Abstract
Homologous chromosomes exchange genetic information through recombination during meiotic synapsis, a process that increases genetic diversity and is fundamental to sexual reproduction. Meiotic studies in mammalian species are scarce and mainly focused on human and mouse. Here, the meiotic recombination events were determined in three species of Platyrrhini monkeys (Cebus libidinosus, Cebus nigritus and Alouatta caraya) by analysing the distribution of MLH1 foci at the stage of pachytene. Moreover, the combination of immunofluorescence and fluorescent in situ hybridisation has enabled us to construct recombination maps of primate chromosomes that are homologous to human chromosomes 13 and 21. Our results show that (a) the overall number of MLH1 foci varies among all three species, (b) the presence of heterochromatin blocks does not have a major influence on the distribution of MLH1 foci and (c) the distribution of crossovers in the homologous chromosomes to human chromosomes 13 and 21 are conserved between species of the same genus (C. libidinosus and C. nigritus) but are significantly different between Cebus and Alouatta. This heterogeneity in recombination behaviour among Ceboidea species may reflect differences in genetic diversity and genome composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meiosis is a specialised cell division by which haploid gametes (containing a single copy of each chromosome) are generated. In most eukaryotes, homologous chromosomes exchange genetic information through recombination during meiosis, a process that increases genetic diversity by breaking haplotypes. Recombination provides physical connections between homologues during the first meiotic division, contributing to correct chromosome segregation. Although recombination can occur at the somatic level (such as recombination produced in the immune system), only those recombination events occurring in the germ line will have effects in genome diversity and composition.
Homologous recombination is triggered by programmed double-strand breaks early in meiosis (Bishop and Zickler 2004). Studies in the budding yeast have revealed that the broken ends are processed, and approximately half of them result in double Holliday junctions and crossovers (COs) (Petes 2001). In this organism, COs exhibit three main features: (a) they are not randomly distributed but rather concentrated in hotspots, (b) there is always at least one CO per pair of homologous chromosomes (so-called obligatory chiasmata) to ensure the proper disjunction of homologous chromosomes and (c) they present “interference” (COs tend to follow an evenly spaced distribution) (Petes 2001). Studies in mammals have shown, on the other hand, that the total number and distribution of COs in a specific chromosome depend on several factors, such as chromosomal size, gender and age of the individual under study (Lynn et al. 2004; Paigen and Petkov 2010). Larger chromosomes tend to accumulate larger numbers of COs, and each chromosome arm generally presents at least one CO (Sun et al. 2004). Early studies (Dutrillaux 1986) demonstrated a strong correlation between the number of chiasmata and the haploid number of chromosome arms. Following this view, Pardo-Manuel de Villena and Sapienza (2001) suggested that the recombination rate was proportional to the number of chromosome arms, after analysing a wide range of mammalian species. Such assumptions are of relevance given that perturbations in the recombination pattern have been shown to be linked to non-disjunction problems in humans (Lamb et al. 2005).
Recombination maps elaborated using LD analyses or/and sperm typing are now available for a variety of species (Frazer et al. 2007; Megens et al. 2009; Qanbari et al. 2010; Rogers et al. 2006; Wu et al. 2010). While LD analyses represent indirect evidence of the recombination process, sperm typing directly detects recombinant DNA molecules. LD-based maps estimate the location of recombination events in the progeny (see Lynn et al. 2004 for a review) and represent an integration of population-level processes over several generations (Clark et al. 2010). However, if our intention is to analyse the recombination process as it occurs, alternative methods to genetic linkage analysis should be used. This is the case of the in situ immunolocalisation of recombination proteins on spermatocyte cells (Lynn et al. 2002), which allows for the recognition of physical COs during the first meiotic prophase in each cell individually by analysing the distribution of MLH1 foci, a marker of CO events (Baker et al. 1996). Recombination maps using this last direct approach have been made in humans, both female (Robles et al. 2009; Tease et al. 2002; Lenzi et al. 2005) and male (Barlow and Hulten 1998; Codina-Pascual et al. 2006; Ferguson et al. 2009; Hassold et al. 2004; Lynn et al. 2002; Sun et al. 2004), mouse (Anderson et al. 1999; Froenicke et al. 2002) and, more recently, in birds (Pigozzi 2007; 2008; Pigozzi and Solari 2005), marsupials (Franco et al. 2007), dogs (Basheva et al. 2008), the American mink (Borodin et al. 2009) and the common shrew (Borodin et al. 2008a,b) as well as several rodent species (Dumont and Payseur 2011). However, meiotic studies in non-human primates are scarce and are based on classical techniques, such as Giemsa and silver staining (Armada et al. 1987; Lima and Seuanez 1991; Mudry et al. 1998, 2001; Rahn et al. 1996; Seuánez et al. 1983; Steinberg et al. 2007, 2008). Only until very recently has in situ immunolocalisation of recombination proteins been applied in non-human primates (Garcia-Cruz et al. 2009; Hassold et al. 2009). Therefore, experimental descriptions of recombination maps in other mammalian species are needed if the ultimate goal is to understand the mechanisms underlying chromosomal evolution and speciation.
