Abstract
The aim of the present study was to analyze the mortality from circulatory diseases for about 30,000 members of the Techa River cohort over the period 1950–2003, and to investigate how these rates depend on radiation doses. This population received both external and internal exposures from 90Sr, 89Sr, 137Cs, and other uranium fission products as a result of waterborne releases from the Mayak nuclear facility in the Southern Urals region of the Russian Federation. The analysis included individualized estimates of the total (external plus internal) absorbed dose in muscle calculated based on the Techa River Dosimetry System 2009. The cohort-average dose to muscle tissue was 35 mGy, and the maximum dose was 510 mGy. Between 1950 and 2003, 7,595 deaths from circulatory diseases were registered among cohort members with 901,563 person years at risk. Mortality rates in the cohort were analyzed using a simple parametric excess relative risk (ERR) model. For all circulatory diseases, the estimated excess relative risk per 100 mGy with a 15-year lag period was 3.6 % with a 95 % confidence interval of 0.2–7.5 %, and for ischemic heart disease it was 5.6 % with a 95 % confidence interval of 0.1–11.9 %. A linear ERR model provided the best fit. Analyses with a lag period shorter than 15 years from the beginning of exposure did not reveal any significant risk of mortality from either all circulatory diseases or ischemic heart disease. There was no evidence of an increased mortality risk from cerebrovascular disease (p > 0.5). These results should be regarded as preliminary, since they will be updated after adjustment for smoking and alcohol consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influence of low to moderate doses of exposure to ionizing radiation on the development of non-cancer effects, and in particular on diseases of the circulatory system (DCS), has remained controversial. A detailed study of non-cancer mortality and mortality from DCS among atomic bomb survivors was presented in 2003 (Preston et al. 2003) and later (Shimizu et al. 2010). These works provide clear-cut evidence for a linear dependence of death rates on weighted absorbed dose (where the neutron absorbed dose was given a weight of 10) suggesting an excess risk even at low dose within the range 0–4 Sv. However, it is noted in both papers that there is no direct evidence of radiation effects for doses less than 0.5 Sv. Recently, a statistically significant increase in the risk of mortality from DCS was also described among male workers in the British nuclear fuels industry (McGeoghegan et al. 2008), but the authors have pointed out that there is evidence of inhomogeneity in the apparent dose response arising at cumulative doses in excess 300 mSv and that further efforts are required to investigate the role of factors, other than radiation. Also, in a recent publication (Azizova et al. 2010, 2011), an increased risk of incidence and mortality from ischemic heart disease (IHD) and cerebrovascular diseases (CVD) was described in Mayak PA workers. Studies of Chernobyl accident clean-up workers indicated an increased risk of CVD (Ivanov 2007). Changes in mortality and incidence associated with cardio-vascular pathology were also described for radiologists and radiological technologists, patients receiving radiotherapy, and persons administered diagnostic procedures, involving radiation exposures in different years and countries (McGale and Darby 2005; UNSCEAR Report 2006; Little et al. 2010). At the same time, McGale and Darby (2005) in their review showed that several studies (Darby et al. 1987; Davis et al. 1989; Berrington et al. 2001) had not found appreciable evidence that exposure to low-dose radiation was associated with circulatory diseases.
It is well known that the impact of non-radiation factors such as lifestyle (hypodynamia, smoking, alcohol consumption, and diet), socio-economic status, and health status (obesity, hyperlipidemia, diabetes, and heredity) play a more important role in the development of DCS and IHD as compared with radiation exposure. That is why one should be confident that the dose response does not depend on these factors. Azizova et al. (2010–2011) investigated the dependence of DCS on non-radiation factors (smoking, body mass index, blood pressure) and showed that the adjustment for these factors had little impact on the estimates of excess relative risk (ERR) per Gy for IHD and CVD, although there was an observable relationship between DCS and smoking, hypertension, and increased body mass, in this cohort. The studies performed by Yamada et al. (2004) based on the adult health study (AHS) cohort of the atomic bomb survivors showed a positive linear dose response for acute myocardial infarction in individuals exposed at the age under 40, while adjustment for such factors as smoking and alcohol did not change the dose-response value.
