in cases of fluorescently labeled antibodies) [4]. In addition, quantification of the signal remains inaccurate due to non-linear characteristics of signal amplification. Furthermore, quantification of chromogenic IHC is performed by analyzing pixel intensities of digital scans of slides instead of measuring the reporter on the antibody [5].
Mass spectrometry IHC (MS-IHC) is an emerging approach with the potential for multiplex analysis of markers directly on tissue section [6]. This technique utilizes an innovative antibody tagging method that was originally developed for extensive multiplex analysis of single cells in mass cytometry [7,8,9,10]. Briefly, the primary antibody is tagged with a combination of rare-earth-metal isotopes and specific metal-chelating polymers instead of enzymatic or fluorescent probes. After incubation with primary antibodies, the entire surface of tissue section is rastered either by laser or ion beam in a coordinated way, using a grid array at a fixed lateral resolution. At each coordinate, the generated ions are analyzed by MS to generate a data set, which is used for reconstruction of ion images (similar to other imaging mass spectrometry technologies, e.g., matrix assisted laser desorption/ionization) [6, 11, 12]. Recently, this method was successfully applied to simultaneously assess 10 and 32 makers in breast cancer tissues by secondary ion mass spectrometry (SIMS) and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS), respectively [13, 14].
In this study, MS-IHC was evaluated as a multiplex method to quantitatively analyze human postmortem formalin-fixed, paraffin embedded (FFPE) samples by LA-ICP-MS for seven markers: troponin T, myoglobin, fibronectin, C5b-9, unphosphorylated connexin 43 (Cx43), JunB, and vascular endothelial growth factor-B (VEGF-B).
Materials and methods
Autopsy specimens
Cardiac samples were collected from six autopsy cases, performed at the University Center of Legal Medicine in Geneva, Switzerland, between December 2010 and November 2014. In all cases (n = 6; 5 males, 1 female; mean age 61.7 years), the cause of death was acute MI, defined by the presence of polymorphonuclear leucocyte infiltration (associated or not with hemorrhagic infiltrates and eventually contraction band necrosis) and cardiomyocyte coagulation necrosis, in sections stained with hematoxylin and eosin (H&E). In order to ensure the presence of ischemic myocardium, samples with evident MI were intentionally selected for this study. In accordance with our standard sampling protocol, eight cardiac regions were evaluated in every case (anterior, lateral, and posterior wall of both ventricles, anterior and posterior septum) but only one region representative of each case was used for further investigation in this study: the region with the most severely infarcted area, as observed by histological examination. In total, one tissue block from each case was examined in this study (the total number of sections per case is illustrated in Supplementary Fig. 1). Tissue samples were fixed in 4% buffered formaldehyde for 24 h, embedded in paraffin, and cut in 5-μm thick sections. The postmortem interval (time from death to autopsy) ranged from 5 to 36 h.
Immunohistochemistry
Chromogenic IHC on serial sections for troponin T, myoglobin, fibronectin, and C5b-9 were used for comparison to MS-IHC by LA-ICP-MS, as “positive controls.” Although chromogenic IHC and MS-IHC are different techniques, the overall pattern for markers’ distribution (accumulation or depletion) was expected to be approximately similar because the same primary antibodies were used in both techniques and evaluated on consecutive sections. In addition, three experimental markers were added to the multiplex panel, namely, Cx43, VEGF-B, and JunB. FFPE tissue sections were deparaffinized and treated for epitope retrieval by either incubation with proteinase K (Sigma-Aldrich®, 0.1 mg/ml in 50 mM Tris-Buffered Saline) for C5b-9 and fibronectin or microwaving for 15 min at 95 °C in Dako Target Retrieval Solution for the other antibodies. Following epitope retrieval, the sections were treated with 1% H2O2 in 10% methanol, blocked with bovine serum albumin, and subsequently probed with primary and secondary antibodies in a humidified chamber. Primary antibodies were against troponin T (mouse monoclonal, Abcam®, 1:250; n = 5), myoglobin (Bond™ mouse monoclonal, Leica, 1:50; n = 6), fibronectin (rabbit polyclonal, Dako, 1:2000; n = 6), C5b-9 (mouse monoclonal, Quidel®, 1:2000; n = 6), Cx43 (mouse monoclonal, Invitrogen™, 1:500; n = 4), JunB (rabbit polyclonal, Abcam®, 1:200; n = 3), VEGF-B (mouse monoclonal, R&D System®, 1:500; n = 2). Secondary antibodies were biotinylated anti-mouse (Vector Laboratories) or anti-rabbit (Vector Laboratories) IgG, followed by incubation with either VECTASTAIN® ABC Complex HRP kit (Vector Laboratories) for JunB or streptavidin/horseradish peroxidase-conjugate (Dako) for the rest. Colorimetric detection was performed using either Vector® DAB (Vector Laboratories) or Vector® AEC (Vector Laboratories) kits, according to the manufacturer’s instructions, with hematoxylin as counter-staining. For chromogenic IHC, negative controls without primary antibody were tested for all antibodies; moreover, negative controls were run in every IHC series and the slides were evaluated only if the negative controls showed negative results.
