Abstract
It has generally been assumed that the neutron and γ-ray absorbed doses in the data from the life span study (LSS) of the Japanese A-bomb survivors are too highly correlated for an independent separation of the all solid cancer risks due to neutrons and due to γ-rays. However, with the release of the most recent data for all solid cancer incidence and the increased statistical power over previous datasets, it is instructive to consider alternatives to the usual approaches. Simple excess relative risk (ERR) models for radiation-induced solid cancer incidence fitted to the LSS epidemiological data have been applied with neutron and γ-ray absorbed doses as separate explanatory covariables. A simple evaluation of the degree of independent effects from γ-ray and neutron absorbed doses on the all solid cancer risk with the hierarchical partitioning (HP) technique is presented here. The degree of multi-collinearity between the γ-ray and neutron absorbed doses has also been considered. The results show that, whereas the partial correlation between the neutron and γ-ray colon absorbed doses may be considered to be high at 0.74, this value is just below the level beyond which remedial action, such as adding the doses together, is usually recommended. The resulting variance inflation factor is 2.2. Applying HP indicates that just under half of the drop in deviance resulting from adding the γ-ray and neutron absorbed doses to the baseline risk model comes from the joint effects of the neutrons and γ-rays—leaving a substantial proportion of this deviance drop accounted for by individual effects of the neutrons and γ-rays. The average ERR/Gy γ-ray absorbed dose and the ERR/Gy neutron absorbed dose that have been obtained here directly for the first time, agree well with previous indirect estimates. The average relative biological effectiveness (RBE) of neutrons relative to γ-rays, calculated directly from fit parameters to the all solid cancer ERR model with both colon absorbed dose covariables, is 65 (95 %CI: 11; 170). Therefore, although the 95 % CI is quite wide, reference to the colon doses with a neutron weighting of 10 may not be optimal as the basis for the determination of all solid cancer risks. Further investigations into the neutron RBE are required, ideally based on the LSS data with organ-specific neutron and γ-ray absorbed doses for all organs rather than the RBE weighted absorbed doses currently provided. The HP method is also suggested for use in other epidemiological cohort analyses that involve correlated explanatory covariables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies on survivors of the World War II atomic bombings over Hiroshima and Nagasaki have been crucial to assessments of the detrimental health risks due to exposures from ionising radiation. The life span study (LSS) of A-bomb survivors continues to provide valuable radiation epidemiological data and quantitative assessments of the radiation-related detrimental health risks (Preston et al. 2003, 2004, 2007; Ozasa et al. 2012). The radiation absorbed doses in the LSS have been determined from information on the mixed γ-ray and neutron field and survivor location at the time of the bombings using the most recent dosimetry system DS02 (Young and Kerr 2005). However, in nearly all past LSS health risk analyses, it has been assumed that the neutron and γ-ray absorbed doses are too highly correlated to be able to separate out the detrimental health effects due to neutrons and γ-rays independently (e.g., Hunter and Charles 2002). Consequently, the widely adopted practice, in nearly all recent analyses, had been to base risk estimates on weighted absorbed doses, that is, γ-ray organ absorbed dose plus a weighting factor for the neutrons multiplied by the organ neutron absorbed dose. However, since the release of the most recent data for all solid cancer incidence which, with 17,448 first primary cancer cases, represents an increase in statistical power over previous datasets (i.e., 8,613 first primary incident solid cancers in Thompson et al. 1994: 9,335 deaths from solid cancer in Preston et al. 2003; and 10,929 deaths from solid cancer in Ozasa et al. 2012), it is instructive to consider alternatives to the usual approach.
Recent developments in the application of information theory and multi-model inference to radiation epidemiology have aided the inferential process by reducing uncertainty associated with model-selection and accounting for increased uncertainties when a multiplicity of models fit the data almost equally well (Walsh 2007; Walsh and Kaiser 2011). However, these approaches focus on comparisons among alternative models and not on the relative importance of the explanatory variables included in the models. Once, a preferred model or set of models has been identified, radiation epidemiologists would often like to know which of the various possibly explanatory covariables included in the models has the strongest influence on the risk response variable.