In an attempt to shed light on the dynamics of mammalian meiosis and its implication in chromosome evolution, we have analysed the recombination features during meiosis in three Ceboidea species: Cebus libidinosus [formerly C. paraguayanus (Groves 2001)], Cebus nigritus and Alouatta caraya (Platyrrhini, Primates). Immunoflorescence against different meiotic proteins involved in the synaptonemal complex (SC) formation and recombination were performed on testicular material of these species. Moreover, the application of fluorescence in situ hybridisation with the 13 and 21 human whole-chromosome painting probes in spermatocytes of all three species allowed for the construction of the recombination map of these specific chromosomes from an evolutionary perspective, since their chromosomal homologies are known (de Oliveira et al. 2002; Garcia et al. 2000, 2002; Stanyon et al. 2011). Using this approach, we were able to investigate whether the cytological distribution of COs in homologous chromosomes is conserved among these species and whether the presence of extracentromeric/interstitial heterochromatin regions influences the location of COs.
Materials and methods
Sample processing
One male individual from each species was included in this study, all of them born in the wild within the southern-most natural geographic distribution. The adult specimen of A. caraya (ACA) was 15 years old and was kept in captivity at the Centro de Reeducación del Mono Aullador Negro (Córdoba, Argentina), where it has sired nine offspring. The typical lifespan of Alouatta has been recorded to be approximately 20 years (Chebez and Ruiz Blanco 2008). Both C. libidinosus (CLI) and C. nigritus (CNI) adult specimens were kept in captivity at the Córdoba Zoo (Córdoba, Argentina). The CLI specimen was 10 years old and has sired one offspring, whereas the CNI specimen was 7 years old and with unrecorded offspring. The lifespan of Cebus can range from 15 to 25 years, depending on the species (Canevari and Fernández Balboa 2003; Fedigan and Jack 2004).
Testicular biopsies were obtained from each male according to the protocols of the “Guide for Care and Use of Experimental Animals”, as promulgated by the Canadian Council of Animal Care and the Principles for the Ethical Treatment of non-human Primates of the American Society of Primatologists (ASP). Biopsies were taken by the veterinarian Miguel Carelo (Córdoba Zoo) after administration of a dose of anaesthetics (Ketamine, Holliday Scott; Argentina; 10 mg kg−1 day−1) and transferred to GIBE (FCEyN, UBA; Buenos Aires), where they were kept at −80°C until use. Testicular tissues were processed as previously described (Garcia-Cruz et al. 2009) in order to obtain spermatocyte spreads.
Immunostaining
Immunostaining of meiocytes was performed following Garcia-Cruz et al. (2009). Different sets of antibodies were used: rabbit poyclonal serum against central element protein SYCP3 (a kind gift of C. Heyting), mouse monoclonal antibody against MLH1 (BD Pharmingen) and human calcinosis, Raynaud’s phenomenon, oesophageal dysfunction, sclerodactyly and telangiectasia (CREST) serum (a kind gift of M. Fritzler). Fluorochrome-conjugated secondary antibodies were used for detection (all from Jackson ImmunoResearch Laboratories). Primary and secondary antibodies were diluted in PBTG (0.2% bovine serum albumin, 0.05% Tween, 0.2% gelatin in phosphate-buffered saline). Primary antibodies were incubated overnight at 4°C or at room temperature in a humid chamber. Secondary antibodies were incubated for 1 h at 37°C in a humid chamber. DNA was counterstained with antifade solution (Vector Laboratories) containing 0.1 μg/ml 4′,6′-diamidino-2-phenylindole (Sigma).
Fluorescence “in situ” hybridisation
Whole-chromosome painting probes specific to human (HSA) chromosomes 13 and 21 (Metasystems) were hybridised on previously immunostained preparations to identify SCs of homologous chromosomes in ACA (chromosomes 20 and 21, respectively) and CLI/CNI (chromosomes 17 and 11, respectively). A first denaturation of the slides was performed in 70% formamide in a 2× saline sodium citrate (SSC) solution for 5 min at 70°C. The slides were washed in water, and treatment with NaSCN 1 M for 3 h at 65°C was applied. A second denaturation step was then performed as stated above. Dehydration of the slides was carried out before applying the denatured probe. Hybridisation was performed at 37°C in a humid chamber for 72 h. Post-hybridisation washes were performed in 4× SSC.
Data processing
Preparations were visualised using a Nikon eclipse 90i epifluorescence microscope equipped with the appropriate filters and a charged coupled device camera. Images were captured and produced by Isis software (Metasystems).