However, many issues concerning epidemiological evidence for non-cancer effects, especially at low to moderate doses, and biological mechanisms of cardio-vascular pathology associated with protracted exposure to ionizing radiation, still remain unclear. Analyses of radiation effects at low and moderate doses among Techa River Cohort (TRC) members have the potential to provide this much needed information. Between 1949 and 1956, about 1017 Bq of uranium fission products were released into the Techa River from the radiochemical plant of the Mayak Production Association (MPA) which was the first site in Russia for the production of weapon-graded plutonium. About 95 % of the total activity was released into the river, between September 1950 and November 1951 (Degteva et al. 2012; Shagina et al. 2012). Villagers living along the river received external exposure due to contamination of the river shoreline and floodplain soils, and internal exposure due to ingestion of 90Sr, 89Sr, 137Cs, and other radionuclides with contaminated water, milk, and local foodstuffs. The first investigation of the radioactive contamination of water and bottom sediments in the upper Techa River was performed in June 1950. Systematic monitoring of the environmental contamination along the river began in July 1951. Maximum levels of radioactive contamination were observed in the early 1950s and decreased with time as well as with the distance from the site of radioactive releases.
The Urals Research Center for Radiation Medicine (URCRM) developed a system for permanent follow-up of exposed persons and established the TRC (Akleyev and Kisselyov 2002; Kossenko et al. 2005). For five decades, the highly qualified medical specialists of the URCRM have been rendering specialized medical service to the exposed residents, and the URCRM researchers have been investigating the consequences of the Techa River contamination.
Materials and methods
Cohort definition
The TRC includes 29,735 individuals born before January 1, 1950, who lived in any of the 41 Techa riverside villages during the period from 1950 to 1960.
The basic sources of information used for identifying cohort members were village tax books which contained detailed information on each of the residents of the Techa riverside villages. In addition, door-to-door surveys of the population of the riverside villages have been conducted in 1950–1960 by the staff of the URCRM.
Mortality follow-up
For all cohort members, the period of follow-up started on January 1, 1950 or at the date of the person’s arrival in one of the villages on the Techa River during the period from January 1, 1950, through December 31, 1960. The date of the end of the follow-up period was December 31, 2003, or an earlier date if the person died at that date, or the date of migration outside the catchment area, or the date of last known vital status in the catchment area.
The mortality catchment area (MCA) encompasses Chelyabinsk and Kurgan oblasts (districts) through which the Techa River flows. In this catchment area, most of the cohort members lived during the whole follow-up period, and it was possible to collect information on dates and causes of death on a systematic basis.
The key sources of vital status information for TRC members were tax books and data obtained from Address Bureaus of Chelyabinsk and Kurgan oblasts. For mortality data, the key sources were copies of medical death certificates obtained from the Oblast Civil Registrar’s offices and statistical bureaus. Unfortunately, there is a lack of information on postmortem examinations. In the framework of the present study, causes of death from all DCS (ICD-9 codes 390–459) and IHD (ICD-9 codes 410–414) were included in the analysis; also, cases of death from CVD (ICD-9 codes 430–438) were considered. Underlying causes of death were coded by trained URCRM staff nosologists based on the rules and codes of International Classification of Diseases (ICD) #9 (ICD-9-CM 2009). Coding was performed using the double-blind method. All cases of disagreement were examined by a commission of experts. A more detailed description of the information collection system was presented in a number of publications (Akleyev and Kisselyov 2002, Kossenko et al. 2005, Akleyev et al. 2008).
More than 80 % of the 29,735 cohort members lived near the river between 1950 and 1953, the period of the highest radiation exposure. The cohort includes individuals of all ages, both genders (women make up 58 %), and two major ethnic groups (with 80 % being of Slavic origin and 20 % of Tartar or Bashkir origin). About 40 % of the cohort members were under 20 when first exposed. At the end of the follow-up, 25 % (7,356) of TRC members were alive, more than 53 % (15,864) had died, about 16 % (4,686) had migrated outside the catchment area, and for 6 % (1,829) residents of the MCA vital status was unknown. Among the 14,311 deaths, 7,595 (53 %) were due to DCS, and for 9.8 % cause of death was unknown.
It can be seen from the data indicated in Table 1 that the proportions of migrants, persons lost to follow-up and alive TRC members comprising the highest-dose groups are almost equal, which suggests that migration will not lead to a bias in the risk estimates. Similarly, a comparable distribution by dose categories can be noted for the deceased cohort members with causes of death known and unknown (Table 2).
