Abstract
The objective of this study is to test the validity of sex determination in children and adolescents using lateral radiographic cephalometry and discriminant function analysis. Fifty male and 50 female cephalograms of Taiwanese children were used (males and females with mean age of 15.52 ± 1.38 and 15.67 ± 1.54 years, respectively). Twenty-two cephalometric measurements were performed using computerized cephalometry. Statistical analysis shows that all measurements were sexually dimorphic (p < 0.05). Nine measurements, statistically validated and clinically relevant, were used for discriminant function analysis. A stepwise discriminant procedure selected seven of the nine variables, producing 95% accuracy in sex determination. Resubstitution classification reveals the same discriminant rate. Cross-validation classification (the leave-one-out method) reveals that the correct sex determination rate is 91%. However, the combination of four variables using both the stepwise procedure and the resubstitution method achieves a 92% accuracy rate. A cross-validation classification procedure with the same four variables resulted in a 91% accuracy rate. Therefore, this study uses four cephalometric measurements as the minimum number of traits yielding the maximum discriminant effectiveness of sex determination in children and adolescents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex determination based on skeletal features plays a crucial role in legal medicine and forensic anthropology. Researchers have proposed two approaches, morphological (nonmetrical) and metrical, for the determination of sexual characteristics from bones. Since morphological attributes are more subjective and sex determination depends on the experience of the investigator, inexperienced workers are likely to make inaccurate assessment of sex based using visual methods [1, 2]. However, combining these two approaches improves accuracy, as they complement each other [1, 2].
Next to the pelvis, the skull is the most easily sexed part of the skeleton [3]. As a general rule, the skeleton does not manifest definitive sexual traits until after the full development of the secondary sex characteristics that appear during puberty. As the female progresses from puberty to adulthood, her skull retains many prepubertal traits, such as smoothness and gracility. The male skull, on the other hand, exhibits more robustness and larger muscle attachment areas, more pronounced supraorbital ridges, etc. The lack or weaker development of frontal and occipital superstructures also causes a fairly characteristic difference in the profiles of female and male crania [3].
Discriminant function analysis of the human skull has become important in legal medicine and forensic anthropology [1, 2, 4, 5]. The quantification of morphological traits makes it possible to use them as metrical variables for subsequent discriminant function analysis. Computer-aided analysis of radiographic cephalometric data extends the utility of this method.
Scholars agree that sex identification can be performed easily and with high accuracy using adult skulls [1, 4]. However, relatively few studies discuss sex determination using child skulls. Therefore, the objective of this study is to test the validity of sex discrimination using lateral radiographic cephalometry and discriminant function analysis in children and adolescents. Discriminant function analysis is used to evaluate the significance of the cephalometric variables and to select the minimum number of traits required to achieve maximum discriminatory effectiveness.
Materials and methods
Lateral cephalometric radiographs were obtained from the files of the Orthodontic Department, Kaohsiung Medical University Hospital, from January 2005 to June 2009. The sample in this study comprised 100 children with ages ranging from 12 years 2 months to 17 years 11 months. The sample included an equal number of males and females with mean age of 15.52 ± 1.38 and 15.67 ± 1.54 years, respectively (Table 1). According to the Cervical Vertebral Maturation (CVM) method for the assessment of skeletal ages [6], the males included ten in CS4, 19 in CS5, and 21 in CS6 stages, while the females included three in CS4, 22 in CS5, and 25 in CS6 stages (Table 1).
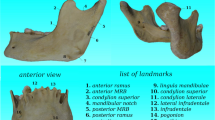
The magnification of linear measurements from each cephalogram used in this study was 10%. Nineteen cephalometric landmarks in each radiograph were identified and digitized (Fig. 1). We chose the sella to nasion (SN) line, the Frankfurt horizontal (FH) plane, and the basion to nasion (BaN) line as reference lines or planes, commonly used in lateral radiographic cephalometric analyses. Calculations of 22 cephalometric measurements (Table 2) were performed using a computerized cephalometric system, Winceph (version 8.0, Rise Co., Japan). Cephalometric data were analyzed using the SAS program (version 9.1; SAS Institute Inc., Cary, NC, USA). Descriptive statistics, including means, standard deviations, and Student’s t test, were obtained for each of the measurements (Table 3).