The purpose of this paper is two fold. The first purpose is to propose and give details of a quantitative technique for obtaining a measure of the relative importance of several main and possibly explanatory covariables in the assessment of detrimental health risks in epidemiological cohorts using the method of hierarchical partitioning (HP) (Chevan and Sutherland 1991). Hierarchical partitioning is considered here because this method has been shown by Murray and Conner (2009) to be the best method for application when correlations between the explanatory variables are present. It is suggested here to apply HP more widely in the field of radiation epidemiology cohort analysis because correlations of concern are often present in the data. The second purpose is to illustrate the application of HP to the covariables for the γ-ray and neutron absorbed doses in the assessment of risks for all solid cancer in the publically available LSS data to see if any extra information regarding the average effectiveness of neutrons relative to γ-rays can be extracted from the data.
Materials and methods
Determination of the degree of independent effects via HP
Hierarchical partitioning represents an add-on to any technique that yields a measure of goodness of fit of a model to a dataset (e.g., the deviance in Poisson regression). It is similar to a technique called “dominance analysis” in other fields of research (e.g., Budescu 1993). The objective of applying HP to multivariate regression is to partition a measure of association, R (e.g., the deviance) between each independent component, I, and a joint component, J, such that R = I + J. The requirements of HP are as follows: an initial measure of fit when no independent variable is present; a final measures of fit when all considered independent variables are present; and all intermediate models with various combinations of independent variables. Given a dependent variable (y) and k explanatory variables (x 1, x 2,…, x k ), the independent effect of covariable x 1 represents the average contribution of covariable x 1 to the variance in y over all 2k possible models. Through the process of HP, the independent effect of each covariable is calculated by comparing the fit of all models containing a particular variable to the fit of all nested models lacking that variable. Although the idea behind HP is simple, the equations illustrating the method can be somewhat abstruse and the application computationally intensive. Therefore, a qualitative description of HP, similar to that provided in German by Dormann and Kühn (2009), has been opted for here.
Consider three independent variables, that is, the explanatory additive covariables A, B and C, resulting in 23 = 8 simple linear models: no covariable (N), A, B, C, A + B, A + C, B + C and A + B + C. This set of models can be considered to have four hierarchical levels (0, 1, 1, 1, 2, 2, 2, 3), respectively. In order to study the effect of A, one considers all models without A, then one adds A to each of these models and computes each of the resulting changes in goodness of fit measure: A versus N, A + B versus B, A + C versus C, A + B + C versus B + C. In the next step, the resulting changes in goodness of fit measure are averaged for each hierarchical level. Finally, these hierarchical level-specific averages are further averaged over all hierarchical levels. The result of this averaging procedure is the independent effect, I, of A on the dependent variable. The joint effects, J, of A can be calculated from the difference in goodness of fit measure of the complete model and the partial model. The application made here is for the simpler case of only two independent variables which are the γ-ray and neutron absorbed doses in the LSS data.
Correlations among explanatory covariables
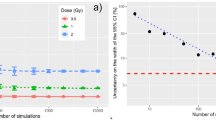
Problems with multi-collinearity (high levels of correlation) between independent variables include instability of fit-parameter estimates, inflated standard errors for model fit parameters with concomitant increases in Type II errors and false rankings of variable importance (see for example Zar 1999; Belsley et al. 2004). Degrees of multi-collinearity can be assessed with variance inflation factors (VIFs) or tolerance values (TOLs) between two (or more) correlated explanatory covariables (x 1, x 2). Calculation of TOLs and VIFs does not vary based on the type of multivariate analysis to be applied because multi-collinearity concerns the relationship between the independent variables, rather than the relationship of the independent variables to the dependent variable. VIFs can be obtained by a linear regression (even if performing Poisson regression) of one covariable onto the other, that is, if x 1 = βx 2 is defined as the regression function and r 2 is the coefficient of determination in this linear regression model, then VIF = 1/(1 − r 2) = 1/TOL, see Fig. 1. The VIF indicates how much the estimated variances of the regression coefficients associated with x 1 and x 2 are increased above what they would be if r 2 = 0. Although suggested cut-off values for the pair-wise correlation coefficient (r) requiring remedial action vary widely, remedial methods are generally not recommended unless r > 0.80 (p 90, Katz 2011), see Fig. 1. According to Katz (2011), TOLs <0.25 are worrisome, <0.10 are serious; VIFs >2.5 may be problematical, and >10 are serious. There are also “rules of thumb” for indicating excessive multi-collinearity associated with the VIF—most commonly the rule of 10—sometimes the rule of 4. O’Brien (2007) has given a thorough review of such rules and elucidated reasons for caution in applying such rules.