The Micromeasure 3.3 computer application (Reeves 2001) was used for the analysis of MLH1 foci positions and for the measurement of the length of the heterochromatin blocks in CLI and CNI chromosomes. For each SC analysed, the position of each MLH1 focus was recorded as a relative position (percentage of total length) from the centromere, identified by the CREST signal in each preparation. To convert the MLH1 foci to genetic distances, the number of MLH1 detected per SC was multiplied by 50 map units (1 crossover = 50 cM). Additionally, the Kolmogorov–Smirnov test (KS test) was used to determine whether the distribution of MLH1 foci differs significantly among species by constructing plots of cumulative frequency.
Results and discussion
In this report, the recombination maps of three primate species (C. libidinosus, C. nigritus and A. caraya) are described by analysing the number and distribution of MLH1 foci in the SCs of pachytene spermatocytes. The Cebus genus belongs to the Cebidae, and it has been regarded as retaining the putative ancestral karyotype of all Platyrrhini species (Clemente et al. 1990; Couturier and Dutrillaux 1981; Garcia et al. 2002; Neusser et al. 2001). Within this genus, C. libidinosus (CLI) and C. nigritus (CNI) share virtually the same karyotype (2n = 54, aFN = 72), with differences mainly due to variation in size of non-centromeric heterochromatic regions (Mudry 1990; Nieves et al. 2010; Ponsà et al. 1995), specifically regarding chromosomes 17 and 11. A. caraya, on the other hand, is a member of the Atelidae family, and it is currently considered that its karyotype (2n = 52, aFN = 66) has suffered multiple reorganisations since its divergence from the Platyrrhini common ancestor (de Oliveira et al. 2002; Stanyon et al. 2011).
In a first experiment, the number of MLH1 foci was scored in a total of 366 cells, 116 in CNI, 85 in CLI and 165 in ACA (Fig. 1). During meiosis, the X and Y chromosomes in Cebus spermatocytes form a sex body with a human-like morphology (Seuánez et al. 1983). A. caraya, on the other hand, presents a complex sex-chromosome system, which forms a convoluted sex body involving four chromosomes (Mudry et al. 1998, 2001; Rahn et al. 1996), which folded back onto itself, not allowing for a correct visualisation of the MLH1 foci (Fig. 2a). Thus, given the complexity of the morphology adopted by the sexual complement in A. caraya spermatocytes, the SCs involved in the sexual complement were not included in the present analysis, and MLH1 counts correspond to autosomal SCs in all three species. The mean MLH1 foci number per autosomal set was found to be significantly different among species (χ 2 = 12.92, p = 0.002): 41.3 ± 4.8 (SD) for CLI, 39.2 ± 3.3 (SD) for CNI and 40.6 ± 4.3 (SD) for ACA, with a range of 31–52, 31–46 and 31–50 MLH1 foci per cell, respectively (Fig. 1). It is well known that recombination rates present inter- and intra-specific variation, although the factor(s) affecting these differences remain elusive (Paigen and Petkov 2010). MLH1 recombination studies similar to the present work performed in humans, macaques and rodents are an example of such diversity (Hassold et al. 2009; Lynn et al. 2002; Sun et al. 2004; Dumont and Payseur 2011). The present data correspond to one representative specimen of each species, and some caution has to be taken when generalising to the whole species. Nevertheless, and assuming that one MLH1 focus can be translated into 50 centimorgans (cM) in genetic length, these recombination events would correspond to autosomal genetic lengths of 2,065 cM for CLI, 1,960 cM for CNI and 2,030 cM for ACA. The values of genetic lengths in the Cebidae and Atelidae species analysed in the present study and in a previous study of our group (Garcia-Cruz et al. 2009) are considerably lower than the 2,500 cM (corresponding to 50 MLH1 foci/cell) estimated for autosomal chromosomes in human males by cytological studies (Sun et al. 2004). A similar reduction in the recombination rate, with respect to human, has been found in the rhesus macaque (1,950 cM) (Hassold et al. 2009).