Tissue and antibody preparation for MS-IHC
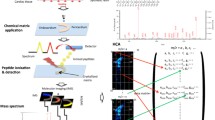
The overall workflow for the entire procedure consisted of three major stages: sample preparation, data acquisition, and data analysis (Fig. 1). The protocol for sample preparation and tissue labeling was similar to the process used in chromogenic IHC and IFM but instead of conjugating the antibody with enzyme or fluorophore, the primary antibody was coupled to a polymer tagged with rare-metal isotope. Sections (5 μm) from human autopsy FFPE samples with infarcted myocardium were deparaffinized, treated for epitope retrieval using microwave (10 min at 95 °C in Dako target retrieval solution), pre-treated with 1% H2O2 in 10% methanol, and blocked with bovine serum albumin. The sections were then incubated for 90 min with a 200-μl mixture (per slide) of seven antibodies that were pre-labeled with a complex containing MAXPAR® X8 Polymer and a rare-metal isotope (MAXPAR® Antibody Labeling Kit, Fluidigm®): C5b-9 tagged with 139La (0.5 μg/ml; n = 6), fibronectin tagged with 141Pr (2.5 μg/ml; n = 6), myoglobin tagged with 144Nd (15.0 μg/ml; n = 6), troponin T tagged with 151Eu (4.0 μg/ml; n = 5), JunB tagged with 159Tb (20.0 μg/ml; n = 5), Cx43 tagged with 175Lu (1.0 μg/ml; n = 5), and VEGF-B tagged with 162Dy (1.0 μg/ml; n = 5). After incubation with the mixture of antibodies, the sections were rinsed in Dako Wash Buffer (Dako) and dried at room temperature prior to MS analysis.
Workflow for quantitative multiplex imaging using a combination of rare-metal-isotope-tagged antibodies and LA-ICP-MS. a Sample preparation: the primary antibodies are tagged with a complex consisting of a polymer chelated with a rare metal. The tissue sample fixed in formaldehyde is embedded in paraffin, sectioned, placed onto a slide, and processed (deparaffinization, epitope retrieval, etc.). Multiplex hybridization is then performed: tissue section is incubated with a mixture of uniquely labeled antibodies. b Data acquisition: the sample is placed into the laser ablation chamber and the sample surface is scanned using focused laser beam (line by line). The laser ablation process generates particles, which are transported to the inductively coupled plasma torch for further digestion and ionization of the samples mass. The released metal tags are detected and recorded as mass spectra. c Data analysis: the acquired data set is analyzed to create ion image reconstruction and obtain quantitative information
MS-IHC data acquisition
Tissue pre-incubated with rare-metal-isotope-tagged antibodies was placed into the laser ablation chamber and was ablated by a Nd:YAG/213-nm laser focused on 8 or 40 μm diameter spot using laser ablation system (NWR-213, New Wave, Fremont, USA) coupled to quadrupole mass analyzer (Agilent 7700 ICP series, Darmstadt, Germany). The generated ablated particles were transported to the ICP ion source by a helium carrier gas of 800 mL/min and exposed to plasma torch. Only the metal tags were able to withstand this step and were subsequently passed to mass spectrometer for detection and quantification. The LA-ICP-MS parameters were optimized to provide the best signal-to-noise ratio while keeping the maximum reproducibility between analyses. In this way, the energy output was set at 23% corresponding to a laser fluency of 0.35 J/cm2. The lateral resolution was fixed at 10 or 50 μm in the raster mode with a scan speed of 10 or 50 μm/s, respectively. The laser repetition frequency was set at 20 Hz. Rare-earth metals (139La, 141Pr, 144Nd, 151Eu, 159Tb, 175Lu, and 162Dy) were monitored for a duty cycle of 1 s.
