Abstract
The main target of this work is to examine blood clearance and external exposure for 177Lu-DOTATATE compared with new emerging 177Lu-PSMA therapy. Blood clearance and radiation exposure of 31 patients treated with 5.5 ± 1.1 GBq 177Lu-DOTATATE were compared to those of 23 patients treated with 7.4 GBq 177Lu-PSMA. Dose rates were measured at several distances and time points up to 120 h after treatment. Blood samples were collected conjunctively after infusion. Caregiver’s cumulative dose was measured by means of an OSL (optically stimulated luminescence) dosimeter for 4–5 days and medical staff’s dose was also estimated using electronic personal dosimeters. Finger dose was determined via ring TLD (Thermoluminescence Dosimeter) for radiopharmacists and nurses. Dose rates due to 177Lu-DOTATATE at a distance of 1 m, 4 h and 6 h after infusion, were 3.0 ± 2.8 and 2 ± 1.9 µSv/(h GBq), respectively, while those due to 177Lu-PSMA were 3.1 ± 0.8 and 2.2 ± 0.9 µSv/(h GBq). Total effective dose of 17 caregivers was 100–200 µSv for 177Lu-DOTATATE therapy. Mean effective doses to nurses and radiopharmacists were 5 and 4 µSv per patient, respectively, while those for physicists and physicians were 2 µSv per patient. For 177Lu-DOTATATE, effective half-life in blood and early elimination phase were 0.31 ± 0.13 and 4.5 ± 1 h, while they were found as 0.4 ± 0.1 and 5 ± 1 h, respectively, for 177Lu-PSMA. The first micturition time following 177Lu-DOTATATE infusion was noted after 36 ± 14 min, while the second and third voiding times were after 74 ± 9 and 128 ± 41 min, respectively. It is concluded that blood clearance and radiation exposure for 177Lu-DOTATATE are very similar to those for 177Lu-PSMA, and both treatment modalities are reasonably reliable for outpatient treatment, since the mean dose rate [2.1 µSv/(h GBq)] decreased below the dose rate that allows release of the patient from the hospital (20 µSv/h) after 6 h at 1 m distance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peptide receptor radionuclide therapy (PRRT) with radio-labeled somatostatin analogues is considered a convincing treatment for patients suffering from a neuroendocrine tumor with high expression of somatostatin receptors. In particular, most neuroendocrine tumors possess specialized somatostatin receptors that bind to the naturally synthesized somatostatin hormone (Pool et al. 2010). Currently, various analogues of somatostatin have been developed with uneven affinity to somatostatin receptor (SSTR) subtypes. Octreotate (TATE) is a synthetic analogue with high affinity for SSTR-2 (Kwekkeboom et al. 2010, 2001; Sward et al. 2010).
Since a long time, the most common radionuclides used for peptide receptor radionuclide therapy have been Indium-111 (111In), Yttrium-90 (90Y), and recently Lutetium-177 (177Lu). The physical properties and the energy of the particles emitted by these radionuclides have a great effect on the tumor response. The energy absorbed by tissue is a pivotal parameter when a certain absorbed dose is required to damage tumor cells, with less damage to the surrounding healthy tissues. 177Lu (physical half-life: 162 h) emits both β (b−) and γ (g) radiation, with a maximum energy of 0.498 MeV for the emitted βb-particles and an average energy of 0.133 MeV. 177Lu shows two main γg-emission lines with an energy of 113 keV (relative abundance 6%) and 208 keV (relative abundance 11%) (Breeman et al. 2006; Bodei et al. 2013). Nowadays 177Lu-labeled octreotate (177Lu-DOTATATE) is of great relevance, due to its specific characteristics that allow for a balance between damage to malignant cells and protection of surrounding critical organs.
177Lu-prostate-specific membrane antigen (PSMA)-617 tracer has been recently introduced in the domain of targeted therapy because it appears to allow for a promising therapy for progressive prostate cancer (Kabasakal et al. 2015). Using 177Lu-PSMA-617 dose rates for 23 patients was evaluated earlier in our department and published in terms of outpatient protocol applicability (Demir et al. 2016).