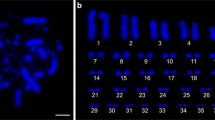
Immunolocalisation of meiotic recombination events. a, b Sequential image of a spermatocyte from ACA depicting a triple immunostaining with SYCP3 (red), MLH1 (green) and CREST (blue) and b fluorescent in situ hybridisation (FISH) with the paintings of human chromosomes 13 (green) and 21 (red). Note how the sexual complement in Alouatta caraya spermatocytes is folded back onto itself, not allowing for a correct visualisation of the MLH1 foci. c, d Selected chromosomes that are homologous to c HSA13 and d HSA21 in ACA, CLI and CNI spermatocytes, as identified by sequential immunostaining (SYCP3 in red, MLH1 in green and CREST in blue) and FISH. Human chromosome 13 is homologous to ACA20, CLI17 and CNI17, whereas human chromosome 21 is homologous to ACA21, CLI11 and CNI11. Note the identification of the heterochromatin blocks (Het) in CLI and CNI chromosomes by the absence of a hybridisation signal with the commercial painting probes
In order to explain the differences found among species, the effect of chromosomal number variation on recombination was explored, given that a strong correlation between the number of COs and the haploid number of chromosome arms has been reported in the literature (Dutrillaux 1986; Pardo-Manuel de Villena and Sapienza 2001). Under this assumption, we would expect Cebus species (aFN = 72, 16 acrocentric, eight submetacentric and two metacentric autosomal chromosome pairs) to have a higher number of COs than A. caraya (aFN = 66, 15 acrocentric, seven submetacentric and two metacentric autosomal chromosome pairs). Our results of MLH1 foci scoring indicate that the minimum requirement of one CO per chromosome is accomplished in all three species, but the recombination rate does not seem related to the number of chromosome arms. Therefore, although our study is based on one specimen as a representative of each species, there must be additional elements, such as the karyotype evolution of each species (the final outcome of diverse fusion/fission events and their own genomic makeup), and not only its fundamental chromosome number, that influence the recombination rate.
Once the cytological distribution of COs was established for each primate species, the distribution of MLH1 foci on specific chromosomes was studied by combining immunostaining and fluorescence in situ hybridisation (FISH) with whole-chromosome paints (Fig. 2a, b). Our efforts were concentrated on those Platyrrhini chromosomes homologous to human chromosomes 13 and 21, both chromosomes frequently involved in human disorders (mainly aneuploidies) and whose recombination and pairing behaviour are well known in male humans (Codina-Pascual et al. 2006; Ferguson et al. 2009). These chromosomes are evolutionarily conserved as a whole entity among the three Platyrrhini species, so their analysis can be used to assess the influence of different genetic backgrounds on meiotic recombination and vice versa. In addition, these chromosomes present differences in size of non-centromeric heterochromatic blocks between C. libidinosus and C. nigritus homologous pairs, but not in A. caraya, where these heterochromatin blocks are absent (Fig. 2c, d). By this way, the comparison of the recombination patterns among homologous chromosomes in all three species was also used to determine the possible effect of heterochromatin on meiotic recombination. Human chromosome 13 (HSA13) is homologous to an entire acrocentric chromosome in all three Platyrrhini species, corresponding to CNI17, CLI17 and ACA20 (de Oliveira et al. 2002; Garcia et al. 2000). Additionally, the homologous chromosomes in both Cebus species present differences in the size of a terminal heterochromatin block, which can be confirmed by the absence of hybridisation signal in Cebus preparations (Fig. 2c). Likewise, the chromosomes homologous to HSA21, corresponding to CNI11, CLI11 and ACA21, are acrocentric in all three species, but associated with a fragment of HSA3 (de Oliveira et al. 2002; Garcia et al. 2000), a conservation of chromosomal synteny (3/21) considered to be ancestral for all eutherian mammals (Robinson and Ruiz-Herrera 2008). C. libidinosus presents a distal or terminal heterochromatin block in chromosome 11, which takes up to 75% of the total chromosome length, while this heterochromatic region is absent in C. nigritus and A. caraya (Fig. 2d).
A total of 403 cells was analysed, 99 in CNI, 107 in CLI and 197 in ACA, with sequential immunofluorescence and FISH. The analysis of MLH1 foci for these specific chromosomes in all three species revealed that chromosomes homologous to HSA13 usually contain two MLH1 foci (mean number of MLH1 foci per SC is 1.85 for CLI, 1.70 for CNI and 1.71 for ACA), while those chromosomes homologous to HSA21 present one MLH1 focus (mean number of MLH1 foci per SC is 1.12 for CLI, 1.02 for CNI and 1.04 for ACA) (Fig. 3). Chromosomes with non-exchanges were either not detected or were very rare. Interestingly, MLH1 frequencies in human chromosomes 13 and 21 (Codina-Pascual et al. 2006; Sun et al. 2004) are similar to those reported here for their CLI, CNI and ACA counterparts (Fig. 3). Thus, the overall reduction in the genetic length found in the spermatocytes of these Platyrrhini species, with respect to human spermatocytes, could not be attributed to a general reduction of the recombination rate in all chromosomes.