The variance inflation factor (VIF) as a function of the degree of correlation (r), between two explanatory covariables. The vertical line shows the level below which remedial actions are typically not recommended. The cross indicates the value from Table 3, for the correlation between the neutron and γ-ray absorbed doses in the Japanese epidemiological incidence data (Preston et al. 2007)
Risk models and dataset
The most recent all solid cancer incidence data for the follow-up 1958–1998 (Preston et al. 2007, data file: ds02can.dat, results file: ds02can.log from www.rerf.or.jp), have been used. The risk models applied here, for radiation-induced solid cancer incidence, are very similar to those already considered and explained in detail in Preston et al. 2007. Use is made of a general rate (hazard) model of the form
for the excess relative risk (ERR), where λ 0(a, e, s, c, nic) is the baseline cancer death rate, at age attained, a, age at exposure e, with indicator variables for gender, s (M = male, F = female), city, c (H = Hiroshima, N = Nagasaki) and “not in either city at the time of the bombs”, nic. The organ absorbed doses from γ-rays and neutrons are d γ, d n, respectively. Organs with separate γ-ray and neutron absorbed doses given in the dataset considered here are colon, liver and marrow (for the other organs only the weighted absorbed doses are available).
Although the baseline rates can be dealt with by stratification, a fully parametric model is adopted here:
where β 0,M, …, β 10,F are fit parameters.
This is a simplified version of the model of Preston et al. (2007) because some terms associated with p values >0.05, including a city parameter relating to differences in baseline cancer rates between Hiroshima and Nagasaki, were not considered here. This was because an application of the likelihood ratio test for nested models indicated that the extra terms did not significantly improve the fit in the current analysis.
The ERR models considered were of the form ERR(d γ, d n) = α 1 d γ + α 2 d n. A set of four models for HP was considered for the main analysis: a baseline model with ERR(d γ = 0, d n = 0); ERR(d γ, d n = 0); ERR(d γ = 0, d n); and ERR(d γ, d n). Additional terms in d 2γ and d 2n were also included and tested in the ERR model to determine if they resulted in a statistically significant improvement in goodness of fit of the model to the data. This approach differs from the usual application of ERR(d), d = d γ + RBE · d n, that is, the organ absorbed doses weighted by the relative biological effectiveness (RBE) of neutrons relative to γ-rays. The value of 10 for RBE has been widely used in the past for weighting the organ absorbed doses in spite of indication for larger values (Kellerer et al. 2006, Rühm and Walsh 2007). In the present work, adjustments in the ERR relating to the explanatory covariables of gender, age attained and age-at-exposure are omitted so that the resulting risks are gender and age averages. This was done because there is clearly a limit to the statistical power associated with this data and so the model was chosen to be parsimonious for the main purpose, that is, the determination of separate average γ-ray and neutron risk estimates, from which an average RBE can be determined.
Model fit parameters and goodness of fit measures were obtained by optimising the models to the data via Poisson regression with the AMFIT module of the EPICURE software (Preston et al. 1993).
Results
The results of applying this technique are shown in Table 1 which gives the deviances of the set of four ERR models for hierarchical partitioning, that is, just baseline model with ERR(d γ = 0, d n = 0), ERR(d γ, d n = 0), ERR(d γ = 0, d n) and ERR(d γ, d n). R (i.e., R γ and R n) is a measure of the zero-order association between the dependent variable (ERR) and the subscripted independent variable, I is the independent component of R and J is the joint component of R, where R = I + J. It can be seen from Table 1 that the joint effect of d γ, and d n is less than the independent effects and only accounts for just less than half of the total effects (R). The total deviance drop from adding the covariables d γ, d n in a linear ERR model relative to the baseline model was 324.4. This deviance drop partitions into an independent contribution from the γ-rays of 177.8 (54.8 %) and an independent contribution from the neutrons of 146.6 (45.2 %), thus providing also a rank of the importance of the dose covariables. The addition of quadratic d γ or d n terms to the ERR model did not lead to either a statistically significant improvement in overall goodness of fit or statistically significant fit parameters (p > 0.5, by the score test) in both cases. The goodness of fit of purely quadratic dose models was much worse than for the linear dose models.