Cephalometric landmarks. 1 Nasion, 2 glabella, 3 V1 (upper parameter of frontal sinus cavity), 4 V2 (lower parameter of frontal sinus cavity), 5 H1 (anterior parameter of the frontal sinus cavity on inner bregma to nasion line), 6 H2 (posterior parameter of the frontal sinus cavity on inner bregma to nasion line), 7 supraglabella, 8 metopion, 9 bregma, 10 opisthocranion, 11 inion, 12 opisthion, 13 mastoidale, 14 B2 (posterior parameter of the mastoid width at the level of cranial base), 15 B1 (anterior parameter of the mastoid width at the level of cranial base), 16 basion, 17 porion, 18 orbitale, 19 sella
All measurements were found to be significantly different between the sexes at the level of p < 0.01, except for inion-opisthocranion (IOp)–FH, glabella-supraglabellare to supraglabellare-metopion (G–Sg–M), and inion to opisthocranion-opisthion (I–OpO), which were found significantly different at the p < 0.05 level. The Sg–glabella-metopion (GM) measurement was too small (males and females with mean value of 0.71 and 0.38 mm, respectively) for clinical relevance. These four measurements were excluded for discriminant function analysis.
Different cephalometric measurements of the same diagnostic region may reveal essentially the same information. This study selects the most adequate measurements that are statistically validated and clinically relevant [7]. The correlation coefficients (r) for GM–BaN vs. GM–SN and GM–BaN vs. GM–FH are 0.93 (p < 0.0001) and 0.62 (p < 0.0001), respectively. The GM–BaN angle between the sexes has the largest t value among the three variables. The correlation coefficients (r) for IOp–BaN vs. IOp–SN, IOp–BaN vs. IOp–FH, and IOp–BaN vs. opisthion-inion to inion-opisthocranion (O–I–Op) are 0.95 (p < 0.0001), 0.71 (p < 0.0001), and 0.54 (p < 0.0001), respectively. The IOp–BaN angle has the largest t value among the four variables. The correlation coefficients (r) for mastoid height from cranial base (MaHt) vs. mastoid width at the level of cranial base (MaWd), MaHt vs. mastoidale to sella-nasion (Ma–SN) and MaHt vs. mastoidale to porion-orbitale (Ma–FH) are 0.76 (p < 0.0001), 0.62 (p < 0.0001), and 0.62 (p < 0.0001), respectively. The MaHt has the largest t value among the four variables. The correlation coefficients (r) for glabella projection index (GPI) vs. glabella to supraglabellare-nasion (G–SgN) and GPI vs. supraglabellare to nasion (Sg–N) are 0.97 (p < 0.0001) and 0.52 (p < 0.0001), respectively. The GPI has the largest t value among the three variables.
Nine of 22 cephalometric measurements were used in discriminant function analysis. These nine cephalometric variables were treated using the SAS program for discriminant analysis with resubstitution classification, cross-validation classification, and stepwise discriminant analysis (Tables 4, 5, and 6).
To assess errors involved in cephalometric digitizing, 20 randomly selected lateral cephalographs were digitized by one investigator. The same cephalographs were redigitized after an interval of 2 weeks by the same investigator. The method errors between the double measurements were then analyzed for both angular and linear measurements. No significant differences appeared between the two sets of repeated measurements. The method errors were between 0.16 and 0.29 mm for linear measurements and between 0.26° and 0.60° for angular measurements, and the reliability coefficients were from 0.973 to 0.990 [8].
