Abstract
The goal of the present study was to evaluate if quantitative postmortem cardiac 3-T magnetic resonance (QPMCMR) T1 and T2 relaxation times and proton density values of histopathological early acute and chronic myocardial infarction differ to the quantitative values of non-pathologic myocardium and other histopathological age stages of myocardial infarction with regard to varying corpse temperatures. In 60 forensic corpses (25 female, 35 male), a cardiac 3-T MR quantification sequence was performed prior to autopsy and cardiac dissection. Core body temperature was assessed during MR examinations. Focal myocardial signal alterations in synthetically generated MR images were measured for their T1, T2, and proton density (PD) values. Locations of signal alteration measurements in PMCMR were targeted at heart dissection, and myocardial tissue specimens were taken for histologic examinations. Quantified signal alterations in QPMCMR were correlated to their according histologic age stage of myocardial infarction, and quantitative values were corrected for a temperature of 37 °C. In QPMCMR, 49 myocardial signal alterations were detected in 43 of 60 investigated hearts. Signal alterations were diagnosed histologically as early acute (n = 16), acute (n = 10), acute with hemorrhagic component (n = 9), subacute (n = 3), and chronic (n = 11) myocardial infarction. Statistical analysis revealed that based on their temperature-corrected quantitative T1, T2, and PD values, a significant difference between early acute, acute, and chronic myocardial infarction can be determined. It can be concluded that quantitative 3-T postmortem cardiac MR based on temperature-corrected T1, T2, and PD values may be feasible for pre-autopsy diagnosis of histopathological early acute, acute, and chronic myocardial infarction, which needs to be confirmed histologically.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For over a decade, postmortem cardiac MRI has been used to support forensic autopsy in assessing myocardial infarction [1,2,3,4,5]. Recently, MRI quantification has been introduced to postmortem cardiac imaging. By use of a particular quantitative MRI sequence, T1 and T2 relaxation times and proton density of myocardial tissue can be assessed [6,7,8,9,10,11,12,13]. A recent quantitative 3-T postmortem study indicated that different histopathological age stages of myocardial infarction such as acute infarction with fiber necrosis and granulocytous infiltration or subacute infarction with granulation tissue may be diagnosable based on quantitative T1 and T2 relaxation times and proton density values [13]. To date, quantitative 3-T postmortem T1, T2, and proton density (PD) values of early acute myocardial infarction aged less than 24 h and chronic myocardial infarction aged more than 3 weeks are unknown. Moreover, it is unknown if the quantitative values of histopathologic early acute and chronic myocardial infarction differ to the quantitative values of non-pathologic myocardium and other histopathological age stages of myocardial infarction. In order to use quantitative 3-T cardiac MRI for a discriminative diagnosis of myocardial infarction in postmortem imaging, the different histopathological age stages, including early acute and later infarction stages, need to be known for 3-T postmortem applications.
In general, quantitative postmortem MRI bears the problem that MR relaxation times, T1 relaxation times in particular, are dependent on body temperature that usually vary in corpses in rather broad ranges [11, 13,14,15,16]. If quantitative MR values of corpses with varying body temperatures are to be used for myocardial tissue characterization and comparison, corpse body temperature has to be measured and temperature correction of quantitative values has to be performed. The goal of the present study was to evaluate if quantitative values of histopathological early acute and chronic myocardial infarction differ to the quantitative values of non-pathologic myocardium and other histopathological age stages of myocardial infarction with regard to varying corpse temperatures.