Data analysis and image visualization
The acquired raw data were extracted using the MassHunter software (Agilent, Darmstadt, Germany). Data files (.d) of IMS experiment were successively converted to mzML and imzML formats using msconvert and imzMLConverter tools, respectively. Ion images were reconstructed and visualized using either the MSIReader v0.06 software or MALDIQuant and Cardinal packages in R environment (http://cran.r-project.org) [15, 16].
Calibration procedure
The concentration ranges for each rare-metal-isotope reporter were calculated using corresponding calibration curves, which were generated using laboratory standards. Briefly, synthetic laboratory standards were prepared from human heart homogenates of analogous control autopsy cases doped with rare-metal isotope at four different concentrations (50, 500, 1000, and 5000 ng/g; Supplementary Table S1 lists these concentrations in nmol/g) as described elsewhere [17, 18]. For each rare-metal isotope, the 80 μm × 100 μm rectangles from the spiked areas were analyzed, i.e., 80 pixels at each concentration, and average intensities of those 80 pixels were calculated for each concentration. Then the average pixel intensity (u.a.) was plotted against the concentration of a metal isotope to generate a calibration curve. The calibration curves were then used to convert the intensity scales recorded by LA-ICP-MS to the concentrations of rare-earth metals, which is directly proportional to the amounts of markers.
Results
Multiplex tissue imaging of MI in human postmortem samples with MS-IHC
Chromogenic IHC results showed cytoplasmic depletion of troponin T and myoglobin from large groups of cardiomyocytes in the infarcted areas and accumulation of fibronectin, C5b-9, Cx43, and JunB (Fig. 2). Fibronectin and C5b-9 accumulation was observed in cytoplasm of cardiomyocytes located in the infarcted area. Increased Cx43 signal localized at gap junctions and in some cardiomyocytes also in cytoplasm. JunB positive reactions were found in nuclei of cardiomyocytes and infiltrating leucocytes. Evaluation of IHC with microscope at high magnification showed some VEGF-B positive reactions only in endothelial cells of few vessels (Supplementary Fig. 2). After analysis of the samples by MS-IHC and data acquisition, image reconstruction was performed for each rare-metal-isotope (Fig. 2, Supplementary Fig. 2). Reconstructed ion images obtained with MS-IHC showed similar distribution patterns as the consecutive sections stained with chromogenic IHC (Fig. 2). Concerning VEGF-B–162Dy, some variations in signal were also observed in reconstructed ion images but due to the expression of this marker in endothelial cells of blood vessels, the accurate interpretation of the LA-ICP-MS data would require analysis at a higher resolution than was selected in this study.
Distribution of six ischemic marker in human postmortem tissue with myocardial infarction: comparison of imaging by MS-IHC with LA-ICP-MS vs. chromogenic IHC performed on serial tissue sections (images from a representative case). a–f Reconstructed ion images obtained with LA-ICP-MS at 10 μm of lateral resolution. The signal intensities for rare-metal isotopes were coded on a color scale: minimum intensity is dark blue; maximum intensity is red. g–l Chromogenic IHC on serial sections at low magnification (× 4); red rectangles outline approximate area that are shown in a–f; black rectangles highlight areas that are shown at higher magnification in m–r. m–r Chromogenic IHC at higher magnification from black rectangles in g–l (× 40)
This approach for multiplex tissue imaging allows reconstruction of composite images. Figure 3 shows two composite reconstructions: (1) troponin T–151Eu and fibronectin–141Pr, and (2) troponin T–151Eu and C5b-9–139La. Increased fibronectin–141Pr and C5b-9–139La levels were evident in the areas with decreased troponin T–151Eu.