Table 2 gives the all solid cancer ERR/Gy of γ-ray absorbed dose and the ERR/Gy of neutrons for the three organ absorbed doses that are included with the dataset applied, that is, colon, liver and marrow. The fit-parameter correlation coefficients between the ERR/Gy of γ-ray absorbed dose and the ERR/Gy of neutrons are −0.826, −0.817 and −0.813 for the risks with respect to colon, liver and marrow absorbed doses, respectively. Using these parameter correlations and the ERR/Gy with uncertainty ranges in Table 2, the RBE of neutrons with respect to γ-rays and the corresponding uncertainty ranges were calculated with Monte-Carlo simulation using 1,000 realizations. The results are RBE values of 65 (95 % confidence interval (CI): 11; 170), 38 (95 % CI: 4; 97) and 29 (95 % CI: 5; 75) when calculated from the fit parameters with respect to colon, liver and marrow absorbed doses, respectively (Table 3).
Table 3 gives the person-year weighted mean absorbed doses and the partial correlation (r) between the neutron and γ-ray absorbed doses. The correlation between neutron and γ-ray colon absorbed doses is r = 0.74. The corresponding VIF is 2.2 and therefore much lower than the commonly assumed threshold values, beyond which remedial measures are often taken (see also Fig. 1). The neutron fraction of the organ absorbed dose, defined as neutron organ absorbed dose/(neutron organ absorbed dose + γ-ray organ absorbed dose) has also been calculated and given in Table 3 for the three types of organ absorbed doses considered here. The mean neutron absorbed dose fractions in per cent are 0.46, 0.69 and 0.86, with respect to colon, liver and marrow absorbed doses, respectively.
Discussion
Hierarchical partitioning is not the only method currently available to quantify covariable importance. A recent study by Murray and Conner (2009) has compared six different measures commonly used; zero-order correlations, partial correlations, semi-partial correlations, standardised regression coefficients, Akaike weights, and independent effects (HP) using simulated test data that included correlated explanatory variables and a spurious variable. Once spurious variables had been identified and eliminated, hierarchical partitioning was found by Murray and Conner (2009) to be the best method for application when correlation between the explanatory variables was included in the test model. For this reason, the HP technique was considered here suitable for application to the LSS epidemiological data that have correlated neutron and γ-ray absorbed doses.
The partial correlation between the LSS neutron and γ-ray colon absorbed doses may be considered to be high at 0.74, but this value is just below the level, beyond which remedial action is usually recommended (see Fig. 1). The resulting VIF is 2.2 and also below levels often assumed to indicate the need for the application of measures for reducing multi-collinearity (such as combining two or more independent variables into a single variable). Applying HP it was found that only just under half of the drop in deviance, resulting from adding the γ-ray and neutron absorbed doses to the baseline risk model, comes from the joint effects of the neutrons and γ-rays. Hence, a substantial proportion of this deviance drop is accounted for by individual effects of the neutrons and γ-rays. It is the presence of this substantial proportion of individual effects that leads to a reliable determination of separate neutron and γ-ray risks and hence average RBE determinations. Under these conditions, average RBE values of 65 (95 % CI: 11; 170), 38 (95 % CI: 4; 97) and 29 (95 % CI: 5; 75) when calculated from the risks (Table 2) correlate inversely with the neutron absorbed dose fractions of 0.46, 0.69 and 0.86 %, where both sets of values are with respect to colon, liver and marrow absorbed doses, respectively. Although the 95 % CIs are quite wide, this pattern in the central estimates of average RBE values is consistent with the physics of the neutron shielding by the human body—that is, the organs with less body shielding have larger neutron absorbed dose fractions because the neutrons are attenuated more than the γ-rays by body shielding (Kellerer et al. 2006).