γ rays emitted from the entire body after PRRT can be used for post-therapy scintigraphy, to trace radiopeptides in tumors and body organs after injecting the prescribed activity (Flemming 1979). Unfortunately, this γ emission results in radiation exposure of caregivers and the general public (e.g., family members). 177Lu-DOTATATE therapy therefore requires isolation of patients in lead-shielded rooms for a certain period of time, to minimize radiation exposures of other people. The International Commission on Radiological Protection (ICRP), the International Atomic Energy Agency (IAEA), the European Atomic Energy Community (EURATOM), and the Turkish Atomic Energy Authority (TAEK) therefore recommended specific standards and guidelines to limit the radiation exposure to tolerable dose. Guidelines propose a measured dose rate of less than 20 µSv/h at a distance of 1 m, before a patient can be released from hospital following radioiodine therapy (The International Commission on Radiological Protection 1990; Demir et al. 2011).
Currently, therapy units in nuclear medicine accepting patients for 177Lu-DOTATATE therapy rely on several regulations for patient release time. According to TAEK, for example, patients must be kept in lead-shielded rooms (lead thickness 1.6 cm, height 2 m) after radioiodine therapy, and patients release is only possible when the dose rate at a distance of 1 m from the patient decreases below 30 µSv/h (Demir et al. 2011). The present standard treatment protocols in Europe require patients to stay in shielded rooms for 1–3 days after the administration of 7.4 GBq 177Lu (Bakker et al. 2006). In Germany where radiation protection regulations are more rigorous, patients should stay for a longer period of time after 177Lu therapy (Turner 2012). Therefore, some patients without governmental or private health assurance are compelled to pay for the expenses of long hospitalization. Furthermore, prolonged isolation overnight might exacerbate the disturbance of patients and accompanying family members. In the same context, a longer stay of old and disabled patients needing persistent surveillance during hospitalization might increase the risk of caregivers and companions of acquiring hospital infections.
The present study describes efforts made at the Nuclear Medicine Department/Cerrahpaşa Medical Faculty of Istanbul University, to quantify radiation exposures to staff and family members related to radiotherapy with 77Lu-DOTATATE and 177Lu-PSMA. Blood clearance was assessed and the cumulative effective dose to caregivers was also estimated. Additionally, the annual effective dose to the medical staff responsible for 177Lu-DOTATATE and 177Lu-PSMA therapy including radiopharmacists, nurses, physicians, physicists, and nuclear medicine technologists was evaluated.
Materials and methods
Activity preparation
Radiopharmaceutical substances were prepared inside a hot lab placed in a D-class room. RP-HPLC (Shimadzu Company. HPLC system) system and thin layer chromatography paper (ITLC-SG) were used for quality control and radiochemical purity test before administration.
Patient preparation
On the day of treatment, a nuclear medicine physicist provided radiation protection instructions for the time during hospitalization and after patient release. The family members and patients received a written form containing all necessary precautionary instructions for radiation safety. In addition, informed consent was collected from each patient according to the Human Research Ethics Institute in Turkey. Patient treatment was performed in the targeted therapy unit of the nuclear medicine department, which included 15 lead-shielded rooms (1.6 cm lead thickness, 2 m height, door’s lead thickness 0.8 cm). Special shielded tanks (two tanks, each with a mass of about 100 tons) were available for collecting nuclear waste and safe disposal.
Dose rate measurement
Radiation dose rates were measured for 17 patients treated with 5.5 ± 1.1 GBq 177Lu-DOTATATE. Dose rates were measured at different distances (0, 0.25, 0.50, 1.0, and 2.0 m) and times (0, 1, 2, 4, 6, 18, 24, 48, and 120 h after termination of infusion). Measurements were performed on a plane perpendicular to the lateral side of the sternum in the mid-thorax while the patients were in a supine position. A Geiger–Müller probe (GM probe, Eberline ESP-2, NM, USA) was employed for the dose rate measurements (calibrated by the Çekmece Nuclear Research and Training Center, Istanbul). The accuracy of the GM probe was calculated to be within ± 3%. The same criteria were applied for 23 patients who received prostate cancer therapy with 7.4 GBq 177Lu-PSMA.