Composite ionic image reconstruction with data obtained by MS-IHC with LA-ICP-MS. a Composite reconstruction of troponin T–151Eu (green) and fibronectin–141Pr (red) at 10 μm of lateral resolution. b Composite reconstruction of troponin T–151Eu (green) and C5b-9–139La (blue) at 10 μm of lateral resolution. c–h Chromogenic IHC for fibronectin (accumulation), troponin T (depletion), and C5b-9 (accumulation); black rectangles in c–e highlight areas that are shown at higher magnification (× 40) in f–h. Fibronectin and C5b-9 accumulated in cardiomyocytes in the infarcted area. In approximately the same area, large groups of cardiomyocytes were depleted of troponin T
Due to its sensitivity, the multiplex MS-IHC allowed in some cases more precise determination of areas with marker’s accumulation or depletion. For example, evaluation of one of the cases for fibronectin by chromogenic IHC showed accumulation of fibronectin (positive staining) in the entire region. However, reconstruction of ion image for fibronectin–141Pr showed obvious distinction between areas with and without signal (Fig. 4). This difference between the two methods and strong signal from chromogenic IHC could possibly be explained by signal amplification in chromogenic IHC during both the incubation with secondary antibody and the exposure to substrate for development of colored precipitate.
Quantification of three markers (fibronectin–141Pr, myoglobin–144Nd, and C5b-9–139La) by MS-IHC with LA-ICP-MS. a–c Chromogenic IHC; black rectangle outlines estimated area used for analysis by LA-ICP-MS; black asterisk marks a region of fibrotic area. d–f Ion image reconstruction using MS-IHC with LA-ICP-MS data set at 10 μm of lateral resolution; intensity color scale was converted to a concentration scale for each rare-metal isotope using calibration curves shown in the bottom panel; white asterisk in d marks a region of fibrotic area. g–i Calibration curves were generated by plotting intensity of standards against their known concentrations
Sensitive method that permits accurate marker quantification
Quantification was performed for selected cases (representative case is shown in Fig. 4). Since the metal isotopes are measured by mass spectrometer and the isotopes are conjugated to the primary antibody, quantification is fairly straightforward. The signal intensity is proportional to the local marker concentration in the tissue section (in contrast to IHC or IFM, where the quantified signal is often amplified by incubations with additional antibodies needed for the detection and visualization). Using calibration curves for each rare-metal isotope, the intensity color scale was converted to concentration scale (Fig. 4, Supplementary Fig. 3). Utilizing the concentration scale already provides a more objective way for comparison. For instance, visual examination of ionic reconstruction for C5b-9–139La and fibronectin–141Pr (Fig. 4) suggests similar distribution of these markers in the selected tissue area. Despite similar distribution, the concentration of C5b-9–139La was much higher (nearly doubled) in comparison to fibronectin–141Pr.
Discussion
Majority of postmortem studies are in agreement that no single marker would be both sensitive and specific enough to precisely detect EMI, and, therefore a panel of markers should be used for accurate diagnostics. But chromogenic IHC (the most widely used tool to evaluate markers in postmortem samples) limits the number of antibodies that can be simultaneously tested on the same tissue sections. Availability of techniques capable of multiplex tissue imaging would be an advantage for evaluating a panel of markers. The main novelty of this study is the evaluation of MS-IHC with LA-ICP-MS as a technique to simultaneously visualize and quantify seven ischemic markers in the same tissue section from human postmortem cardiac tissue with evident MI. Cases with evident MI were intentionally selected in order to ensure the presence of ischemic myocardium. The only two previous MS-IHC studies assessed markers in breast cancer and did not investigate the absolute quantification capability of the approach [13, 14]. Primary antibodies tagged directly with the innovative reporters (rare-earth-metal isotopes combined with metal-chelating polymer) have the potential to greatly surpass multiplex options available with IHC and IFM and to improve multiplex tissue imaging in the same tissue cross section to a new high-throughput level.
