Abstract
Aspartic acid racemisation (AAR) results in an age-dependent accumulation of d-aspartic acid in durable human proteins and can be used as a basis for age estimation. Routinely, age estimation based on AAR is performed by analysis of dentine. However, in forensic practise, teeth are not always available. Non-dental tissues for age estimation may be suitable for age estimation based on AAR if they contain durable proteins that can be purified and analysed. Elastin is such a durable protein. To clarify if purified elastin from arteries is a suitable sample for biochemical age estimation, AAR was determined in purified elastin from arteries from individuals of known age (n = 68 individuals, including n = 15 putrefied corpses), considering the influence of different stages of atherosclerosis and putrefaction on the AAR values. AAR was found to increase with age. The relationship between AAR and age was good enough to serve as basis for age estimation, but worse than known from dentinal proteins. Intravital and post-mortem degradation of elastin may have a moderate effect on the AAR values. Age estimation based on AAR in purified elastin from arteries may be a valuable additional tool in the identification of unidentified cadavers, especially in cases where other methods cannot be applied (e.g., no available teeth and body parts).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age estimation for forensic purposes is commonly used to assess the age of young subjects (age threshold for criminal prosecution and application of adult criminal law) or to estimate the age of unknown cadavers as one of the first steps for identification. Several age-estimation techniques with different accuracy are presently available, and most of these require specialised and trained scientists. Nevertheless, one major disadvantage of these techniques using morphological features (skeletal or dental development) is that the accuracy depends on the skills and the experience of the ordered expert [6, 13]. Morphological features mirror the biological variance in a population, e.g., an accelerated ossification of the wrist depends and is related to the socio-economic status in modern populations [31, 32]. In the light of these uncertainties, the Study Group on Forensic Age Diagnostics recommended the implementation of three morphologically independent features in each single case to obtain age estimates as precisely as possible [33].
Aspartic acid racemisation (AAR) is an inevitable consequence of the physiological ageing of proteins and can be observed in numerous proteins of different human tissues (for details see [24]). It leads to an age-dependent accumulation of d-aspartic acidFootnote 1 in durable human proteins, which can be used as a basis of biochemical age estimation.
Age estimation based on AAR is both highly accurate and reproducible, as confirmed by numerous publications from different groups (e.g., [2, 8–12, 15, 16, 20–23, 25, 27–29]). Meanwhile, age estimation based on AAR by analysis of dentine is now performed routinely. Because of its high content of durable proteins and its relatively stable biochemical composition, it is an ideal tissue for biochemical age estimation. In forensic practise, however, there are cases when teeth are not present. Apart from dentine, other tissues for age estimation may be suitable for age estimation based on AAR, if they would contain durable proteins that can be purified and analysed. Such a protein is represented by elastin: it is an extremely durable, if not permanent, protein.
A close relationship between AAR and age indicate, that a lifetime residence for mature elastin (for details, see [24]) was described for purified elastin from human lung parenchyma, yellow ligaments, skin, and arteries [17, 27, 28, 34]. The yellow ligament containing an extremely high amount of elastin represents a tissue well-suited for age estimation based on AAR in purified elastin [27].
Data published by Powell et al. [17] have proven a close relationship between AAR in purified elastin from arteries and age. However, this data set consisted of only 12 cases, was based on coarse purification (characterised by amino acid composition) and did not consider potential influences of pathology (atherosclerosis) and post-mortem conditions (putrefaction).
To clarify if purified elastin from arteries is a suitable target protein for biochemical age estimation, AAR was determined in purified elastin from arteries from individuals of known age (n = 68, including n = 15 putrefied corpses) taking possible effects of atherosclerosis and putrefaction into account.
Material and methods
AAR was determined in purified elastin from the abdominal aorta of 53 individuals aged between 6 weeks and 91 years, and the relationship between AAR and age was tested. All tissue samples were graded according to the degree of atherosclerotic lesions. To assess the impact of putridity on AAR in elastin, 15 samples from arteries of putrefied deceased were analysed.