Comparison of the percentage of cells with a different number of MLH1 foci in conserved chromosomes. Data from the homologous human counterparts have been extracted from Codina-Pascual et al. (2006). HSA Homo sapiens, CLI C. libidinosus, CNI C. nigritus, ACA A. caraya
After the total number of MLH1 foci per chromosome was determined for each chromosome homologous to HSA13 and HSA21, their relative positions along the SC were analysed (Fig. 4, left panel). Using the centromere as a reference, the positions of each MLH1 focus were calculated along the SC, from the centromere to the telomere. In those chromosomes with non-centromeric heterochromatin blocks (CLI11, CLI17 and CNI17), MLH1 foci were always found to be located within the euchromatic regions. Thus, for comparison, the MLH1 position was expressed as the relative position of each focus in the euchromatin of the chromosome. For each chromosome, the length of the SC has been divided into 5% intervals. The general pattern of MLH1 distribution in these chromosomes varies among species depending on the chromosome studied (Fig. 4). The recombination pattern in primate chromosomes homologous to HSA13 shows the effect of interference, which leads to the highest rates of recombination in two separate regions, the first one proximal to the centromere, with the other one being more terminal (Fig. 4). It is important to note, however, that in the case of both Cebus species, a small percentage of COs is also localised in the central region of the SC. This distribution shows similar features with the male recombination map of human chromosome 13 inferred from genome-wide single-nucleotide polymorphism (SNP) data at a resolution of 10 kilobases (kb) (Kong et al. 2010) (right panel in Fig. 4). Although SNP data and MLH1 foci represent different levels of resolution and units of measurement (axis length for the cytological measurements vs. DNA length for the genetic analysis), similar patterns can be appreciated. In the case of chromosomes homologous to HSA21, COs are more evenly distributed in all instances, although differences among species were found. In both Cebus species, COs are distributed along the whole length of the SCs, whereas in ACA, the MLH1 foci concentrate in the terminal region of the chromosome. These differences among species are more evident when plotting the data into cumulative frequency graphs (Fig. 5). This type of representation allows for the statistical estimation of similitudes between MLH1 distributions among homologous chromosomes of the three species. The analysis revealed that the distribution of MLH1 in chromosomes homologous to HSA13 and HSA21 was similar between both Cebus, regardless of whether chromosomes contain a heterochromatin block in both species (p = 0.727 for chromosomes homologous to HSA13, Kolmogrov–Smirnov) or not (p = 0.953 for chromosomes homologous to HSA21, Kolmogrov–Smirnov). However, the distribution of MLH1 in these chromosomes differs between both Cebus species and ACA (p < 0.01 for both chromosomes, Kolmogrov–Smirnov). In chromosomes homologous to HSA13, which normally contain two MLH1 foci in all three species, the first focus tends to appear closer to the centromere while the second focus tends to appear slightly closer to the telomere in ACA, when compared to Cebus. Nevertheless, differences in MLH1 distribution between the species are more evident in chromosomes homologous to HSA21. In that chromosome, which normally contains one MLH1 focus, exchanges are displaced towards distal positions in ACA, when compared to the homologous chromosome in Cebus. In any case, our results indicate that recombination is suppressed at the centromeric region in all species (Fig. 5), mirroring results previously obtained in human (Ferguson et al. 2009) and mouse (Froenicke et al. 2002).
Chromosomal distributions of recombination events. Left panel Histogrammes showing the distribution of MLH1 foci in chromosomes, which are homologous to HSA13 and HSA21 in all three Platyrrhini species (ACA, CLI and CNI). The X-axis represents the length of the synaptonemal complex (SC) from the centromere to the telomere, while the Y-axis shows the percentage of total MLH1 foci. Each chromosome is divided into 5% intervals of SC length. Note that SC length refers to the SC length of the euchromatic region. Arrows in the X-axis show the position of heterochromatin blocks (Het) in chromosomes homologous to HSA13 (in CLI and CNI) and HSA21 (in CLI). Right panel Male recombination maps for human chromosomes 13 and 21 based on SNP data (data extracted from Kong et al. 2010). The X-axis represents the length of each human chromosome expressed in base pairs (bp), whereas the Y-axis indicates the standard recombination rate
Cumulative frequency plots in conserved chromosomes in the Platyrrhini species. Note that SC length refers to the SC length of the euchromatic region. CLI C. libidinosus, CNI C. nigritus, ACA A. caraya. The inset in each plot represents the G-banding pattern for each of the chromosomes analysed. Arrows in the X-axis show the position of heterochromatin blocks (Het) in chromosomes homologous to HSA13 (in CLI and CNI) and HSA21 (in CLI)
From our data, it is also inferred that the presence or absence of the interstitial or terminal heterochromatin blocks does not have a major influence on the distribution of MLH1 foci in euchromatic region of homologous chromosomes in either Cebus species. Heterochromatin shows a particular behaviour during meiotic pairing and synapsis. In humans, Codina-Pascual et al. (2006) noted that non-centromeric heterochromatin regions are the last regions to synapse and, when unsynapsed, gaps and splits are observed as in the SCs, showing differences in timing along synapsis, depending on the chromosomes analysed. Mirroring these results, a previous study from our research group in another specimen of C. libidinosus (=C. paraguayanus) (Garcia-Cruz et al. 2009) showed how this pattern of delayed pairing and synapsis is also conserved in non-human primates. Despite this particular behaviour, heterochromatic regions do not interfere with the general progress of pairing and synapsis (Garcia-Cruz et al. 2009) nor the recombination process itself (present data).