Results
No significant differences were found between sexes in chronological ages (p > 0.05, t test) and in skeletal ages (p > 0.05, x 2 test) (Table 1).
Table 3 shows the descriptive statistics of 22 cephalometric variables from the sample of 100 Taiwanese children and adolescents. As indicated above, the mean differences for all measurements were statistically significant (p < 0.05), indicating the presence of sexual dimorphism in the skull. Mean male values for all linear measurements and proportional measurement were larger than mean female values, but mean male values for all angular measurements were smaller than female values.
For stepwise discriminant function analysis, this study used the SAS computer program to select a combination of measurements that best determine sex from the measurements entered. Since the chi-square value is not significant at the 0.1 level, a pooled covariance matrix was used in the discriminant function with one, two, three, and four cephalometric variables (yielding a linear function) (x 2 = 0.2159, p = 0.6422; x 2 = 4.1882, p = 0.2418; x 2 = 7.6554, p = 0.2645; x 2 = 12.5544, p = 0.2497; respectively). Because of unequal group covariance matrices (x 2 = 39.9428, p = 0.0005; x 2 = 42.7465, p = 0.0034; x 2 = 62.8354, p = 0.0002; respectively), the discriminant function with five, six, and seven variables was based on the individual within-group covariance matrices (yielding a quadratic function) [9].
The computer program selected the best seven out of nine measurements in stepwise analysis. Including additional variables fails to substantially improve sex determination. The model with the GM–BaN alone classifies 73% of the sexes correctly. The models with two variables [GM–BaN and basi-bregmatic height (Ba–Br)] and three variables (GM–BaN, Ba–Br, and MaHt) classify the sexes with 84% and 90% accuracy, respectively, whereas the models with four, five, six, and seven cephalometric variables [GM–BaN, Ba–Br, MaHt, foramen magnum length (Ba–O), GPI, IOP–BaN, and frontal sinus width on inner bregma to nasion line (FSWd)] classify the sexes with accuracy ranging from 92% to 95% (Table 4). Using the same variable models, the correct sex determination rate of the resubstitution approach is the same as that in stepwise discriminant analysis (Table 5).
The cross-validation methods (the leave-one-out classification) with one, two, and three variables yield correct sex determination rates of 73%, 84%, and 90%, respectively, as in the stepwise discriminant analysis and resubstitution classification. However, the classification accuracy of cross-validation procedures with four, five, six, and seven variables all produce the same rate of 91% (Table 6).
Discussion
Age estimation of individuals has received considerable attention in the legal medicine and forensic anthropology [10–15]. Dental and skeletal maturity can be used to estimate the age of individuals in children [16, 17]. Dental maturity is generally accepted as a reliable method to estimate the chronological age of children. The most frequently used method is based on the Demirjian’s stages of dental development. Age estimation is more accurate under 14 years of age [17]. When the subjects are older than 14 years with completely developed permanent teeth (at terminal stage H), the errors between estimated and actual age would increase. Age estimation involving examination of the development of the third molar, which is related to the measurement of the open apices, has been used for determining adult age. It is not an ideal age indicator in identifying individuals being 18 years of age or older [10].
For children and adolescents, the preferred biological process to be considered in the context of an age diagnosis is the skeletal age [18]. Skeletal age assessment can be recorded on lateral cephalometric radiographs, eliminating the need for an additional radiograph of the hand and wrist. Cervical vertebral development was evaluated using the Cervical Vertebral Maturation method [6, 19] based on maturational changes appearing in the second to fourth cervical vertebrae. The CS4 stage represents the decelerating phase of the pubertal growth spurt following the peak height growth. The CS5 stage represents the terminal phase of pubertal growth, and pubertal growth is completed at stage CS6. Therefore, the study subjects were on the deceleration, maturation, or completion maturational stages of skeletal growth [6, 19]. We need further study on the applicability of the clinically applied skeletal age determination of CVM method for forensic age estimation in individuals.