Methods
Study population
In a prospective postmortem study from October 2015 to September 2016, 71 forensic cases displaying no relevant external signs of putrefaction were chosen that presented with either a known diagnosis of myocardial infarction in individual’s medical history or symptoms of acute coronary syndrome and did not receive cardiopulmonary resuscitation prior to death. Information about known cardiac diagnoses or symptoms of acute coronary syndrome was received from paramedic reports, police, or relatives of the deceased. Ten non-putrefied control cases that were not known for pre-existent cardiac pathologies (control group 5 male, 5 female; mean age 48.7 years, SD 11.3) were chosen that died in circumstances making a primary cardiac-related death improbable (n = 6 intoxication cases and n = 4 strangulation cases). Cases with known diagnosis of myocardial infarction or symptoms of acute coronary syndrome (n = 71) and control group (n = 10) underwent whole-body postmortem computed tomography (PMCT) as part of routine forensic examinations. Eleven of 71 cases showed relevant amounts of gas formations in PMCT and were excluded to avoid gas-related quantitative measurement falsification in MRI. The remaining 60 cases (study group 35 male, 25 female; mean age 52.4 years, SD 15.3) and the control group underwent MR scanning of the heart prior to autopsy. Autopsies were ordered by the local police bureau. Postmortem imaging was approved by the local ethics committee (number/ID of approval Dnr 2016/200-31). Postmortem interval (time frame between time of death according to autopsy reports and MR scan) ranged between 1 and 4 days. Bodies either received MR scan and autopsy few hours after time of death or were cooled in a refrigerator before imaging and autopsy.
Postmortem MRI
Corpses were MR scanned (Philips, Ingenia 3.0 T) in a linen sheet in supine position. Applied MR sequences were a cardiac short-axis quantification sequence (SyMRI®, Synthetic MR, Linköping/Sweden) [17] and a conventional cardiac short-axis T2-weighted sequence (slice thickness 3 mm, gap 0.3 mm, TR 2700 ms, TE 90 ms). The MR quantification sequence used was a multi-echo, multi-slice turbo spin echo (TSE) sequence, with a saturation pre-pulse. Each acquisition was performed with two different echo times at 23 and 105 ms. Four different saturation delay times were acquired at 210, 610, 1820, and 3830 ms, using a TR of 4020 ms, resulting in a matrix of 2 × 4 images with various effects of T1 and T2 relaxation. The FOV was 230 × 246 mm with 51-mm oversampling, acquired at a resolution of 0.8 × 0.9 mm and reconstructed at a resolution of 0.4 mm. The slice thickness was 4 mm (gap 0.4 mm); 20 slices were acquired. The scan time was 10 min and 20 s, using a SENSE factor 2. The raw images were post-processed using the commercially available SyMRI® 8.0 (Synthetic MR, Linköping/Sweden) to retrieve T1 and T2 relaxation maps, as well as PD maps. The effects of B1 inhomogeneity, coil sensitivity profile, spurious echoes, and imperfect pulse profile effects were automatically corrected for. Based on the maps, synthetic T1-weighted, T2-weighted, and PD-weighted images could be generated.
During MR examination, corpse temperature was assessed in real time by placing a MR proof temperature probe (Temperature Transmitter FTX-300, Fiber Optic Temperature Probe PRB-MR1, Osensa Innovations, Canada) into the esophagus immediately before MR examinations [11, 13, 14].
T1, T2, and PD measurements
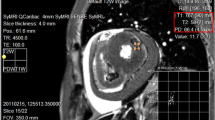
SyMRI® post-processing software for PACS was used for quantitative T1, T2, and PD measurements in synthetically generated cardiac short-axis images of corpses. Synthetic T1-weighted, T2-weighted, and PD-weighted cardiac images were evaluated for visible focal hypointense and/or hyperintense signal alterations in the left and right ventricle of the study group (n = 60). ROIs (size according to magnitude of focal signal alteration but at least 0.5 cm and maximum of 1.5 cm in each dimension) were placed in signal alterations to obtain T1, T2 (in ms), and PD (as %) values as shown in Fig. 1. Only signal alterations with pre-defined size (expansion over at least five consecutive slices and allowing placing at least three adjacent ROIs in one slice) were measured to ensure successful targeting and specimen taking of measured myocardial areas at heart dissection for histologic analysis. In total, five ROIs per signal alteration were placed in at least two different slices. In the control group (n = 10), five ROIs were placed in the right and left ventricle at the anterior wall at midventricular level. Measurements were conducted by a forensic pathologist (observer 1) with 8 years of experience in postmortem MR imaging. Arithmetic means out of five single measurements in MR signal alterations in the study group (n = 60) and arithmetic means out of five single myocardial measurements in the control group (n = 10) were calculated for T1, T2, and PD.