The relative distribution of MLH1 foci in homologous chromosomes does not show significant differences in the same genus, regardless of the presence or absence of heterochromatin blocks, while it differs in homologous chromosomes from different families. These differences among species may be caused by the intrinsic genetic characteristics of each chromosome as a result of their different evolutionary histories since their common divergence. Atelidae and Cebidae Platyrrhini families are thought to have diverged approximately 20 million years ago (Opazo et al. 2006), enough time to accumulate genetic divergences and chromosomal reorganisations. The conserved karyotype in both Cebus species may explain the similarities in their CO distribution when analysing specific chromosomes. On the other hand, it is currently considered that the karyotype of A. caraya has suffered multiple chromosomal rearrangements since its divergence from the Platyrrhini common ancestor, and its genomic landscape in terms of chromosomal homologies is different from its Cebidae relatives. Indeed, the fact that recombination hotspots are not conserved among primate species has been described in previous studies based on linkage disequilibrium analysis in human and chimpanzees (Paigen and Petkov 2010; Ptak et al. 2005). Although both species share 99% of genetic similarities, their karyotypes differ in chromosome number due to several inter- and intra-chromosomal reorganisations (Clemente et al. 1990; Feuk et al. 2005; Yunis and Prakash 1982). In light of our data, the recombination rate as well as the number of COs per individual chromosome might be influenced by the chromosomal reorganisations that are shaping the genomic evolutionary landscape of each species. Further analysis including other primate species would be of great interest to deepen our knowledge of the influence of meiotic recombination in chromosomal evolution and vice versa.
References
Anderson LK, Reeves A, Webb LM, Ashley T (1999) Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 151:1569–1579
Armada JL, Barroso C, Lima M, Muniz J, Seuanez HN (1987) Chromosome studies in Alouatta belzebul. Am J Primatol 13:283–296
Baker SM, Plug AW, Prolla TA et al (1996) Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet 13:336–342
Barlow AL, Hulten MA (1998) Crossing over analysis at pachytene in man. Eur J Hum Genet 6:350–358
Basheva EA, Bidau CJ, Borodin PM (2008) General pattern of meiotic recombination in male dogs estimated by MLH1 and RAD51 immunolocalization. Chromosome Res 16:709–719
Bishop DK, Zickler D (2004) Early decision; meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117:9–15
Borodin PM, Karamysheva TV, Belonogova NM, Torgasheva AA, Rubtsov NB, Searle JB (2008a) Recombination map of the common shrew, Sorex araneus (Eulipotyphla, Mammalia). Genetics 178:621–632
Borodin PM, Karamysheva TV, Rubtsov NB (2008b) Immunofluorescent analysis of meiotic recombination and interference in the domestic cat. Tsitologiia 50:62–66
Borodin PM, Basheva EA, Zhelezova AI (2009) Immunocytological analysis of meiotic recombination in the American mink (Mustela vison). Anim Genet 40:235–238
Canevari M, Fernández Balboa C (2003) 100 mamíferos argentinos. Ed Albatros, Buenos Aires
Chebez J, Ruiz Blanco M (2008) Carayá-pitá. In: Chebez JC. Los que se van. Fauna argentina amenazada, vol. 3. Albatros, Buenos Aires, pp 64–69
Clark AG, Wang X, Matise T (2010) Contrasting methods of quantifying fine structure of human recombination. Annu Rev Genomics Hum Genet 11:45–64
Clemente IC, Ponsa M, Garcia M, Egozcue J (1990) Evolution of the Simiiformes and the phylogeny of human chromosomes. Hum Genet 84:493–506
Codina-Pascual M, Campillo M, Kraus J, Speicher MR, Egozcue J, Navarro J, Benet J (2006) Crossover frequency and synaptonemal complex length: their variability and effects on human male meiosis. Mol Hum Reprod 12:123–133
Couturier J, Dutrillaux B (1981) Conservation of replication chronology of homologous chromosome bands between four species of the genus Cebus and man. Cytogenet Cell Genet 29:233–240
de Oliveira EH, Neusser M, Figueiredo WB et al (2002) The phylogeny of howler monkeys (Alouatta, Platyrrhini): reconstruction by multicolor cross-species chromosome painting. Chromosome Res 10:669–683
Dumont BL, Payseur BA (2011) Evolution of the genomic recombination rate in murid rodents. Genetics 187:643–657
Dutrillaux B (1986) Role of chromosomes in evolution: a new interpretation. Ann Genet 29:69–75