Elastin preparation and grading of atherosclerotic lesions
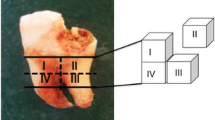
Specimens from the abdominal aorta of 53 individuals were collected during autopsy. In these cases, the post-mortem interval (pmi) was short and no signs of putrefaction were recorded. The samples were prepared avoiding contamination with adjacent tissues. Prior to further preparation, arterial walls were graded with regard to the extent of atherosclerosis according to the following qualitative criteria [4]: state 1, fatty lesions (fatty streaks or predominantly fatty plaques); state 2, fibrous plaques or diffuse thickening of the intima; and state 3, complicated lesions (old or recent haemorrhage, ulceration, thrombosis, and calcification).
To test the impact of putrefaction on AAR in elastin, 15 samples from putrefied deceased were taken from the same location and prepared identically. In these cases collected from April to November 2008, the post-mortem interval varied from some days to several weeks (Table 1).
Rating of putrefaction, determination of post mortem interval
Corpses displaying signs of putrefaction were rated into four stages, principally basing on the changed morphology of the internal organs (compare Table 1). Stage 0 was assigned to bodies displaying no putrefaction-associated abnormalities; stage 1 (beginning putrefaction) displays first signs of changing colour of the skin and the viscera, only marginally affected on macroscopic and histological level. Advanced putrefaction (stage 2) was allocated to findings of moderate signs of putrefaction of the viscera. High-grade putrefaction (stage 3) was allocated to viscera which were macroscopically and, in histological slides, extensively destroyed. Apart from one case (committed suicide), all corpses were found in their domiciles. The duration of the pmi and the stages of putrefaction were obtained from the files of enquiry and autopsy reports.
Purification of elastin
Purification of the highly stable and insoluble cross-linked elastin was performed grossly by solubilization and removal of all other proteins.
The tissue samples were processed following the procedure of Ritz-Timme et al. [28], which, in brief, includes the following steps: specimens were washed in 15% NaCl/protease inhibitor solution overnight, defatted in ethanol/ether for 15 min, again washed in excess aqua dest. (each step at 4°C), then freeze-dried overnight and stored at −20°C before further purification. The tissue samples were weighed and suspended in formic acid (5 ml per 100 mg tissue) containing excess CNBr (200 mg in 10 ml of 98% formic acid) and shaken at room temperature for 5 h. After centrifugation and washing with 10-mmol EDTA until a neutral pH was reached, the samples were digested twice with bacterial collagenase (Sigma type 7, 125 U per 100 mg tissue, Sigma-Aldrich, Taufkirchen, Germany) for 24 h at 37°C. At the end of each collagenase digestion, the samples were rinsed thoroughly in cold aqua dest. and digested by trypsin (133 µg per 1 mg tissue) for 18 h at 37°. Finally the samples were rinsed in distilled water, washed in 8-M urea/0.1 M mercaptoethanol for 18 h at room temperature, rinsed in aqua dest., freeze-dried, weighed for the calculation of elastin yield, and stored at −20°C until further processing.
Assessment of the quality of elastin purification
The quality of the elastin purification was controlled by examination of stained histological paraffin sections (Elastica van Gieson stain) and by amino acid analysis. Amino acid analyses were performed on aliquots of the purified elastin and compared to database entries. The samples were hydrolysed in 6 M HCl for 24 h at 110°C. Analyses were performed by high-performance liquid chromatography (HPLC; Agilent 1100 Series, consisting of a programmable injector unit, quaternary pump, and fluorescence detector, Agilent Technologies, Böblingen, Germany). A combined pre-column derivatisation with OPA/MPA (o-phthaldialdehyde/3-mercaptopropionic acid) and FMOC (9-flourenylmethylchloroformate) allowed the detection of primary and secondary amino acids (Pro, Hyp) in a single run (modified after [3, 5]). Separation was achieved on a HPLC column type Hypersil BDS C18 250 × 3 mm, 5 µm (Thermo Electron GmbH, Dreieich, Germany) with a flow of 1 ml min−1 at 40°C, using a gradient from 90% mobile phase A (40 mM sodium dihydrogen phosphate monohydrate, NaH2PO4), 1.5 mM sodium azide (NaN3), adjusted to pH 7.8 and 10% mobile phase B (450 ml methanol, 450 ml acetonitrile, 100 ml water; all chemicals HPLC grade) from 100-43% A within 40 min. The post-run consisted of flushing the column with 100% B for 7 min, followed by an equilibration of 6 min duration at a flow rate of 1.2 ml min−1. For the detection of primary amino acids, the fluorescence detector was adjusted to an excitation wavelength of 340 nm and a detection wavelength of 450 nm. After 35–39 min the detector was switched to 266 nm and 305 nm for the detection of secondary amino acids. Calibrated external standards were used for signal identification and quantification.