Caregivers were instructed to stay far away from the patients during the first two hours of administration, and entrance of the treatment room was only permitted in case of urgent call. In the patient’s room, caregivers were asked to keep at least 1 m distance from the patient, to reduce radiation exposure. Family members who remained beside the patients during therapy were asked to carry an optically stimulated luminescence (OSL) dosimeter whenever they approached the patients. After patient release, caregivers were asked to wear dosimeters also at home, to measure the dose up to 4–5 days. OSL detectors were sensitive for doses ranging from 0.05 mSv to 10.0 Sv with 8% uncertainty. They were calibrated in terms of personal dose equivalent [for Hp (10)].
Electronic personnel dosimeters (EPD: Polimaster, PM 1621, POLIMASTER Inc, Arlington, VA, USA) were utilized to estimate the total effective dose of members of the medical team responsible for radionuclide labeling and administration including radiopharmacists, physicists, physicians, nurses, and nuclear medicine technologists. This device was suitable for measuring radiation doses during short exposure times. The minimum dose measureable by the EPD was 1 μSv, and its accuracy 15%, as provided by the manufacturer. Finger doses of radiopharmacists and nurses were determined using ring thermoluminescence dosimeters (TLD: Harshaw LiF TLD-100; Saint-Gobain Industrial Ceramics, Solon, OH, USA) worn during activity preparation and infusion. The TLDs were sensitive for doses ranging from 100 μGy to 1 Gy with 12% uncertainty, calibrated in terms of Hp (0.07).
Blood clearance
Assessment of activity clearance in blood was done for a separate group of 14 patients treated with 7.4 GBq of 177Lu-DOTATATE. Multiple blood samples (2 ml) were taken via venous cannula at 3, 10, 20, 40, 60, 90 min and at 2, 3, 24, 48, 72 h following infusion. A γ well counter (Capintec CRC25) was used for 177Lu quantification. All measurements were made consecutively and repeated three times to reduce the relative statistical error to less than 5%. The raw counts were corrected for physical decay during the period between blood sampling and the time of measurement. Based on the corrected counts and the calibration factor of the detector, the corresponding activity was deduced.
Results
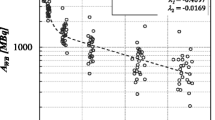
The radiochemical purity test revealed a high ratio of radiolabeled lutetium in 177Lu-DOTATATE (98.96%) and 177Lu-PSMA (99.74%) prepared activity. Table 1 shows the average dose rate per unit administered activity (GBq) measured at different time points and distances in both 177Lu-DOTATATE and 177Lu-PSMA therapy. Dose rate was very high at the beginning of radionuclide infusion close to the patients. Few hours later, the dose rate was already much lower due to the rapid excretion of 177Lu via kidneys. The dose limit recommended by the European Guidelines for patient release (20 µSv/h) after radioiodine therapy was also employed in this study. In other words, it was assumed that patients can be released from the hospital after 177Lu-DOTATATE and 177Lu-PSMA therapy when the dose rate is below 20 µSv/h. Figures 1 and 2 demonstrate the decrease in dose rate after 177Lu-DOTATATE and 177Lu-PSMA therapy from the commencement of administration until 6 h after therapy, at 1 m distance from the patients. An exponential fit describes the decrease in dose rate over time reasonably well. In general, dose rate values differ slightly between both types of therapy (see also Table 1). Figure 3 illustrates the association between mean dose rate values of 177Lu-DOTATATE (n = 17) and 177Lu-PSMA (n = 23) at 1 m distance for all time points.
Dose rates (µSv/h), at a distance of 1 m from patients (n = 23) after 7.4 GBq 177Lu-PSMA therapy (Demir et al. 2016). Gray symbols—dose rate after 177Lu-PSMA therapy; dotted line—exponential fit; solid line—limit of patient release from hospital
The blood samples showed a rapid clearance of radioactivity after infusion, and the mean effective half-life in blood and early elimination phase (during the first 24 h) was found as 0.3 ± 0.1 and 4.5 ± 1 h, respectively in 177Lu-DOTATATE therapy. These values were similar to those in 177Lu-PSMA therapy, which were 0.4 ± 0.1 and 5 ± 1 h, respectively (Demir et al. 2016). Furthermore, the mean first micturition time was noted after 36 ± 14 min of 177Lu-DOTATATE infusion while the mean second and third voiding times were at 74 ± 9 and 128 ± 41 min, respectively. An attempt was made to quantify any difference between the retention ratio of 177Lu-DOTATATE and 177Lu-PSMA following administration (Fig. 4), based on the decrease of mean dose rates recorded at 1 m distance (Table 1).