Example measurement of signal alteration in T2-weighted synthetically generated postmortem image using SyMRI® post-processing software for PACS. a ROIs were placed in signal alterations of sufficient size (green arrow). Quantitative T1, T2, and PD values were assessed for measured regions (red box, upper left corner). In this case, histologic diagnosis of early acute myocardial infarction with beginning necrosis was made (b)
Temperature correction of quantitative values
Means of assessed T1, T2, and PD values were plotted against measured temperature in the esophagus. These plots were used to generate equations for temperature correction of quantitative T1, T2, and PD values to 37 °C; the according corpse temperatures were subtracted from 37 °C (Δ temperatures). Δ temperatures were applied in the equations generated from the quantitative values/temperature relations to gain ΔT1, ΔT2, and Δ PD. Δ values were summated to the uncorrected T1, T2, and PD values to gain temperature-corrected values [11, 13, 14].
Myocardial tissue sampling and histologic analysis
Autopsies were conducted immediately after MR examinations. Hearts were dissected by base parallel slicing to match cardiac MR short-axis images. In the study group, the locations of measured myocardial signal alterations in QPMCMR were targeted at dissected heart slices and myocardial tissue specimens were taken from those locations for histologic examinations (Fig. 1). Observer 1 conducted myocardial location targeting and myocardial tissue securement at autopsy. Location targeting and tissue securement were based on comparison of MR images and dissected heart slices for common morphological traits, and the papillary muscles of the left ventricle were used as landmarks. In the control group, myocardial specimens for histologic examinations were taken from dissected heart slices at regions where ROIs were placed in unremarkable myocardium in synthetically generated MR images. Myocardial tissue specimens were processed for histologic examinations using hematoxylin-eosin-saffron staining. Board-certified forensic pathologists that were blinded for MR examination results conducted histologic diagnoses for different age stages of myocardial infarction [3, 5, 12, 18]. Early acute myocardial infarction (approximately 6–24 h): loss of cross striation and beginning necrosis within myocardial fibers; acute infarction (approximately 1–7 days): coagulation necrosis with granulocyte infiltration with or without hemorrhagic component; subacute infarction (approximately 7–28 days): fibroblasts with loose connective tissue formation and angiogenesis. Chronic infarction (approximately >28 days): nucleus-free and blood vessel-free collagenous fibers. Assessed T1, T2, and PD values of each measured signal alterations in the study group were assigned to corresponding histomorphological diagnoses of age of myocardial infarction.
Statistical analysis
SPSS® (version 23.0) was used to perform a series of one-way Welch F test ANOVAs with post hoc analysis to evaluate significant differentiability of temperature-corrected quantitative T1, T2, and PD values between different age stages of myocardial infarction and between different age stages and non-pathologic myocardium of the control group. Receiver operator characteristic (ROC) curve approach was applied to give accuracy of discrimination between tested age stages of myocardial infarction. Accuracy was measured by the area under the ROC curve (AUC) with the traditional academic point system: 0.90–0.1 = excellent, 0.80–0.90 = good, 0.70–0.80 = fair, 0.60–0.70 = poor, and 0.50–0.60 = fail. ROC curve analysis was conducted to determine temperature-corrected T1, T2, and PD cutoff values with highest sensitivity and specificity in each investigated infarction age group compared to temperature-corrected non-pathologic myocardium of the control group.
To estimate intra-observer variability, 20 random cases with visible signal alteration were measured again by observer 1 after a time period of 4 weeks. For estimation of inter-observer variability, two observers (observer 1 and a forensic pathologist with 1 year of experience in postmortem imaging (observer2)) independently measured the same signal alterations (n = 20) in 20 cases. The intra-observer and inter-observer error was estimated calculating the measuring differences of T1, T2, and PD (not corrected for temperature) in each of the two measurement series. ANOVA testing was applied to test the significance of the measuring differences.