The selected panel of seven markers consisted of four established postmortem markers (C5b-9, fibronectin, troponin T, and myoglobin, where troponin T and myoglobin are also currently used as serum markers for the clinical diagnosis of MI), and three experimental markers (Cx43, JunB, and VEGF-B). Our results for the established four markers (C5b-9, fibronectin, troponin T, and myoglobin) were consistent with previously published studies: C5b-9 and fibronectin accumulated in MI tissue and troponin T and myoglobin were depleted [1, 19,20,21,22,23,24,25,26]. Cx43, JunB, and VEGF-B are markers that were recently shown in experimental research to be influenced by ischemia, already during its onset [2, 27,28,29,30,31]. Connexin43 is gap junctional protein. Cardiac gap junction channels are located at the intercalated disks and are responsible for intercellular exchange of ions and small regulatory molecules. EMI was shown to induce Cx43 and to lead to its accumulation at the intercalated disks as early as 15 min after ischemia onset [2]. Our results concerning the changes of Cx43 in MI are consistent with the only one other study that also evaluated protein level of Cx43 in human postmortem MI samples [26]. The function of JunB during EMI is not yet well understood but it was shown to be induced by EMI [2, 32]. VEGF-B is one of the five secreted glycoproteins in the VEGF family. This released growth factor appears to play a role in the growth of coronary arteries, enlargement of capillaries, and cardiac hypertrophy [29]. Overall, previous studies performed on human postmortem MI samples investigated various combinations of these markers. But only a maximum of four markers were investigated in two separate studies and not in the same section but using consecutive sections [1, 21]. All other postmortem MI investigations evaluated two or three markers at a time in a given study.
Identifying various potential ischemic markers is currently needed in order to improve knowledge about this acute pathology and select a panel of targets that could be used for diagnosis and chronological dating of EMI [33]. Although we investigated only six cases, our results demonstrate that MS-IHC is suitable for performing multiplex tissue imaging in a larger study. Future application of MS-IHC offers a great potential to accelerate research utilizing human postmortem EMI samples. The ultimate goal is to implement this methodology for development of a diagnostic panel of markers and potentially use it as a tool to detect EMI. While seven markers were evaluated in this study, the method allows for a more high-throughput analysis: not only a panel of 32 tags was already successfully evaluated using similar approach in the context of tumor biology but also nearly 100 rare-earth-metal isotopes might be available in the future [14]. Several aspects of this multiplex approach are currently being developed, including the number of available isotopes and metal-chelating chemistries [14]. Innovations in these areas are likely to further improve the multiplexing ability and accuracy of this method. But the persisting challenge for any techniques using rare-earth-metal-tagged antibodies will remain to be the availability of specific primary antibody for a given target.
In imaging MS, the selected criteria are often a compromise between resolution and the analysis time: higher resolution can be achieved but with longer duration for data acquisition [12]. In this study, resolution at cellular level was chosen (either 10 or 50 μm) to evaluate broader area within reasonable amount of time. Assessment of a larger tissue area is more relevant in EMI and in several other forensic investigations. But higher resolution can be obtained with this method. Using similar approach, resolution at 1 μm was recently reported but assessment of 0.5 mm × 0.5 mm area took about 3.5 h [14]. In the near future, innovations in designs of laser ablation chamber and laser properties will improve the efficiency and allow more rapid scanning [34].
In addition to high-throughput multiplexing, other advantages of this technique include single incubation step for labeling with multiple antibodies, elimination of labeling with secondary antibody without losing sensitivity, and improved quantification of the signal. Importantly, this method is highly sensitive and has a wide dynamic range, which allows absolute and precise quantification of the markers [35]. In comparison to MS-IHC, other available multiplex methods employ lengthy and laborious staining techniques with less precise quantification. Quantitative analysis of tissue samples by LA-ICP-MS has been successfully demonstrated showing powerful quantification capabilities [36, 37]. Improved marker quantification within the exact same section is particularly powerful in research context because it allows more precise statistical analysis between study cases and controls, more accurate evaluation of affected area, and establishing cutoff values for marker’s concentration. Since the initiation of this study, several additional markers have been proposed for EMI (e.g., GAL1, GAL3, HIF-1α, MIF, MCP-1) [33, 38,39,40,41]. The challenge for selecting the most useful panel of diagnostic markers is comparison of specificity and sensitivity among all of them. In this case, accurate quantification will be useful for performing appropriate statistical tests to determine the optimal combination of markers and to validate their usefulness. Furthermore, quantification based on external calibration makes it possible to establish a threshold value, similarly to other standard laboratory tests that utilize calibration. In forensic and clinical pathology, implementation of threshold values for ischemic markers in routine work would allow a more accurate comparison of data from different samples and a precise diagnosis of this acute pathology, which otherwise remains undiagnosed, misdiagnosed, or, in the best scenario, only hypothesized. Moreover, quantification of the markers could give information about the severity of the ischemic insult (infarction size), duration of ischemia prior to death, and the time of death. These data are undoubtedly crucial for researchers, clinicians, and pathologists. Besides EMI, accurate marker quantification can be applied to a variety of pathological conditions in order to improve their diagnosis and to obtain information about their time of appearance, evolution, vitality, and contribution to death. Wound vitality and wound age estimation are an example of the possible applications. Lastly, quantitative multiplex tissue imaging allows more efficient and accurate way to study changes in markers’ level over time. Temporal study can be performed in a relatively short amount of time: several targets can be analyzed within one-day procedure (a process that would take several days to test with chromogenic IHC). At the same time, accurate quantification is dependent on reliable calibrations curves. Prior to incorporating this approach for routine work in forensic medicine, the method needs to be validated according to the international guidelines.