The calculations of all solid cancer risks with reference to the colon, as adopted in most recent LSS analyses such as Preston et al. (2003, 2004, 2007) and Ozasa et al. (2012) with the assumed RBE weight of 10, apply an RBE that is at about the lower 95 % confidence limit of the RBE determined here of 65 (95 % CI: 11; 170). Also, the level of correlation between the γ-ray and neutron absorbed doses is not high enough to indicate that the linear combination of these absorbed doses into organ-weighted absorbed doses is absolutely necessary on the grounds of remedy for multi-collinearity.
An important qualification with respect to the partial correlation between the LSS neutron and γ-ray colon absorbed doses of 0.74 should be considered here. A large part of the inhomogeneity between neutron and γ-ray absorbed doses is due to a systematic difference between Hiroshima and Nagasaki. The partial correlations for the two cities are 0.89 and 0.94 for Hiroshima and Nagasaki, respectively. Consequently, it is a possibility that the portion of any dose–response difference between neutron and γ-ray absorbed doses that is due to the difference in neutron versus γ-ray absorbed doses in the two cities could be confounded with a city effect due to other factors, such as a city-specific genetic predisposition to one type of cancer (e.g., it is known that adult T cell leukaemia is endemic in Nagasaki (Arisawa et al. 2002)). However, the author is not aware of any such factors that could have confounded the current analysis based on all solid cancers.
A previous method (Kellerer and Walsh 2001, 2002) uncoupled the solid cancer mortality risk coefficient for neutrons from the low dose estimates of the RBE of neutrons and the γ-ray risk coefficient. This was achieved by relating the solid cancer risk in terms of organ-averaged absorbed doses—rather than the colon absorbed doses—to two directly assessable quantities, namely the excess relative risk (ERR1) due to an intermediate reference dose D 1 = 1 Gy of γ-rays and the RBE of neutrons, R 1, against this reference dose. It was concluded that the neutrons have caused 18 or 35 % of the total effect at 1 Gy for tentatively assumed R 1 values between 20 and 50. The corresponding solid cancer mortality ERR for neutrons was found to be between 8/Gy and 16/Gy of organ-averaged absorbed dose. The latter risks are more numerically compatible with the ERR/Gy of neutrons presented here based on liver absorbed dose (17.3, 95 %CI: 3.1; 32.1) and marrow absorbed dose (12.6, 95 %CI: 2.2; 23.5) than central risk estimates based on colon absorbed dose (30.4, 95 %CI: 6.5; 55.3). Also, the average RBE values of 65 (95 % CI: 11; 170), 38 (95 % CI: 4; 97) and 29 (95 % CI: 5; 75) obtained with reference to the colon, liver and marrow, respectively, are, if one equates R 1 to the average RBE, more compatible numerically for average RBE values based on liver and marrow absorbed doses. The average RBE values of 38 and 29 based on liver and marrow absorbed doses, respectively, are also more compatible with ICRP 103 (2007) (neutron weighting factor values in the range of 5–20 depending on energy), the results of Rühm and Walsh (2007) and biological experiments (RBE in the range 5–50, e.g., Edwards 1999) than the average RBE value of 65 based on colon absorbed doses. Therefore, reference to the colon may not be the best organ on which to base all solid cancer risk estimates. Reference to either a less shielded organ than the colon or organ-specific doses may be more appropriate.
The application of organ-specific doses to the grouped, publicly available LSS solid cancer data for the purpose of determining the all solid cancer risk per unit dose is not entirely trivial. Pierce et al. (1996) noted that “It is impossible to use more specific organ doses for solid cancers as a class, since there is no designated organ for those not dying of cancer.” However, this difficulty has been resolved (Walsh et al. 2004) by treating each person as a set of a number of sub-units at risk, where each sub-unit belongs to one organ category.
Kellerer et al. (2006) presented indications for a higher neutron RBE with respect to γ-rays, than previously assumed. Kellerer et al. (2006) obtained organ-specific ERRs relative to the RBE weighted organ absorbed dose, ERR/Gy. These risks were then plotted against the neutron fraction of the absorbed organ dose, which decreases with the depth of the organ in the human body. It was found that the risks calculated with RBE = 10 are larger for organs closer to body surface and that this trend, although inconspicuous, was highly statistically significant. This trend can be explained by underestimation of the neutron RBE since the statistical significance of the trend was found to decrease for larger RBE values.