The total effective dose to 17 caregivers was found within a range of 100–200 µSv in 177Lu-DOTATATE therapy after infusion of 5.5 GBq, in which the majority of the caregivers received doses between 150 and 200 µSv, and 5% received doses between 100 and 150 µSv. In contrast, caregivers’ total effective dose in 177Lu-PSMA therapy was within a range of 120–265 µSv after infusion of 7.4 GBq. The radiation doses to the medical team involved in 177Lu-DOTATATE and 177Lu-PSMA therapy including a radiopharmacist, a nurse, a physicist, a physician, and a nuclear medicine technologist are summarized in Table 2. The radiopharmacist and the nurse received the highest mean effective dose per patient in both 177Lu-DOTATATE (4 and 5 µSv/patient, respectively) and 177Lu-PSMA therapy (4 and 6 µSv/patient, respectively). Also, the mean finger dose per patient of the radiopharmacist was higher (33 µSv/patient) than that of the nurse (3 µSv/patient) in 177Lu-DOTATATE therapy. The mean average effective dose per patient for the rest of the medical team comprising a physicist, a physician, and a nuclear medicine technologist was 2, 2, and 3 µSv, respectively. These results were similar to those reported in 177Lu-PSMA therapy (Table 2).
Furthermore, the contribution of the yearly received dose during 177Lu-DOTATATE and 177Lu-PSMA therapies to the annual occupational effective dose of the staff was evaluated. The annual occupational effective doses were as follows: for physicist (2.2 mSv/year), radiopharmacist (4.6 mSv/year), physician (1.9 mSv/year), nurse (5.1 mSv/year), technologist (4.1 mSv/year). For the radiopharmacist, the dose from the use of 177Lu-DOTATATE contributed 8% to his total occupational annual effective dose, whereas the corresponding numbers are 6% for the nurse and 2.4% for the technologist, 1.5% for the physician and 2.4% for the physicist. Similar numbers were found for the use of 177Lu-PSMA.
Discussion
Both 177Lu-DOTATATE and 177Lu-PSMA are well tolerated, but acute side-effects like nausea and vomiting might appear after the onset of 177Lu-DOTATATE administration and during amino acid infusion. This situation can be managed with medications or by slowing down amino acid infusion. Severe radiation-induced complications are so far rare, but may occur due to excessive irradiation of organs at risk like kidneys and red marrow (Melis et al. 2005). On one hand, the ongoing improvement of quantification standards and hybrid imaging modalities in the last few years has optimized the dose estimation of critical organs, and paved the way for effective and safe patient-specific therapy. On the other hand, the main aim of the present article is to provide realistic experimental results on external exposure during 177Lu-DOTATATE therapy compared to 177Lu-PSMA. Also, the present study might help to diminish the discrepancy between international regularities regarding patient isolation and hospital release as addressed in the previous section. The dose rates measured across several distances as a function of time and the blood clearance allow for quantification of the levels of radiation exposure after infusion. The present findings demonstrate a rapid clearance of blood activity with an effective half-life of 0.3 ± 0.1 h in 177Lu-DOTATATE and 0.4 ± 0.1 h in 177Lu-PSMA therapy. Moreover, the mean activity was reduced by approximately 69% during the first 6 h after infusion, as estimated from the measured dose rate decrease, in 177Lu-DOTATATE therapy, while it was reduced by 56% in 177Lu-PSMA therapy. Similar results were reported in a group of six patients who underwent 177Lu-PSMA therapy, in which the average eliminated radioactivity in the same interval (6 h) by urine collection was 45% of the prescribed activity (Demir et al. 2016).