In summary, the method presented in this investigation offers the advantage of simultaneously multiplexing and quantifying markers in the same tissue section, in human postmortem cases of MI. Current technologies for biomarker discovery that utilize untargeted strategies are rapidly generating candidate markers that can potentially improve diagnostics. MS-IHC has a great potential for accelerating translational research by simultaneously validating multiple markers of interest and also for improving clinical and forensic diagnostic of pathologies that require a multiplex evaluation of markers.
References
Campobasso CP, Dell’Erba AS, Addante A, Zotti F, Marzullo A, Colonna MF (2008) Sudden cardiac death and myocardial ischemia indicators: a comparative study of four immunohistochemical markers. Am J Forensic Med Pathol 29(2):154–161. https://doi.org/10.1097/PAF.0b013e318177eab7
Sabatasso S, Mangin P, Fracasso T, Moretti M, Docquier M, Djonov V (2016) Early markers for myocardial ischemia and sudden cardiac death. Int J Legal Med 130(5):1265–1280. https://doi.org/10.1007/s00414-016-1401-9
Fineschi V (2015) Measuring myocyte oxidative stress and targeting cytokines to evaluate inflammatory response and cardiac repair after myocardial infarction. Curr Vasc Pharmacol 13(1):3–5
Stack EC, Wang C, Roman KA, Hoyt CC (2014) Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods (San Diego, Calif) 70(1):46–58. https://doi.org/10.1016/j.ymeth.2014.08.016
Dixon AR, Bathany C, Tsuei M, White J, Barald KF, Takayama S (2015) Recent developments in multiplexing techniques for immunohistochemistry. Expert Rev Mol Diagn 15(9):1171–1186. https://doi.org/10.1586/14737159.2015.1069182
Levenson RM, Borowsky AD, Angelo M (2015) Immunohistochemistry and mass spectrometry for highly multiplexed cellular molecular imaging. Lab Investig 95(4):397–405. https://doi.org/10.1038/labinvest.2015.2
Newell EW, Davis MM (2014) Beyond model antigens: high-dimensional methods for the analysis of antigen-specific T cells. Nat Biotechnol 32(2):149–157. https://doi.org/10.1038/nbt.2783
Chattopadhyay PK, Gierahn TM, Roederer M, Love JC (2014) Single-cell technologies for monitoring immune systems. Nat Immunol 15(2):128–135. https://doi.org/10.1038/ni.2796
Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, Pavlov S, Vorobiev S, Dick JE, Tanner SD (2009) Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal Chem 81(16):6813–6822. https://doi.org/10.1021/ac901049w
Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe'er D, Tanner SD, Nolan GP (2011) Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science (New York, NY) 332(6030):687–696. https://doi.org/10.1126/science.1198704
Lauer E, Villa M, Jotterand M, Vilarino R, Bollmann M, Michaud K, Grabherr S, Augsburger M, Thomas A (2017) Imaging mass spectrometry of elements in forensic cases by LA-ICP-MS. Int J Legal Med 131(2):497–500. https://doi.org/10.1007/s00414-016-1414-4
Thomas A, Chaurand P (2014) Advances in tissue section preparation for MALDI imaging MS. Bioanalysis 6(7):967–982. https://doi.org/10.4155/bio.14.63
Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, Levenson RM, Lowe JB, Liu SD, Zhao S, Natkunam Y, Nolan GP (2014) Multiplexed ion beam imaging of human breast tumors. Nat Med 20(4):436–442. https://doi.org/10.1038/nm.3488
Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, Schuffler PJ, Grolimund D, Buhmann JM, Brandt S, Varga Z, Wild PJ, Gunther D, Bodenmiller B (2014) Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods 11(4):417–422. https://doi.org/10.1038/nmeth.2869