Determination of AAR in purified elastin; evaluation of the relationship between AAR and age
The extent of aspartic acid racemisation was determined as described by Ritz et al. [21] and Ritz-Timme [23], taking into account defined quality standards [26]. Briefly, 5 mg of purified and lyophilized elastin samples were hydrolysed in 6 N HCl at 100°C for 6 h. Hydrochloric acid and water were removed in a vacuum and the dry hydrolysate was esterified with isopropanol/sulfuric acid (10:1) for 1 h at 110°C. After alkaline extraction with dichloromethane, acetylation was performed with trifluoroacetic anhydride (TFAA) at 60°C for 15 min. Amino acids were now present as TFA isopropyl esters. The ratio of d-aspartic acid to l-aspartic acid was determined after separation of the amino acids by gas chromatography (GC-2014, Shimadzu, Duisburg, Germany) on a chiral capillary column (Chirasil-L-Val 25 m × 0.25 mm, Varian, Middelburg, The Netherlands) using a flame ionisation detector, with hydrogen as carrier gas. The samples were analysed at least in duplicate. Signals were identified using external standard substances and the extent of AAR was calculated as the d/l ratio.
Results
Purity of the analysed elastin samples
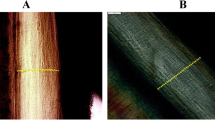
The histological examination of the purified elastin revealed no detectable collagen contamination at the morphological level (Fig. 1). The results of the total amino acid analysis of purified elastin from arterial walls are displayed in Fig. 2 in comparison with the data for the elastin standard and a predicted amino acid composition of human elastin, calculated from the cDNA of tropoelastin [1]. The amino acid composition of the purified elastin samples was reproducible and in excellent agreement with the composition of the elastin standard.
AAR in purified elastin from arteries
AAR in purified elastin from arterial samples increased with age (Fig. 3). The relationship between AAR and age (r = 0.90) can be described by the linear regression equation: y = 0.1121x + 12.621, where, y = d/l-Asx (%) and x = individual age. The mean standard error on the estimated age was 15.48 years using the method of inverse prediction [36]. The linear regression considers only 45 fully adolescent individuals of 24 years or older and neglects potential impacts of atherosclerosis.
AAR in samples from arteries with arteriosclerotic lesions
From 45 fully adolescent individuals, eight revealed no signs of atherosclerosis (grade 0, at the age between 25 and 55 years). Thirty-seven samples exhibited arterioslerotic lesions (n = 14 with grade 1, age between 24 and 89 years; n = 15 with grade 2, age between 43 and 91 years; n = 8 with grade 3, 53–93 years). They did not exhibit systemically lower or higher AAR values, only a tendency to a greater scattering was observed (Fig. 4). The severity of atherosclerosis generally increased with age and its different stages were distributed over a broad interval of age (Fig. 4, insert).
AAR in putrefied samples
Figure 5 depicts the results of the analysis of putrefied samples in comparison to the data of non-putrefied samples. Despite the extended pmi and putrefaction, these AAR data mostly fitted fairly well into the data set of the A. infrarenalis obtained from individuals under short pmi conditions. However, in isolated cases of advanced putrefaction, the AAR showed extreme scattering in both directions. The stages of putrefaction exhibited a wide overlap in the post mortem interval (compare Table 1), for instance stage 1 (n = 5) was allocated to pmi of 2–5 days, stage 3 (n = 3) was already observed in an individual having been discovered after 5 days as well. Morphological changes attributed to stage 2 (n = 6) were found in individuals, who displayed pmi ranging from 3–10 days.