In resubstitution classification of the discriminant analysis, the same data set is used both to derive and evaluate the discriminant function. In general, the resulting estimates are optimistically biased. The selection process of the stepwise discriminant method is not necessarily the best possible model because the selection process does not take into account the relationships between variables that have not yet been selected. Cross-validation is a common way to reduce classification bias. The procedure is repeated n times, omitting a different observation each time. Careful cross-validation can be a valuable aid in selecting a discriminant model [9, 20].
In this study, the cross-validation procedures with four, five, six, and seven variables show a drop in classification accuracy of 1% to 4% compared to the stepwise procedure and resubstitution method and maintain the same accuracy rate of 91%. Therefore, this study selects four cephalometric measurements as the minimum number of traits required to obtain the maximum discriminant effectiveness (91%) with the least possible risk of error. Among these measurements, the GM–BaN variable is an angular measurement of glabella-metopion to basion-nasion. The GM–BaN angle has the largest t value. The large GM–BaN angle in the female corresponds with steeper forehead ascension in the female skull than in the male one [3]. The male glabella-metopion line proceeds from a well-developed glabella (or supraorbital ridges) to a receding forehead, producing a smaller GM–BaN angle. Inversely, the female GM line proceeds from a less developed glabella to a round forehead, creating a larger GM–BaN angle [1]. The other three variables are linear measurements. The Ba–Br is the basi-bregmatic height, MaHt is the mastoid height, and Ba–O is the foramen magnum length, which are expected to differ in size between sexes [2, 4, 21].
Our previous study conducts a discriminant function analysis of lateral cephalometric radiographs from a sample of 50 male and 50 female Taiwanese adults [22]. Using 18 cephalometric variables obtained from the lateral cephalometric radiographs, it is possible to determine the sex of an individual with 100% accuracy. Furthermore, three of the 18 variables are more indicative than the others. Using these three variables alone can determine the sex of a subject to 98% accuracy. In a similar study by Patil and Mody [23], ten cephalometric measurements were used in discriminant function analysis of 150 adults in a central Indian population, and they provided 99% reliability in sex determination. Later, Veyre-Goulet et al. [24] aimed to validate our previous method on a European population. The results showed that sex was determined with 95.6% accuracy in a sample of 114 adult dry skulls using discriminant function derived from the same 18 cephalometric measurements [22]. They concluded that skull-sexing methods using lateral cephalometric radiographs with discriminant function analysis seem always suitable, but the most indicative variables could differ relative to the ethnic population concerned.
Craniofacial morphology has a significant genetic component. When assessing the relative importance of the groups of morphological features, one can generally say that genetically determined morphological features weigh more than metric traits [18]. Yet, cephalometry has the advantage of being quantifiable by the effective means of discriminant analysis. A vital prerequisite of accurate sex identification in skeleton is information about their ethnic origin, so that the most indicative cephalometric variables can be selected.
In conclusion, this study selects four cephalometric measurements as the minimum number of traits required to obtain the maximum discriminant effectiveness of sex determination in children and adolescents. In the future, this discriminant method will be tested on different ethnic populations to confirm its reliability.