Fedigan L, Jack K (2004) The demographic and reproductive context of male replacements in Cebus capucinus. Behaviour 141:755–775
Ferguson KA, Leung S, Jiang D, Ma S (2009) Distribution of MLH1 foci and inter-focal distances in spermatocytes of infertile men. Hum Reprod 24:1313–1321
Feuk L, MacDonald JR, Tang T et al (2005) Discovery of human inversion polymorphisms by comparative analysis of human and chimpanzee DNA sequence assemblies. PLoS Genet 1:e56
Franco MJ, Sciurano RB, Solari AJ (2007) Protein immunolocalization supports the presence of identical mechanisms of XY body formation in eutherians and marsupials. Chromosome Res 15:815–824
Frazer KA, Ballinger DG, Cox DR et al (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449:851–861
Froenicke L, Anderson LK, Wienberg J, Ashley T (2002) Male mouse recombination maps for each autosome identified by chromosome painting. Am J Hum Genet 71:1353–1368
Garcia F, Nogues C, Ponsa M, Ruiz-Herrera A, Egozcue J, Garcia Caldes M (2000) Chromosomal homologies between humans and Cebus apella (Primates) revealed by ZOO-FISH. Mamm Genome 11:399–401
Garcia F, Ruiz-Herrera A, Egozcue J, Ponsa M, Garcia M (2002) Chromosomal homologies between Cebus and Ateles (primates) based on ZOO-FISH and G-banding comparisons. Am J Primatol 57:177–188
Garcia-Cruz R, Robles P, Steinberg ER, Camats N, Brieno MA, Garcia-Caldes M, Mudry MD (2009) Pairing and recombination features during meiosis in Cebus paraguayanus (Primates: Platyrrhini). BMC Genet 10:25
Groves C (2001) Primate taxonomy. Smithsonian Institution Press, Washington, p 350
Hassold T, Judis L, Chan ER, Schwartz S, Seftel A, Lynn A (2004) Cytological studies of meiotic recombination in human males. Cytogenet Genome Res 107:249–255
Hassold T, Hansen T, Hunt P, VandeVoort C (2009) Cytological studies of recombination in rhesus males. Cytogenet Genome Res 124:132–138
Kong A, Thorleifsson G, Gudbjartsson DF et al (2010) Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467:1099–1103
Lamb NE, Sherman SL, Hassold TJ (2005) Effect of meiotic recombination on the production of aneuploid gametes in humans. Cytogenet Genome Res 111:250–255
Lenzi ML, Smith J, Snowden T, Kim M, Fishel R, Poulos BK, Cohen PE (2005) Extreme heterogeneity in the molecular events leading to the establishment of chiasmata during meiosis i in human oocytes. Am J Hum Genet 76:112–127
Lima MM, Seuanez HN (1991) Chromosome studies in the red howler monkey, Alouatta seniculus stramineus (Platyrrhini, Primates): description of an X1X2Y1Y2/X1X1X2X2 sex-chromosome system and karyological comparisons with other subspecies. Cytogenet Cell Genet 57:151–156
Lynn A, Koehler KE, Judis L et al (2002) Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science 296:2222–2225
Lynn A, Ashley T, Hassold T (2004) Variation in human meiotic recombination. Annu Rev Genomics Hum Genet 5:317–349
Megens HJ, Crooijmans RP, Bastiaansen JW et al (2009) Comparison of linkage disequilibrium and haplotype diversity on macro- and microchromosomes in chicken. BMC Genet 10:86
Mudry MD (1990) Cytogenetic variability within and across populations of Cebus apella in Argentina. Folia Primatol (Basel) 54:206–216
Mudry MD, Rahn M, Gorostiaga M, Hick A, Merani MS, Solari AJ (1998) Revised karyotype of Alouatta caraya (Primates: Platyrrhini) based on synaptonemal complex and banding analyses. Hereditas 128:9–16
Mudry MD, Rahn IM, Solari AJ (2001) Meiosis and chromosome painting of sex chromosome systems in Ceboidea. Am J Primatol 54:65–78
Neusser M, Stanyon R, Bigoni F, Wienberg J, Muller S (2001) Molecular cytotaxonomy of New World monkeys (Platyrrhini)—comparative analysis of five species by multi-color chromosome painting gives evidence for a classification of Callimico goeldii within the family of Callithrichidae. Cytogenet Cell Genet 94:206–215
Nieves M, Muhlmann M, Mudry MD (2010) Cebus paraguayanus and Cebus nigritus (Primates, Platyrrhini): a comparative genomic hybridization analysis. Cytogenet Genome Res 128:214–220
Opazo JC, Wildman DE, Prychitko T, Johnson RM, Goodman M (2006) Phylogenetic relationships and divergence times among New World monkeys (Platyrrhini, Primates). Mol Phylogenet Evol 40:274–280
Paigen K, Petkov P (2010) Mammalian recombination hot spots: properties, control and evolution. Nat Rev Genet 11:221–233
Pardo-Manuel de Villena F, Sapienza C (2001) Recombination is proportional to the number of chromosome arms in mammals. Mamm Genome 12:318–322
Petes TD (2001) Meiotic recombination hot spots and cold spots. Nat Rev Genet 2:360–369