Discussion
The purity of the analysed elastin samples
If biochemical age estimation is to be performed on purified proteins, the purity of the samples is essential for the quality of age estimation. Contaminating proteins will have negative effects on the results as the kinetics of AAR depends on the molecular structure of a protein (see [24]). Thus, the absence of collagen is crucial for a correct analysis of AAR in elastin. Elastin contains 1.5–5.2 Asx/1,000 residues (dependent on splice variant) compared to 42 Asx/1,000 residues in arterial alpha 1 (III) collagen [7, 28]. The slower racemisation kinetics of Asx in collagen will result in an underestimation of the AAR in collagen-contaminated elastin samples. Histological analysis did not indicate any collagen contamination. With the purification protocol applied here, an elastin preparation with a reproducible amino acid composition was obtained, which was in close agreement with the composition of the elastin standard and with the predicted amino acid composition of human elastin from sequence data (Fig. 2). Amino acid compositions displayed a low amount of hydroxyproline and biochemically confirmed the absence of contaminating collagen in the elastin specimens. However, some prolyl-residues in elastin are transformed through post-translational hydroxylation [18] but to a considerably lower amount as compared with collagen. Thus, the detected low amount of hydroxyprolin residues in combination with the absence of methionine is a strong indicator for elastin purity. Tryptophan, theoretically an additional marker for the presence of collagen, is destroyed during hydrolysis and, thus, was not detected. Overall, the elastin preparation appeared to be suitable for age estimation based on AAR, a fact confirmed by the AAR data.
AAR in purified elastin from arteries
Analysis of the purified elastin revealed a relatively rapid accumulation of d-Asx and a close relationship with age in adults. In younger individuals, elastin synthesis still persists. This explains a logarithmical progression of the accumulation of d-Asx in elastin in adolescent individuals which changes to linearity with the end of this process (Fig. 3). These data support the data of Powell et al. [17] and confirm the longevity of elastin in the arterial wall (for details, see [24]). The age-dependent accumulation of modified aspartic acid residues is a common feature in ageing elastin regardless of the tissue source. In fact, in healthy adult tissues, elastin production is suppressed by a post-transcriptional mechanism mediating a rapid decay of the tropoelastin mRNA [14, 35], however, re-initiation of elastin production has been observed after tissue damage [30]. This is apparently a contradiction to the assumption that elastin is a permanent protein without relevant turnover, as indicated by the AAR data. Newly synthesised elastic fibres have been described as highly disorganised, non-cross-linked, immature, and of a low elastin content [30]; these incomplete fibres should be removed by the purification steps employed (see also [17]). It is an interesting, but as yet unsolved question, whether the accumulation of post-translationally modified elastin molecules with age has functional implications for the elasticity of the artery wall.
Age estimation based on AAR in elastin from arteries
The relationship between AAR in elastin from arteries was close (r = 0.90, Fig. 3), but considerably worse than in the cases of the "routine sample" dentine or even in elastin from yellow ligaments [27]. This can be readily explained by much more complex biochemical and physical influences on the elastin molecules in the arterial wall, which may result in an intravital degradation of the molecule. Degradation changes the molecular structure and may have effects on AAR [24]. This fact is obviously also the reason for the tendency to a higher scattering of values in samples with advanced atherosclerosis and for divergent values in putrefied samples (Figs. 4 and 5).
However, the relationship between AAR in purified elastin from arteries is still close enough to serve as basis for biochemical age estimations. The attainable accuracy is better than in most other methods for age estimation in adults [25], despite of the effects of atherosclerosis. Age estimation based on AAR in elastin from arteries may be a valuable additional tool in the identification of unidentified cadavers, especially in cases where other methods cannot be applied (e.g., no available teeth and body parts). To minimise the influences of intravital degradation effects, samples displaying severe signs of atherosclerosis should not be analysed. In case of advanced putrefaction, the risk of a false AAR values has to be considered; however, very large discrepancies should not be expected when putrefaction is at an early stage. It is dispensed with a detailed statistical analysis, which links these impacts on the AAR. The analysis of the present AAR dataset, with respect to the impact of atherosclerosis and putrefaction, results for both in a broad heterogeneity and small quantities per single category (compare Fig. 4 and Table 1). In subsequent works focussing single effectors, this should be caught up and experimental approaches should supplement the present preliminary results.