Robichaud G, Garrard KP, Barry JA, Muddiman DC (2013) MSiReader: an open-source interface to view and analyze high resolving power MS imaging files on Matlab platform. J Am Soc Mass Spectrom 24(5):718–721. https://doi.org/10.1007/s13361-013-0607-z
Bemis KD, Harry A, Eberlin LS, Ferreira C, van de Ven SM, Mallick P, Stolowitz M, Vitek O (2015) Cardinal: an R package for statistical analysis of mass spectrometry-based imaging experiments. Bioinformatics 31(14):2418–2420. https://doi.org/10.1093/bioinformatics/btv146
Matusch A, Depboylu C, Palm C, Wu B, Hoglinger GU, Schafer MK, Becker JS (2010) Cerebral bioimaging of Cu, Fe, Zn, and Mn in the MPTP mouse model of Parkinson’s disease using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). J Am Soc Mass Spectrom 21(1):161–171. https://doi.org/10.1016/j.jasms.2009.09.022
Becker JS, Zoriy MV, Pickhardt C, Palomero-Gallagher N, Zilles K (2005) Imaging of copper, zinc, and other elements in thin section of human brain samples (hippocampus) by laser ablation inductively coupled plasma mass spectrometry. Anal Chem 77(10):3208–3216. https://doi.org/10.1021/ac040184q
Jasra SK, Badian C, Macri I, Ra P (2012) Recognition of early myocardial infarction by immunohistochemical staining with cardiac troponin-I and complement C9. J Forensic Sci 57(6):1595–1600. https://doi.org/10.1111/j.1556-4029.2012.02172.x
Jenkins CP, Cardona DM, Bowers JN, Oliai BR, Allan RW, Normann SJ (2010) The utility of C4d, C9, and troponin T immunohistochemistry in acute myocardial infarction. Arch Pathol Lab Med 134(2):256–263. https://doi.org/10.1043/1543-2165-134.2.256
Ortmann C, Pfeiffer H, Brinkmann B (2000) A comparative study on the immunohistochemical detection of early myocardial damage. Int J Legal Med 113(4):215–220
Brinkmann B, Sepulchre MA, Fechner G (1993) The application of selected histochemical and immunohistochemical markers and procedures to the diagnosis of early myocardial damage. Int J Legal Med 106(3):135–141
Piercecchi-Marti MD, Lepidi H, Leonetti G, Vire O, Cianfarani F, Pellissier JF (2001) Immunostaining by complement C9: a tool for early diagnosis of myocardial infarction and application in forensic medicine. J Forensic Sci 46(2):328–334
Edston E, Kawa K (1995) Immunohistochemical detection of early myocardial infarction. An evaluation of antibodies against the terminal complement complex (C5b-9). Int J Legal Med 108(1):27–30
Schafer H, Mathey D, Hugo F, Bhakdi S (1986) Deposition of the terminal C5b-9 complement complex in infarcted areas of human myocardium. J Immunol 137(6):1945–1949
Kawamoto O, Michiue T, Ishikawa T, Maeda H (2014) Immunohistochemistry of connexin43 and zonula occludens-1 in the myocardium as markers of early ischemia in autopsy material. Histol Histopathol 29(6):767–775. https://doi.org/10.14670/HH-29.767
Matsushita T, Takamatsu T (1997) Ischaemia-induced temporal expression of connexin43 in rat heart. Virchows Arch 431(6):453–458
Hatanaka K, Kawata H, Toyofuku T, Yoshida K (2004) Down-regulation of connexin43 in early myocardial ischemia and protective effect by ischemic preconditioning in rat hearts in vivo. Jpn Heart J 45(6):1007–1019
Bry M, Kivela R, Leppanen VM, Alitalo K (2014) Vascular endothelial growth factor-B in physiology and disease. Physiol Rev 94(3):779–794. https://doi.org/10.1152/physrev.00028.2013
Ogawa H, Suefuji H, Soejima H, Nishiyama K, Misumi K, Takazoe K, Miyamoto S, Kajiwara I, Sumida H, Sakamoto T, Yoshimura M, Kugiyama K, Yasue H, Matsuo K (2000) Increased blood vascular endothelial growth factor levels in patients with acute myocardial infarction. Cardiology 93(1–2):93–99
Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA (2000) Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med 342(9):626–633. https://doi.org/10.1056/NEJM200003023420904