Dose rate readings in 177Lu-DOTATATE therapy demonstrated the possibility of standing near patients in the isolation room at the moment of infusion keeping more than 2 m distance, since at this distance the dose rate [3.2 ± 1.1 (µSv/h GBq)] was markedly below 20 µSv/h. Similarly, normalization of dose rate per unit activity for 177Lu-PSMA therapy leads to a similar result [3.3 ± 1.1 (µSv/h GBq)]. Such a low exposure level is of great importance especially for critical patients who need constant care during infusion. Six hours following the onset of infusion, dose rate at 1 m was as low as 2 ± 0.8 (µSv/h GBq) in 177Lu-DOTATATE and 2.2 ± 0.9 (µSv/h GBq) in 177Lu-PSMA therapy. According to EURATOM and TAEK recommendations, this dose rate could be considered safe as it falls below the permissible exposure level after use of 5.5–7.4 GBq 177Lu (Demir et al. 2011; Turner 2012). Further, the dose rate declined to 2.8 ± 0.7 (µSv/h GBq) after 24 h at 0.5 m distance in 177Lu-DOTATATE and to 3 ± 0.8 (µSv/h GBq) in 177Lu-PSMA therapy. This would generally allow caregivers to remain close to patients, especially in case of old and disabled patients during the course of therapy. Figure 1 illustrates that dose rates decreased below the release limit after 5 h for most of the patients who had undergone 177Lu-DOTATATE therapy. In contrast, the recently started 177Lu-PSMA therapy showed a mean dose rate of 23 ± 6 μSv/h at 1 m distance after 4 h, and 15 ± 4 μSv/h after 6 h following 7.4 GBq 177Lu-PSMA infusion (Demir et al. 2016).
Figure 4 shows the relative decrease of the mean relative retention obtained from the effective dose rate along 120 h at 1 m distance for both 177Lu-DOTATATE and 177Lu-PSMA therapy. The data suggest that the elimination of 177Lu-DOTATATE is not largely different from that of 177Lu-PSMA.
Regarding the effective dose to caregivers, total effective dose range of 100–200 µSv was obtained for 17 caregivers in 177Lu-DOTATATE therapy, whereas the mean total effective dose to caregivers in 177Lu-PSMA therapy was 202 ± 43 µSv (23 patients). The cumulative effective doses to the caregivers in both 177Lu-DOTATATE and 177Lu-PSMA therapy were much less than the maximum permissible dose recommended by IAEA (5 mSv/year) (Marriott et al. 2007). However, it was not possible to confirm whether the caregivers followed the radiation protection instructions carefully in both 177Lu-DOTATATE and 177Lu-PSMA therapies. It is noted that there will be no serious radiation exposure as long as the radiation precautions are well applied during the course of the therapy. The present results are consistent with other regulations, such as the Canadian guidelines of the radiation protection standards, which recommend public dose limit of 1 mSv/year and annual dose limit of 5 mSv for caregivers (Demir et al. 2016).
The mean effective dose to medical staff carrying out 177Lu-DOTATATE and 177Lu-PSMA therapies was also evaluated including a radiopharmacist who accomplished the task of synthesizing and labeling radioactivity for each patient. The mean effective dose to the radiopharmacist (4 μSv/patient) was compared to that to the physicist (2 μSv/patient), physician (2 μSv/patient), and technologist (3 μSv/patient) in 177Lu-DOTATATE therapy. These findings are similar to those reported for 177Lu-PSMA therapy (Demir et al. 2016). Due to the necessity of nurse attendance during infusion and medical monitoring after termination of infusion, the mean effective dose to the nurse in 177Lu-DOTATATE therapy was similar to that in 177Lu-PSMA therapy, and resulted in the highest dose compared with those to the other caregivers in both 177Lu-DOTATATE and 177Lu-PSMA therapy.
Ultimately, the clinical findings proposed here emphasize the safety of 177Lu-DOTATATE and 177Lu-PSMA therapy as well-tolerated treatment and could possibly be realized for patient released after therapy when the precautions are carefully followed. The current study also indicates no major difference between 177Lu-DOTATATE and 177Lu-PSMA therapy, both in terms of blood clearance and external exposure. The effective doses to the medical team and to caregivers in both types of therapy are within acceptable dose limits making patient isolation for a long period of time less important.