The necessity of high quality elastin purification makes the method much more sophisticated than the analysis of the routine sample dentine. Even more than for dentine, the use of quality standards [26] is important, and the result of the elastin preparation has to be controlled strictly by analysis of the amino acid composition.
Nevertheless, the present preliminary and previous results from elastin containing tissues (aorta, [17]; intervertebral discs, [19]; yellow ligaments, skin, [27, 28]; and lung, [34]) suggest that AAR should give coherent age estimates from purified elastin independent of the type of tissue, if the prerequisites mentioned above are fulfilled.
Notes
In proteins, the so-called racemisation of aspartic acid involves both aspargine and aspartic acid that decompose via a succimide ring to the same four residues, namely l-aspartyl, d-aspartyl, l-isoaspartyl, and d-isoaspartyl residues, all of which are in chemical equilibrium via the succimide ring (for overview, see [24]). Asparingyl, aspartyl, isoaspartyl, and succinimidyl residues are all converted to free aspartic acid during acid hydrolysis, a preparative step in chromatographic amino acid analysis for biochemical age estimation.
References
Daamen WF, Hafmans T, Veerkamp JH, van Kuppevelt TH (2001) Comparison of five procedures for the purification of insoluble elastin. Biomaterials 22:1997–2005
Fu S-J, Fan C-C, Song H-W, Wei F-Q (1995) Age estimation using a modified HPLC determination of ratio of aspartic acid in dentin. Forensic Sci Int 73:35–40
Heems D, Luck G, Fradeau C, Vérette E (1998) Fully automated precolumn derivatization, online dialysis and high-performance liquid chromatography analysis of amino acids in food, beverages and foodstuff. J Chromatogr A 789:9–17
Holman RL, Brown BW, Gore I et al (1960) An index for the evaluation of arteriosclerotic lesions in the abdominal aorta. Circulation 27:1137–1143
Kaufman DS, Manley WF (1998) A new procedure for determining DL amino acid ratios in fossils using reverse phase liquid chromatography. Quat Geochronol 17:987–1000
Landa MI, Garamendi PM, Botella MC, Alemán I (2009) Application of the method of Kvaal et al. to digital orthopantomograms. Int J Legal Med 123:123–128
Miller EJ (1984) Chemistry of the collagens and their distribution. In: Piez KA, Reddi AH (eds) Extracellular matrix proteins. Elsevier, New York, Amsterdam, Oxford, pp 40–81
Mörnstad H, Pfeiffer H, Teivens A (1994) Estimation of dental age using HPLC technique to determine the degree of aspartic acid racemization. J Forensic Sci 39:1425–1431
Ogino T, Ogino H, Nagy B (1985) Application of aspartic acid racemization to forensic odontology: post mortem designation of age of death. Forensic Sci Int 29:259–267
Ohtani S (1995) Estimation of age from the teeth of unidentified corpses using the amino acid racemization method with reference to actual cases. Am J Forensic Med Pathol 16:238–242
Ohtani S (1995) Estimation of age from dentin by using the racemization reaction of aspartic acid. Am J Forensic Med Pathol 16:158–161
Ohtani S, Yamamoto K (1987) Age estimation using the racemisation of aspartic acid in human dentin. Nippon Hoigaku Zasshi 41:181–190
Paewinsky E, Pfeiffer H, Brinkmann B (2005) Quantification of secondary dentine formation from orthopantomograms—a contribution to forensic age estimation in adults. Int J Legal Med 119:27–30
Parks WC (1997) Posttranscriptional regulation of lung elastin production. Am J Respir Cell Mol Biol 17:1–2
Pfeiffer H, Mörnstad H, Teivens A (1995) Estimation of chronological age using the aspartic-acid racemization method. 1. On human rib cartilage. Int J Legal Med 108:19–23
Pfeiffer H, Mörnstad H, Teivens A (1995) Estimation of chronological age using the aspartic-acid racemization method. 2. On human cortical bone. Int J Legal Med 108:24–26