Harpster MH, Bandyopadhyay S, Thomas DP, Ivanov PS, Keele JA, Pineguina N, Gao B, Amarendran V, Gomelsky M, McCormick RJ, Stayton MM (2006) Earliest changes in the left ventricular transcriptome postmyocardial infarction. Mamm Genome 17(7):701–715. https://doi.org/10.1007/s00335-005-0120-1
Turillazzi E, Pomara C, Bello S, Neri M, Riezzo I, Fineschi V (2015) The meaning of different forms of structural myocardial injury, immune response and timing of infarct necrosis and cardiac repair. Curr Vasc Pharmacol 13(1):6–19
Ogrinc Potocnik N, Porta T, Becker M, Heeren RM, Ellis SR (2015) Use of advantageous, volatile matrices enabled by next-generation high-speed matrix-assisted laser desorption/ionization time-of-flight imaging employing a scanning laser beam. Rapid Commun Mass Spectrom 29(23):2195–2203. https://doi.org/10.1002/rcm.7379
Dobrowolska J, Dehnhardt M, Matusch A, Zoriy M, Palomero-Gallagher N, Koscielniak P, Zilles K, Becker JS (2008) Quantitative imaging of zinc, copper and lead in three distinct regions of the human brain by laser ablation inductively coupled plasma mass spectrometry. Talanta 74(4):717–723. https://doi.org/10.1016/j.talanta.2007.06.051
Sabine Becker J (2013) Imaging of metals in biological tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS): state of the art and future developments. Journal of Mass Spectrometry : JMS 48(2):255–268. https://doi.org/10.1002/jms.3168
Becker JS, Zoriy M, Matusch A, Wu B, Salber D, Palm C, Becker JS (2010) Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom Rev 29(1):156–175. https://doi.org/10.1002/mas.20239
Al-Salam S, Hashmi S (2014) Galectin-1 in early acute myocardial infarction. PLoS One 9(1):e86994. https://doi.org/10.1371/journal.pone.0086994
Hashmi S, Al-Salam S (2015) Galectin-3 is expressed in the myocardium very early post-myocardial infarction. Cardiovasc Pathol 24(4):213–223. https://doi.org/10.1016/j.carpath.2014.12.001
Chan W, White DA, Wang XY, Bai RF, Liu Y, Yu HY, Zhang YY, Fan F, Schneider HG, Duffy SJ, Taylor AJ, Du XJ, Gao W, Gao XM, Dart AM (2013) Macrophage migration inhibitory factor for the early prediction of infarct size. J Am Heart Assoc 2(5):e000226. https://doi.org/10.1161/JAHA.113.000226
White DA, Fang L, Chan W, Morand EF, Kiriazis H, Duffy SJ, Taylor AJ, Dart AM, Du XJ, Gao XM (2013) Pro-inflammatory action of MIF in acute myocardial infarction via activation of peripheral blood mononuclear cells. PLoS One 8(10):e76206. https://doi.org/10.1371/journal.pone.0076206
Acknowledgements
We would like to thank Max Villa and Catia Pomponio for the support and assistance with LA-ICP-MS and immunohistochemistry, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required. All cases included in this study were obtained from the autopsy database in our center. In agreement with the local ethics committee and the local general prosecutor, these cases can be included in this type of studies, provided that they are anonymized. In this investigation, no information allowing the identification of a person is given. People, who had previously refused, in a written form, their consent to bequeath their body parts for research use, were excluded from the study.
Electronic supplementary material

Supplementary Fig1
(JPEG 732 kb)

Supplementary Fig2
(JPEG 1653 kb)

Supplementary Fig3
(JPEG 285 kb)
Table S1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Aljakna, A., Lauer, E., Lenglet, S. et al. Multiplex quantitative imaging of human myocardial infarction by mass spectrometry-immunohistochemistry. Int J Legal Med 132, 1675–1684 (2018). https://doi.org/10.1007/s00414-018-1813-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-018-1813-9