Conclusion
The present results suggest very similar radiation exposure and blood clearance following 177Lu-DOTATATE therapy compared to 177Lu-PSMA therapy. The study supports patients to be released from hospital 6 h after therapy, to avoid unnecessary patient suffering and extra expenses from longer hospital stay.
References
Bakker WH, Breeman WA, Kwekkeboom DJ, De Jong LC, Krenning EP (2006) Practical aspects of peptide receptor radionuclide therapy with [177Lu] [DOTA0, Tyr3] octreotate. Q J Nucl Med Mol Imaging 50:265–271
Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O’Dorisio MS, O’Dorisio TM, Howe JR, Cremonesi M, Kwekkeboom DJ, Zaknun JJ (2013) The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumors. J Nucl Med Mol Imaging 40:800–816
Breeman W, De Blois E, Bakker W, Krenning E (2006) Radiolabeling DOTA-peptides with Y-90 and Lu-177 to a high specific activity. In: Chinol M, Paganelli G (eds) Radionuclide peptide cancer therapy. Taylor and Francis, New York, pp 19–26
Demir M, Demir B, Sayman H, Sager S, Sabbir Ahmed A, Uslu I (2011) Radiation protection for accompanying person and radiation workers in PET/CT. Radiat Prot Dosim 147:528–532
Demir M, Abuqbeitah M, Uslu-Besli L, Yildirim O, Yeyin N, Cavdar I, Vatankulu B, Gündüz H, Kabasakal L (2016) Evaluation of radiation safety in (177)Lu-PSMA therapy and development of outpatient treatment protocol. J Radiol Prot 36:269–278
Fleming JS (1979) A technique for the absolute measurement of activity using a gamma camera and computer. Phys Med Biol 24:176–80
International Commission on Radiological Protection (1990) Recommendations of the International Commission on Radiological Protection Annals of the ICRP. ICRP Publication 60 ed. Pergamon Press, Oxford, pp 1–201
Kabasakal L, AbuQbeitah M, Aygun A, Yeyin N, Ocak M, Demirci E, Toklu T (2015) Pre-therapeutic dosimetry of normal organs and tissues of Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. J Nucl Med Mol Imaging 42:1976–1983
Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL, Schmidt MA, Bugaj JL, de Jong M, Krenning EP (2001) [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med 28:1319–1325
Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, de Jong M, de Herder WW, Krenning EP (2010) Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Rel Cancer 17:R53–R73
Marriott CJ, Webber CE, Gulenchyn KY (2007) Radiation exposure for ‘caregivers’ during high-dose outpatient radioiodine therapy. Radiat Prot Dosim 123:62–67
Melis M, Krenning EP, Bernard BF, Barone R, Visser TJ, de Jong M (2005) Localisation and mechanism of renal retention of radiolabeled somatostatin analogues. J Nucl Med Mol Imaging 32:1136–1143
Pool SE, Krenning EP, Koning GA, van Eijck CH, Teunissen JJ, Kam B, Valkema R, Kwekkeboom DJ, de Jong M (2010) Preclinical and clinical studies of peptide receptor radionuclide therapy. Sem Nucl Med 40:209–218
Sward C, Bernhardt P, Ahlman H, Wangberg B, Forssell-Aronsson E, Larsson M, Svensson J, Rossi-Norrlund R, Kölby L (2010) [177Lu-DOTA 0-Tyr 3]-octreotate treatment in patients with disseminated gastroenteropancreatic neuroendocrine tumors: the value of measuring absorbed dose to the kidney. World J Surg 34:1368–1372
Turner JH (2012) Outpatient therapeutic nuclear oncology. Ann Nucl Med 26:289–97
Acknowledgements
The authors would like to express their sincere gratitude to the Head of Nuclear Medicine Department in Cerrahpaşa Medical Faculty for his cordial support, and to all colleagues who provided insight and professional advice to achieve this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by Istanbul University Cerrahpaşa Medical Faculty Clinical Research Ethics Committee (document number: 83045809/604/5855).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Abuqbeitah, M., Demir, M., Uslu-Beşli, L. et al. Blood clearance and occupational exposure for 177Lu-DOTATATE compared to 177Lu-PSMA radionuclide therapy. Radiat Environ Biophys 57, 55–61 (2018). https://doi.org/10.1007/s00411-017-0721-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-017-0721-6