Powell JT, Vine N, Crossman M (1992) On the accumulation of d-aspartate in elastin and other proteins of the aging aorta. Atherosclerosis 97:201–208
Prockop DJ, Kivirikko KI, Tuderman L, Guzman NA (1979) Biosynthesis of collagen and its disorders. N Engl J Med 301(13–23):77–85
Ritz S, Schütz HW (1993) Aspartic acid racemization in intervertebral discs as an aid to post mortem estimation of age at death. J Forensic Sci 38:633–640
Ritz S, Schütz H-W, Schwarzer B (1990) The extent of aspartic acid racemization in dentin: a possible method for a more accurate determination of age at death? Z Rechtsmed 103:457–462
Ritz S, Schütz HW, Peper C (1993) Postmortem estimation of age at death based on aspartic acid racemization in dentin: its applicability for root dentin. Int J Legal Med 105:289–293
Ritz S, Stock R, Schütz HW, Kaatsch H-J (1995) Age estimation in biopsy specimens of dentin. Int J Legal Med 108:135–139
Ritz-Timme S (1999) Lebensaltersbestimmung aufgrund des Razemisierungsgrades von Asparaginsäure. Grundlagen, Methodik, Möglichkeiten, Grenzen, Anwendungsbereiche. In: Berg S, Brinkmann B (eds) Arbeitsmethoden der medizinischen und naturwissenschaftlichen Kriminalistik, Band 23. Lübeck, Schmidt-Römhild, pp 15–41
Ritz-Timme S, Collins MJ (2002) Racemization of aspartic acid in human proteins. Age Res Rev 1:43–59
Ritz-Timme S, Cattaneo C, Collins M, Waite ER, Schütz HW, Kaatsch H-J, Borrman HIM (2000) Age estimation: the state of the art in relation to the specific demands of forensic practise. Int J Legal Med 113:129–136
Ritz-Timme S, Rochholz G, Schütz HW, Collins MJ, Waite ER, Cattaneo C, Kaatsch HJ (2000) Quality assurance in age estimation based on aspartic acid racemization. Int J Legal Med 114:83–86
Ritz-Timme S, Laumeier I, Collins M (2003) Age estimation on aspartic acid racemization in elastin from the yellow ligaments. Int J Legal Med 117:96–101
Ritz-Timme S, Laumeier I, Collins M (2003) Aspartic acid racemization: evidence for marked longevitiy of elastin in human skin. Br J Dermatol 149:951–959
Rösing FW, Kvaal SI (1998) Dental age in adults. A review of estimation methods. In: Rösing FW, Kvaal SI, Alt KW, Rösing FW, Teschler-Nicola M (eds) Dental anthropology. Fundamentals, limits, and prospects Springer, Wien New York, pp 443–469
Rucker RB, Dubick MA (1984) Elastin metabolism and chemistry: potential roles in lung development and structure. Env Hlth Pers 55:179–191
Schmeling A, Reisinger W, Loreck D, Vendura K, Markus W, Geserick G (2000) Effects of ethnicity on skeletal maturation: consequences for forensic age estimations. Int J Legal Med 113:253–258
Schmeling A, Schulz R, Danner B, Rösing FW (2006) The impact of economic progress and modernization in medicine on the ossification of hand and wrist. Int J Legal Med 120:121–126
Schmeling A, Grundmann C, Fuhrmann A, Kaatsch H-J, Knell B, Ramsthaler F, Reisinger W, Riepert T, Ritz-Timme S, Rösing FW, Rötzscher K, Geserick G (2008) Criteria for age estimatin in living individuals. Int J Legal Med 122:457–460
Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ (1991) Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of d-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 87:1828–1834
Zhang M, Pierce RA, Wachi H, Mecham RP, Parks WC (1999) An open reading frame element mediates posttranscriptional regulation of tropoelastin and responsiveness to transforming growth factor β1. Mol Cell Biol 19:7314–7326
Zar JH (1984) Biostatistical analysis. Prentice Hall, Englewood Cliffs, pp 276–277
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dobberstein, R.C., Tung, SM. & Ritz-Timme, S. Aspartic acid racemisation in purified elastin from arteries as basis for age estimation. Int J Legal Med 124, 269–275 (2010). https://doi.org/10.1007/s00414-009-0392-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-009-0392-1