Results
In 43 (22 male, 21 female; mean age 62.70 years, SD 11.69) of 60 investigated hearts of the study group, a total of 49 MR signal alterations were detected. Six of 17 cases that did not present with MR signal alterations exhibited ambiguous focal pale myocardial areas at heart dissection but appeared unremarkable at histology. The remaining 11 cases without MR signal alterations showed unremarkable myocardium at heart dissection.
Table 1 gives information about localization of detected MR signal alterations and causes of death in each case. All measured signal alterations presented with histopathological findings. Myocardial signal alterations were diagnosed histologically as follows: early acute infarction (n = 16), acute infarction (n = 10), acute infarction with hemorrhagic component (n = 9), subacute infarction (n = 3), and chronic infarction (n = 11). No signal alterations of pre-defined size were detected in the right ventricle. Subacute lesions were omitted from statistical analysis due to low case numbers. Solely, non-pathologic myocardium without pre-existent cardiac pathologies was diagnosed in the 10 control cases. Toxicology was routinely performed in study group and control group. Neither of the two groups showed relevant intoxications.
Table 2 gives linear equations generated from relations of quantitative T1, T2, and PD values and temperature in non-pathologic myocardium of the control group and histologically confirmed different age stages of myocardial infarction of the study group. Temperature dependence was observed mainly for T1 values that were increasing with rising temperature in non-pathologic myocardium. T2 and PD values were only slightly influenced by temperature. Individual variation of the temperature/quantitative T1, T2, and PD value relations was observed in all investigated age stages of myocardial infarction.
Table 3 depicts the mean quantitative T1, T2, and PD values for the histologically identified different age stages of myocardial infarction in the study group and non-pathologic myocardium in the control group as well as the T1, T2, and PD cutoff values of the different age groups of myocardial infarction compared to non-pathologic myocardium. Figure 2 depicts plots visualizing the mean quantitative temperature-corrected T1, T2, and PD values of non-pathologic myocardium and the different age stages of myocardial infarction.
Table 4 shows that based on assessed quantitative temperature-corrected T1, T2, and PD values, a significant difference between the different tested histopathological age groups of myocardial infarction, as well as between non-pathologic myocardium and all age groups, were verified by statistical analysis. Inter- and intra-observer error analysis indicated non-significant inter- or intra-observer variability of measured signal alterations (Table 5).
Discussion
As a relevant new finding, the results of the present study indicate that histopathological early acute myocardial infarction without hemorrhage or leucocyte migration as well as chronic myocardial infarction with blood vessel-free collagenous fibers may be diagnosed using quantitative 3-T MRI quantification based on T1, T2, and PD values. Moreover, 3-T T1, T2, and PD values of early acute and chronic myocardial infarction stages may be differentiable to values of non-pathologic myocardium and acute myocardial infarction aged more than 24 h. Similar findings were presented in a recent postmortem quantitative 1.5-T study investigating myocardial infarction [12]. However, 1.5-T MRI quantification is different to quantitative 3-T quantification since one and the same tissue exhibits different T1 and T2 relaxation times when exposed to different magnetic field strengths [12, 19]. Accordingly, recent quantitative postmortem studies showed that 1.5-T T1 and T2 relaxation times of myocardium differ to 3-T myocardial relaxation times [11,12,13,14]. The advantage of the quantification approach is that quantitative values are not vendor specific but expected to be within the same range between different MRI machines [6, 10]. Hence, in a recent 3-T QPMCMR study using simultaneous T1, T2, and PD quantification, the values of non-pathologic myocardium and acute myocardial infarction were in the range of the measured values of the present study. However, this previous 3-T QPMCMR study had only very few case numbers and temperature correction of quantitative values was not conducted [13]. Consistent with previous studies, the present study showed that postmortem myocardial relaxation times, T1 relaxation times in particular, are temperature dependent [11, 12]. In order to compare quantitative values of different corpses, temperature correction is inevitable. The present study provides equations that may be used for myocardial temperature correction of relaxation times in postmortem 3-T applications.