Non-radiation risk factors
Actually, a lot of factors influence the development of diseases of the circulatory system, and the dose of ionizing radiation is not the most important of them. Currently, access to complete information on tobacco smoking, alcohol consumption, hypodynamia, and concurrent diseases that can influence a DCS rate is limited; therefore, their effects cannot be assessed. However, in the present analyses of the baseline death rates, it was possible to check potential effects of non-radiation risk factors such as gender, age at initial exposure, attained age, calendar period, oblast of first exposure, ethnicity, and birth cohort effect.
Dosimetry
A special dosimetry system named the Techa River Dosimetry System (TRDS) was developed to support the epidemiological studies on the TRC (Degteva et al. 2000a, b, 2006a). For each individual from the TRC, the TRDS uses personal information on age and residence history, to provide annual age-dependent residence-specific estimates of individualized internal and external doses. Recent improvements in dose reconstruction (Degteva et al. 2006b; Tolstykh et al. 2006, 2011) have been implemented into the latest version of the dosimetry system TRDS-2009.
Among the TRC members, dose distribution in the body was heterogeneous: elevated doses were absorbed in bone marrow, bone surfaces, and large intestine from intakes of 89,90Sr. Other tissues (including muscles) were exposed almost homogeneously mainly from external exposures and dietary intakes of 137Cs. Of all the organ doses, it is the muscle dose which, in the authors’ opinion, can serve as the most reliable representative of the dose that could affect the heart (a muscular organ) and the blood vessels (consisting of muscular and connective tissues). This is supported by the fact that diseases of the musculoskeletal system and connective tissue are confined within one class of ICD-9 (ICD-9-CM 2009).
Contributions of external and internal exposures to the total muscle dose varied significantly from individual to individual depending on the residence history. On average, external dose in different Techa riverside villages was 3–10 times higher in comparison with internal dose. The dose rate was maximum in 1951–1953 and then decreased over time. Thus, the annual increase in cumulative dose was significant in the 1950s and became insignificant after 1960.
The mean and maximum absorbed doses to muscular tissue calculated with TRDS-2009 for the TRC members were 35 mGy and 510 mGy, respectively (Degteva et al. 2009). For about 54 % of cohort members, absorbed doses in muscular tissue were below 10 mGy, for 31 % they were from 10 to 50 mGy, and for about 15 % they exceeded 50 mGy. Individual cumulated doses were provided on a year-by-year basis during the period of the individual’s exposure. Because currently there is no clear notion of the time period it takes the DCS to develop after the onset of radiation exposure, specifically under chronic exposure, we estimated ERR using different effect-lag periods (0, 5, 10, 15, and 20 years). This means that the dose we used for risk estimation had accumulated by year n (0, 5, 10, 15 or 20 years) before the end of the follow-up period. In other words, the lag period is the minimum time required for clinical manifestation of dose response.
Statistical model
Mortality rates for DCS were analyzed using simple parametric ERR models (Preston et al. 1993). The basic ERR model for age-specific rates can be written as:
where a is the age at diagnosis, d is the muscle absorbed dose (in mGy), z 0 represents factors (such as sex, birth cohort, ethnicity, oblast of first exposure, or time period) that can modify the baseline rates λ 0 , and z 1 represents factors modifying the ERR. The excess risk was described as a product of a dose response function ρ(d) and an effect modification function (ε(z 1 )). The dose response function was generally taken as a linear function of dose (β 1 d).
Tests for non-linearity in the dose response were based on comparison of the linear model and with a linear-quadratic dose response model (β 1 d + β 2 d 2 ) and a pure quadratic model (β 1 d 2). Significance tests and confidence intervals (CI) were determined directly from the likelihood. Log-linear models were used to describe radiation effect modifiers. The primary analyses were based on dose response models without effect modification. The nature of the effect modification was assessed by adding potential effect modifiers to the model. Analyses of background rates and excess risks were carried out using the Poisson regression method in a highly stratified table of cases and person years (Preston et al. 1993). The factors defining these cross-classifications included gender, ethnicity, oblast of initial exposure, age at the beginning of exposure (5-year groups up to age 60 and one age group above), attained age (5-year groups up to age 80 and one age group above), birth cohort (persons born before or after 1925), calendar period (with lower bounds 1950, 1970, 1990), time since arrival in the catchment area, and lagged cumulative dose (with 0, 5-, 10-, 15-, 20-year lags). There were eight dose categories in the person-year table (with lower bounds of 0, 5, 10, 50, 100, 150, 250, and 300 mGy). The ERR was estimated using five lag periods (0, 5, 10, 15, and 20 years).