It is noted that whereas the LSS incidence data analysed by Preston et al. (2007) contains organ-specific γ-ray and neutron absorbed doses for the three types of organ absorbed doses considered here (colon, liver and marrow), the publicly available data for the LSS mortality follow-up period 1950–2003, analysed by Ozasa et al. (2012) is limited by only including RBE = 10 weighted absorbed doses. Therefore, further research into the neutron RBE, based on the Ozasa et al. 2012 publicly available dataset, is precluded due to this limitation.
Only the grouped data can be made publicly available because Japanese human rights protection laws, related to the extremely important issue of privacy for the atomic bomb survivors, understandably require a rigorous programme of de-identifying and assuring the security of individual data at the Radiation Effects Research Foundation (RERF) before allowing it to be shared with outside investigators. Currently the RBE weighted absorbed doses, with a weight of 10, for all organs of interest are included in the publically available data. Further investigations into the neutron RBE with the publically available grouped LSS data are limited by a lack of availability of organ-specific neutron and γ-ray absorbed doses for all organs of interest. Additional improvements in the precision by which the average neutron RBE could be determined, although they may not be substantial, could also come from a direct application of the methods described here to the ungrouped epidemiological data. Indications from Kellerer and Walsh (2001), Kellerer et al. (2006) and the present study deserve further investigation and should be treated seriously since, for example, some proton therapy patients receive an additional neutron dose as an unwanted by-product (FP-7-EU-ANDANTE for more information see http://www.sciencenet-mv.de/index.php/kb_746/io_2905/io.html). For these reasons, the author would like to encourage RERF scientists with access to the individual data to improve the precision on current average neutron RBE determinations as far as scientifically possible.
Conclusion
A simple evaluation of the degree of independent effects from γ-ray and neutron absorbed doses on the all solid cancer risk with the HP technique is presented here. The HP method is also generally recommended for use in other epidemiological cohort analyses that involve correlated explanatory covariables. The degree of correlation between the γ-ray and neutron absorbed doses has also been considered. The partial correlation between the neutron and γ-ray colon absorbed doses (r = 0.74) and the resulting VIF (2.2) are both below the levels beyond which remedial action is usually recommended. Applying HP to the models, it was found that just under half of the drop in deviance resulting from adding the γ-ray and neutron absorbed doses to the baseline risk model, comes from the joint effects of the neutrons and γ-rays—leaving a substantial proportion of the deviance drop accounted for by individual effects. The average ERR/Gy γ-ray absorbed dose and the average ERR/Gy neutron absorbed dose obtained directly here for the first time, agree well with previous indirect estimates. The average RBE of neutrons relative to γ-rays, calculated from fit parameters to the ERR all solid cancer model with both colon absorbed dose covariables is 65 (95 %CI: 11; 170). Therefore, the determination of all solid cancer risks based on reference to the colon absorbed doses with a neutron weighting of 10 may not be optimal, and this practice should be reviewed. Any future improvements in neutron RBE precision could have important public-health consequences, for example, for the types of proton therapy that produce unwanted by-product neutron doses.