Currently, both 1.5- and 3-T MR machines are being used in postmortem imaging [10,11,12]. If the technique of postmortem quantitative cardiac MRI is to be used in the future for routine diagnosis of myocardial infarction, the quantitative values of the different histopathological age stages of myocardial infarction need to be known for 1.5- and 3-T applications. In a next step, databases taken from large study sample are needed to establish representative quantitative values of the different age stages of myocardial infarction for 3- and 1.5-T applications.
To date, it is unclear if quantitative cardiac MRI may be feasible for detection of myocardial infarction aged less than 6 h without visible pathologic alterations in conventional histology. Currently, detection of very early myocardial damage aged less than 6 h may only be possible by combined use of immunohistochemistry, Western blots, and gene expression analyses [20]. However, it is likely to hypothesize that very early myocardial tissue damage would only cause minor changes of T1 and T2 relaxation times that might not significantly differ to relaxation times of non-pathologic myocardium.
The present study showed that visible histomorphological changes of myocardial tissue composition which can be found at acute infarction with a relevant hemorrhagic component (leading to a significant increase of T1, T2, and PD) or in myocardial scars do influence myocardial T1 and T2 relaxation times in rather large ranges (Table 3). What exactly causes changes of relaxation times in early acute myocardial infarction aged more than 6 h is only the issue of speculation. It may be assumed that changes in intracellular and extracellular water due to movement of water molecules and structural changes of cell integrity are responsible for changes of T1 and T2 relaxation times and proton density. Specific variation in the extent of present water and non-water-related changes of the intracellular and extracellular matrix, such as pH value, may be the cause of differing values between early myocardial infarction less than 24 h and acute myocardial infarction older than 24 h but without hemorrhage [2, 4, 5].
Limitations
Overall quantitative postmortem MRI is only feasible if there is no relevant putrefaction. In the present study, myocardial tissue showed no signs of relevant putrefaction due to exclusion criteria. It is likely to hypothesize that putrefaction-related changes of myocardial tissue composition and larger putrefaction gas accumulations in the heart chambers would cause significant changes of quantitative T1, T2, and PD values.
The pre-defined minimal size of measured MR signal alterations was chosen rather larger. The reason for that was to ensure successful targeting of measured myocardial areas at heart dissection for histologic analysis. Targeting of myocardial specimens for histologic examinations was not blinded but done by the observer who conducted quantitative measurements of myocardial signal alterations in QPMCMR. This methodological approach was chosen to assure that the measured myocardial regions in QPMCMR truly matched the taken myocardial specimens for histologic diagnosis.
Conclusions
Quantitative 3-T postmortem cardiac MR based on temperature-corrected T1, T2, and PD values may be feasible for pre-autopsy diagnosis of histopathological early acute, acute, and chronic myocardial infarction which needs to be confirmed histologically.