The dependence of baseline death rates on non-radiation factors was different for mortality from all DCS and from IHD. The baseline death rates from all DCS were modeled with the function:
where
while the baseline death rates from IHD were also modeled with Eq. (2) where
Testing the same baseline model for CVD has shown a significant dependence on all the parameters, except for calendar period (thus, in Eq. (3) the term \( \beta_{7} period_{1} + \beta_{8} period_{2} \) was excluded).
Results
For a 54-year period of follow-up, 901,563 person years at risk and 7,595 death cases of DCS were registered in the catchment area among TRC members. Among them, there were 3,194 (42 %) death cases from IHD, 1,933 death cases from CVD, and the remainder deaths from DCS amounted to 2,468.
Baseline risks
All parameters included in baseline model of all DCS and calculated based on the linear non-threshold (LNT) model of ERR show a significant effect on death rates in the TRC. Rates for women were almost a factor of 2 smaller than those for men, and the relative risk (RR) was 0.57. Death rates increased with attained age both for men and women. Rates for Slavs were slightly higher than those for Tartars and Bashkirs (RR = 1.1). Death rates for cohort members who were born after 1925 were slightly lower (RR = 0.97) compared with those for cohort members born before 1925. Cohort members whose first exposure was in Kurgan oblast had higher death rates than cohort members first exposed in Chelyabinsk oblast (RR = 1.2). Death rates from the DCS are increasing with each 20-year period of time).
A significant influence (p < 0.001) on baseline mortality rates from IHD was exerted by the following factors: gender (female rate is 0.49 of male rate), attained age (rates are increasing with age), birth cohort (rates of those born after 1925 are 1.38 of the rates of those born before 1925), calendar period (rates after 1986 are 0.62 times those before 1986), and oblast of initial exposure (Kurgan oblast rates were 1.37 times higher than those for Chelyabinsk oblast). The baseline effect of ethnicity was found to be statistically insignificant for deaths associated with IHD.
As indicated in the “Materials and methods” section, the calendar period has not been included in the baseline model for ERR calculation of CVD mortality. Baseline CVD rates for women were lower than those for men, and for Tartars and Bashkirs, they were lower compared with those for Slavs. Among individuals who were born after 1925, the rates were higher compared with those born before 1925, the rates in Kurgan oblast were lower than those in Chelyabinsk oblast, the rates were increasing with age, and the increase was more rapid among women.
Radiation risk estimates
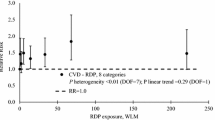
A separate analysis of dose effects did not reveal any significant dose dependence of mortality from CVD. The excess relative risk (ERR) of death from CVD did not show any statistically significant linear or non-linear dependence on doses received whatever lag period used (e.g., the ERR/100 mGy was −1.4 %; 95 % CI:−6.4 %; 4.6 %; p > 0.5 for a 15-year lag period, and it was almost the same for all other lag periods investigated). For this reason, only mortality risks from all DCS and from IHD are discussed in detail below.
As was noted above, the ERR of mortality from all DCS and IHD was calculated using different lag periods. Tables 3 and 4 present ERR and attributive risk values and statistical significance of the rates, as well as excess cases calculated using a simple parametric ERR model for different lag periods for death from all DCS, and for death from IHD. It can be seen that the ERR/100 mGy value and the attributive risk value increase with increasing lag period (from 0 to 20 years), and the p value decreases with increasing lag time. Beginning from the 15-year lag period, ERR becomes significant for mortality from both all DCS (Table 3) and from IHD (Table 4).
Table 5 shows values of ERR/100 mGy for deaths from all DCS and from IHD using different effect dependences. The analysis suggests that despite a number of limitations, the current TRC data provide a significant linear dose response for mortality risk from all DCS (ERR/100 mGy = 3.6 %) and from IHD (ERR/100 mGy = 5.6 %). Based on the likelihood ratio test, addition of a quadratic component to the linear model did not improve the statistical power of the model both for deaths from all DCS and for those from IHD (p > 0.5). A test for purely quadratic dependence for all DCS also indicated a significant ERR/100 mGy value (8.2 %), but the linear model provided a slightly better fit. For IHD, the purely quadratic dependence was statistically insignificant (Table 5).