References
Arisawa K, Soda M, Shirahama S, Saito H, Takamura N, Yamaguchi M, Odagiri K, Nakagoe T, Suyama A, Doi H (2002) Geographic distribution of the incidence of adult T-cell leukemia/lymphoma and other malignancies in Nagasaki Prefecture, Japan. Jpn J Clin Oncol 32:301–306
Belsley DA, Kuh E, Welsch RE (2004) Regression diagnostics: identifying influential data and sources of collinearity. Wiley, Hoboken
Budescu DV (1993) Dominance analysis: a new approach to the problem of relative importance of predictors in multiple regression. Psychol Methods 8:542–551
Chevan A, Sutherland M (1991) Hierarchical Partitioning. The American Statistician 45(2):90–96
Dormann CF, Kühn I (2009) Angewandte Statistik für die biologischen Wissenschaften 2. durchgesehene, aktualisierte, überarbeitete und erweiterte Auflage 2009
Edwards AA (1999) Neutron RBE values and their relationship to judgements in radiological protection. J Radiol Prot 19(2):93–105
Hunter N, Charles MW (2002) The impact of possible modifications to the DS86 dosimetry on neutron risk and relative biological effectiveness. J Radiol Protect 22(4):357
International Commission on Radiological Protection (2007) the 2007 recommendations of the international commission on radiological protection. ICRP_103 Annals of the ICRP 37:2–4
Katz MH (2011) Multivariable analysis: a practical guide for clinicial and public health researchers, 3rd edn. Cambridge University Press, New York
Kellerer AM, Walsh L (2001) Risk estimation for fast neutrons with regard to solid cancer. Radiat Res 156:708–717
Kellerer AM, Walsh L (2002) Solid cancer risk coefficient for fast neutrons, in terms of effective dose. Radiat Res 158:61–68
Kellerer AM, Rühm W, Walsh L (2006) Indications of the neutron effect contribution in the solid cancer data of the A-bomb survivors. Health Phys 90(6):554–564
Murray K, Conner M (2009) Methods to quantify variable importance: implications for the analysis of noisy ecological data. Ecology 90(2):348–355
O’Brien RM (2007) A caution regarding rules of thumb for variance inflation factors. Qual Quant 41:673–690
Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K (2012) Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 177(3):229–243
Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K (1996) Studies of the mortality of atomic bomb survivors. Report 12, Part 1. Cancer 1950–1990. Radiat Res 146:1-27
Preston DL, Lubin JH, Pierce DA (1993) Epicure user’s guide. HiroSoft International Corp, Seattle
Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K (2003) Studies of the mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 160:381–407
Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, Kodama K (2004) Effects of recent changes in atomic bomb survivors dosimetry on cancer mortality risk estimates. Radiat Res 162:377–389
Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168:1–64
Rühm W, Walsh L (2007) Current risk estimates based on the A-bomb survivors data—a discussion in terms of the ICRP recommendations on the neutron weighting factor. Proceedings of the Tenth Symposium on Neutro Dosimetry, Uppsala, Sweden. June 12–16, 2006. Radiat Prot Dosim 126(1–4):423–431
Thompson DE, Mabuchi K, Ron E, Soda M, Tokunaga M, Ochikubo S, Sugimoto S, Ikeda T, Terasaki M, Izumi S, Preston DL (1994) Cancer incidence in atomic bomb survivors. Part II: solid Tumors, 1958–1987. Radiat Res 137:S17–S67
Walsh L (2007) A short review of model selection techniques for radiation epidemiology. Radiat Environ Biophys 46:205–213
Walsh L, Kaiser JC (2011) Multi-model inference of adult and childhood leukaemia excess relative risks based on the Japanese A-bomb survivors mortality data (1950–2000). Radiat Environ Biophys 50:21–35
Walsh L, Rühm W, Kellerer AM (2004) Cancer risk estimates for γ-rays with regard to organ specific doses, part I: all solid cancers combined. Radiat Environ Biophys 43:145–151
Young R, Kerr GD (eds) (2005) DS02: Reassessment of the atomic bomb radiation dosimetry for Hiroshima and Nagasaki, Dosimetry System 2002, DS02 vols 1 and 2, Radiation Effects Research Foundation, Hiroshima
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Acknowledgments
The author is grateful to Prof. Robert M. O’Brien, University of Oregon, U.S.A., Prof. William Kleiber, University of Colorado at Boulder, U.S.A. and Prof. Werner Rühm for useful discussions. This work has been partially supported by the European Union project FP7-ANDANTE (Multidisciplinary evaluation of the cancer risk from neutrons relative to photons using stem cells and the analysis of second malignant neoplasms following paediatric radiation therapy). This work makes use of the data obtained from the Radiation Effects Research Foundation (RERF) in Hiroshima, Japan. RERF is a private foundation funded equally by the Japanese Ministry of Health and Welfare and the US Department of Energy through the US National Academy of Sciences. The conclusions in this work are those of the author and do not necessarily reflect the scientific judgement of RERF or its funding agencies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walsh, L. Neutron relative biological effectiveness for solid cancer incidence in the Japanese A-bomb survivors: an analysis considering the degree of independent effects from γ-ray and neutron absorbed doses with hierarchical partitioning. Radiat Environ Biophys 52, 29–36 (2013). https://doi.org/10.1007/s00411-012-0445-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-012-0445-6