Abbreviations
- ms:
-
Milliseconds
- MRI:
-
Magnetic resonance imaging
- PMI:
-
Postmortem interval
- QPMCMR:
-
Quantitative postmortem cardiac magnetic resonance
- ROC:
-
Receiver operator characteristic
- ROI:
-
Region of interest
- T:
-
Tesla
- T2w:
-
T2-weighted
- TE:
-
Echo time
- TR:
-
Repetition time
- TSE:
-
Turbo spin echo
- PD:
-
Proton density
References
Jackowski C, Schweitzer W, Thali MJ et al (2005) Virtopsy: postmortem imaging of the human heart in situ using MSCT and MRI. Forensic Sci Int 149:11–23
Ruder TD, Ebert LC, Khattab AA, Rieben R, Thali MJ, Kamat P (2013) Edema is a sign of early acute myocardial infarction on post-mortem magnetic resonance imaging. Forensic Sci Med Pathol 9:501–505
Jackowski C, Christe A, Sonnenschein M, Aghayev E, Thali MJ (2006) Postmortem unenhanced magnetic resonance imaging of myocardial infarction in correlation to histological infarction age characterization. Eur Heart J 27:2459–2467
Winklhofer S, Stoeck CT, Berger N et al (2014) Post-mortem cardiac diffusion tensor imaging: detection of myocardial infarction and remodeling of myofiber architecture. Eur Radiol 24(11):2810–2818
Jackowski C, Schwendener N, Grabherr S, Persson A (2013) Postmortem cardiac 3T magnetic resonance imaging: visualizing the sudden cardiac death? J Am Coll Cardiol 62:617–629
Warntjes JB, Dahlqvist O, Lundberg P (2007) Novel method for rapid, simultaneous T1, T*2, and proton density quantification. Magn Reson Med 57:528–537
Warntjes JB, Leinhard OD, West J, Lundberg P (2008) Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med 60:320–329
Warntjes MJ, Kihlberg J, Engvall J (2010) Rapid T1 quantification based on 3D phase sensitive inversion recovery. BMC Med Imaging 10:19
Warntjes MJ, Engström M, Tisell A, Lundberg P (2015) Modelling the presence of myelin and oedema in the brain based on multi-parametric quantitative MRI. Front Neurol 7:16
Jackowski C, Warntjes MJ, Kihlberg J, Berge J, Thali MJ, Persson A (2011) Quantitative MRI in isotropic spatial resolution for forensic soft tissue documentation. Why and how? J Forensic Sci 56:208–215
Zech WD, Schwendener N, Persson A, Warntjes MJ, Jackowski C (2015) Temperature dependence of postmortem MR quantification for soft tissue discrimination. Eur Radiol 25:2381–2389
Schwendener N, Jackowski C, Persson A, Warntjes MJ, Schuster F, Riva F, Zech WD (2016) Detection and differentiation of early acute and following age stages of myocardial infarction with quantitative post-mortem cardiac 1.5 T MR. Forensic Sci Int. doi:10.1016/j.forsciint.2016.10.014
Zech WD, Schwendener N, Persson A, Warntjes MJ, Jackowski C (2015) Postmortem MR quantification of the heart for characterization and differentiation of ischaemic myocardial lesions. Eur Radiol 25:2067–2073
Zech WD, Hottinger AL, Schwendener N, Schuster F, Persson A, Warntjes MJ, Jackowski C (2016) Post-mortem 1.5 T MR quantification of regular anatomical brain structures. Int J Legal Med 130(4):1071–1080
Shiotani S, Kobayashi T, Hayakawa H, Homma K, Sakahara H (2015) Hepatic relaxation times from postmortem MR imaging of adult humans. Magn Reson Med Sci 15(3):281–287
Tashiro K, Shiotani S, Kobayashi T et al (2015) Cerebral relaxation times from postmortem MR imaging of adults. Magn Reson Med Sci 14(1):51–56
Synthetic MR products website. Available at: http://www.syntheticmr.com. Accessed December 20th, 2016
Schoen F, Mitchell R (2014) The heart. In: Kumar V, Abbas A, Aster J (eds) Robbins and Cotran pathologic basis of disease. Elsevier Saunders, Philadelphia, pp 712–798
Haacke ME, Brown RW, Thompson MR, Venkatesh N (1999) Magnetic resonance imaging-physical principles and sequence design. John Wiley & Sons, New York
Sabatasso S, Mangin P, Fracasso T, Moretti M, Docquier M, Djonov V (2016) Early markers for myocardial ischemia and sudden cardiac death. Int J Legal Med 130(5):1265–1280
Acknowledgements
We would like to thank our teams of forensic pathologists, forensic technicians, histology technicians, and radiologic nurses for their support with case handling. We also thank Mats Fredrikson for his support with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Persson, A., Baeckmann, J., Berge, J. et al. Temperature-corrected postmortem 3-T MR quantification of histopathological early acute and chronic myocardial infarction: a feasibility study. Int J Legal Med 132, 541–549 (2018). https://doi.org/10.1007/s00414-017-1614-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-017-1614-6