Pigozzi MI (2007) Localization of single-copy sequences on chicken synaptonemal complex spreads using fluorescence in situ hybridization (FISH). Cytogenet Genome Res 119:105–112
Pigozzi MI (2008) Relationship between physical and genetic distances along the zebra finch Z chromosome. Chromosome Res 16:839–849
Pigozzi MI, Solari AJ (2005) The germ-line-restricted chromosome in the zebra finch: recombination in females and elimination in males. Chromosoma 114:403–409
Ponsà M, Garcia M, Borrell A et al (1995) Heterochromatin and cytogenetic polymorphisms in Cebus apella (Cebidae, Platyrrhini). Am J Primatol 37:325–331
Ptak SE, Hinds DA, Koehler K et al (2005) Fine-scale recombination patterns differ between chimpanzees and humans. Nat Genet 37:429–434
Qanbari S, Pimentel EC, Tetens J, Thaller G, Lichtner P, Sharifi AR, Simianer H (2010) The pattern of linkage disequilibrium in German Holstein cattle. Anim Genet 41:346–356
Rahn MI, Mudry M, Merani MS, Solari AJ (1996) Meiotic behavior of the X1X2Y1Y2 quadrivalent of the primate Alouatta caraya. Chromosome Res 4:350–356
Reeves A (2001) MicroMeasure: a new computer program for the collection and analysis of cytogenetic data. Genome 44:439–443
Robinson TJ, Ruiz-Herrera A (2008) Defining the ancestral eutherian karyotype: a cladistic interpretation of chromosome painting and genome sequence assembly data. Chromosome Res 16:1133–1141
Robles P, Roig I, Garcia R et al (2009) Analysis of recombination along chromosome 21 during human female pachytene stage. Reprod Biomed Online 18:784–794
Rogers J, Garcia R, Shelledy W et al (2006) An initial genetic linkage map of the rhesus macaque (Macaca mulatta) genome using human microsatellite loci. Genomics 87:30–38
Seuánez HN, Armada JL, Barroso C, Rezende C, da Silva VF (1983) The meiotic chromosomes of Cebus apella (Cebidae, Platyrrhini). Cytogenet Cell Genet 36:517–524
Stanyon R, Garofalo F, Steinberg ER, Capozzi O, Di Marco S, Nieves M, Archidiacono N, Mudry MD (2011) Chromosome painting in two genera of South American monkeys: species identification, conservation and management. Cytogenet Genome Res 134:40–50
Steinberg ER, Nieves M, Mudry MD (2007) Meiotic characterization and sex determination system of neotropical primates: Bolivian squirrel monkey Saimiri boliviensis (primates: Cebidae). Am J Primatol 69:1236–1241
Steinberg ER, Cortes-Ortiz L, Nieves M et al (2008) The karyotype of Alouatta pigra (Primates: Platyrrhini): mitotic and meiotic analyses. Cytogenet Genome Res 122:103–109
Sun F, Oliver-Bonet M, Liehr T et al (2004) Human male recombination maps for individual chromosomes. Am J Hum Genet 74:521–531
Tease C, Hartshorne GM, Hulten MA (2002) Patterns of meiotic recombination in human fetal oocytes. Am J Hum Genet 70:1469–1479
Wu ZK, Getun IV, Bois PR (2010) Anatomy of mouse recombination hot spots. Nucleic Acids Res 38:2346–2354
Yunis J, Prakash O (1982) The origin of man: a chromosomal pictorial legacy. Science 215:1525–1530
Acknowledgments
We are grateful to Dr. C. Heyting and Dr. M. Fritzler for their generous gifts of antibodies. We thank L.Uroz, G. Elkorchi and J. del Rey for technical support. Ignasi Roig is acknowledged for comments on an earlier version of this manuscript and Mariela Nieves for her suggestions on the last draft. We are also thankful to the editor and two anonymous reviewers whose comments and suggestions improved the manuscript. All research reported in this manuscript has met the appropriate national and institutional guidelines for the legal acquisition and use of laboratory animals and authorised study of wild animals. The authors also adhered to the Guide for Care and Use of Experimental Animals, as promulgated by the Canadian Council of Animal Care, and to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-human Primates. The work, which took place in Argentina, was done in accordance with the laws of Argentina. We have received funds from PCI2005-A7-O428, MDM PIP 5012, UBACyTX107, PRP2006-02, BFU2006-12951, 2009SGR1107 and CGL2010-20170. The English of this manuscript has been proofread by a native, English-speaking University Instructor of English.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by: S. Keeney
A. Ruiz-Herrera and M. Garcia-Caldés contributed equally to this work.
Rights and permissions
About this article
Cite this article
Garcia-Cruz, R., Pacheco, S., Brieño, M.A. et al. A comparative study of the recombination pattern in three species of Platyrrhini monkeys (primates). Chromosoma 120, 521–530 (2011). https://doi.org/10.1007/s00412-011-0329-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-011-0329-6