References
Inoue M, Inoue T, Fushimi Y, Okada K (1992) Sex determination by discriminant function analysis of lateral cranial form. Forensic Sci Int 57:109–117
Walker PL (2008) Sexing skulls using discriminant function analysis of visually assessed traits. Am J Phys Anthropol 136:39–50
DuBrul EL (1988) Sicher and DuBrul’s oral anatomy, 8th edn. Ishiyaku EuroAmerica, St. Louis
Giles E, Elliot O (1963) Sex determination by discriminant function analysis of crania. Am J Phys Anthropol 21:53–68
Kalmey JK, Rathbun TA (1996) Sex determination by discriminant function analysis of the petrous portion of the temporal bone. J Forensic Sci 41:865–867
Baccetti T, Franchi L, McNamara JA Jr (2005) The cervical vertebral maturation (CVM) method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin Orthod 11:119–129
Chang HP (1985) Evaluation of cephalometric analysis. J Formos Dent Assoc 8:2–13
Houston WJB (1983) The analysis of errors in orthodontic measurements. Am J Orthod 83:382–390
Morrison DF (2005) Multivariate statistical methods, 4th edn. Brooks/Cole Thomson Learning, Belmont
Cameriere R, Ferrante L, De Angelis D, Scarpino F, Galli F (2008) The comparison between measurement of open apices of third molars and Demirjian stages to test chronological age of over 18 year olds in living subjects. Int J Legal Med 122:493–497
Schmidt S, Baumann U, Schulz R, Reisinger W, Schmeling A (2008) Study of age dependence of epiphyseal ossification of the hand skeleton. Int J Legal Med 122:51–54
Schmidt S, Nitz I, Schulz R, Schmeling A (2008) Applicability of the skeletal age determination method of Tanner and Whitehouse for forensic age diagnostics. Int J Legal Med 122:309–314
Schulz R, Mühler M, Reisinger W, Schmidt S, Schmeling A (2008) Radiographic staging of ossification of the medial clavicular epiphysis. Int J Legal Med 122:55–58
Schulz R, Zwiesigk P, Schiborr M, Schmidt S, Schmeling A (2008) Ultrasound studies on the time course of clavicular ossification. Int J Legal Med 122:163–167
Landa MI, Garamendi PM, Botella MC, Alemán I (2009) Application of the method of Kvaal et al. to digital orthopantomograms. Int J Legal Med 123:123–128
Ritz-Timme S, Cattaneo C, Collins MJ, Waite ER, Schütz HW, Kaatsch HJ, Borrman HI (2000) Age estimation: the state of the art in relation to the specific demands of forensic practice. Int J Legal Med 113:129–136
Lee SE, Lee SH, Lee JY, Park HK, Kim YK (2008) Age estimation of Korean children based on dental maturity. Forensic Sci Int 178:125–131
Rösing FW, Graw M, Marré B, Ritz-Timme S, Rothschild MA, Rötzscher K, Schmeling A, Schröder I, Geserick G (2007) Recommendations for the forensic diagnosis of sex and age from skeleton. Homo 58:75–89
Chang HP, Liao CH, Yang YH, Chang HF, Chen KC (2001) Correlation of cervical vertebra maturation with hand–wrist maturation in children. Kaohsiung J Med Sci 17:29–35
McLachlan GJ (2004) Discriminant analysis and statistical recognition. Wiley, New York
Gapert R, Black S, Last J (2009) Sex determination from the foramen magnum: discriminant function analysis in an eighteenth and nineteenth century British sample. Int J Legal Med 123:25–33
Hsiao TH, Chang HP, Liu KM (1996) Sex determination by discriminant function analysis of lateral radiographic cephalometry. J Forensic Sci 41:792–795
Patil KR, Mody RN (2005) Determination of sex by discriminant function analysis and stature by regression analysis: a lateral cephalometric study. Forensic Sci Int 29;147:175–180
Veyre-Goulet SA, Mercier C, Robin O, Guérin C (2008) Recent human sexual dimorphism study using cephalometric plots on lateral teleradiography and discriminant function analysis. J Forensic Sci 53:786–789
Acknowledgements
This work was supported by a research grant from the National Science Council of Taiwan (NSC 85-2331-B-037-086).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hong-Po Chang and Hong-Sen Chen equally contributed to this work as corresponding authors.
Rights and permissions
About this article
Cite this article
Hsiao, TH., Tsai, SM., Chou, ST. et al. Sex determination using discriminant function analysis in children and adolescents: a lateral cephalometric study. Int J Legal Med 124, 155–160 (2010). https://doi.org/10.1007/s00414-009-0412-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-009-0412-1