Table 6 gives the number of person years (over 900 thousand), the number of observed and excess death cases from DCS and from IHD, and the proportion of excessive death cases (i.e., the attributive risk) by dose groups. It can be seen that the proportion of excess cases calculated based on the linear ERR model increases with dose. On the whole, only 1 % of radiation-related deaths from DCS and 1.5 % deaths from IHD can be expected among all TRC members over the whole follow-up period. This small portion of excess deaths related to exposure is probably due to the fact that the mean dose accumulated by TRC members is low.
Effect modification
In the present study, the probability of dose effect modification under the influence of non-radiation factors relevant for all DCS and for IHD was also analyzed. The following factors were considered as modifiers: oblast of first exposure, ethnicity, gender, age at onset of exposure, attained age, and calendar period.
The factor that exhibited a significant influence on ERR of death from all DCS (Table 7) is ethnicity: the value for the ERR/100 mGy for Tartars and Bashkirs is 6 times higher compared with that for Slavs. For mortality from IHD, this tendency is still visible, but insignificant (Table 8). For IHD mortality, statistically significant effect-modifying factors are attained age (ERR is decreasing with increasing attained age, p = 0.002) and age at initial exposure (ERR is decreasing with increasing age of initial exposure by 83 % per decade and ERR was higher for individuals aged 20 than for those aged 40: 10 % and 2 %, p < 0.001, respectively). The same trend with age modification was shown in the life span study (LSS) cohort (Preston et al. 2003, Shimizu et al. 2010).
Discussion
In earlier studies on the TRC cohort, results of risk analyses of solid cancer and leukemia incidence and mortality showed a statistically significant increase in the risk of cancer development over the period of 50 years or more (Krestinina et al. 2005, 2007, 2010). The present paper represents the first publication of the results from mortality risk analysis for all DCS in the TRC over the period from 1950 through 2003, obtained by means of a retrospective epidemiological cohort study.
As expected, the study of baseline death rates showed that mortality from all DCS, IHD, and CVD is significantly lower for women than that for men, and increases with attained age. The rates for Slavs are slightly higher than those for Tartars and Bashkirs (including a significant effect for all DCS and CVD and a marginal effect for IHD), the rates for Kurgan residents are slightly higher than those for Chelyabinsk residents, and the rates are slightly lower for individuals who were born after 1925 as compared with those who were born before 1925. The mortality rates from all DCS are increasing with time.
The gender effect observed is consistent with many other studies (Azizova et al. 2010, 2011; Shimizu et al. 2010), while an increasing death rates from DCS with calendar years is a common trend for Russia. Higher death rates from DCS and lower rates from cancer among Kurgan oblast residents compared with residents of Chelyabinsk oblast are consistent with our previous analyses (Krestinina et al. 2005, 2007). This may reflect the fact that most of Kurgan residents included in the TRC lived in rural localities and were older than the Chelyabinsk oblast residents, and it can be assumed that they were more often diagnosed with atherosclerosis which is in general a common disease among aged persons. However, some other factors unknown to us may also be involved. Effects of ethnicity and birth cohort on baseline rates are small (1–3 % differences) and variable. Note that calculation of radiation risk values included adjustment of the baseline rates by all of these parameters.
The present preliminary study of dose response has shown a statistically significant dose-dependent increase in mortality risk from all DCS, for the TRC which is characterized by a combined chronic external and internal exposure with muscular tissue doses in the range of 0–510 mGy. It should be noted that a statistically significant risk of mortality from DCS and IHD was obtained only when a 15-year (or more) lag since first exposure was used. If the lag period used in the analysis was less than 15 years (0, 5, or 10 years), estimates of the ERR per unit dose were positive, but statistically insignificant. On the contrary, the values of ERR/Gy for death from CVD did not reveal any dose dependence, regardless of the lag period used.
The risk value of mortality from all DCS is consistent with that obtained for the LSS cohort (Shimizu et al. 2010): for the TRC, the ERR per 100 mGy was 3.6 % (95 % CI: 0.2–7.5 %) for a 15-year lag period, while it was 1.1 % (95 % CI 0.5–1.7 %) in the LSS cohort. The TRC estimates are very close (but insignificant) to those of the LSS if the analysis was performed without a lag period (1.3 %; 95 % CI: −1.6; 4.6). The recent studies of the Mayak worker cohort (Azizova et al. 2010) also indicated that the ERR values (associated with external dose) for IHD incidence among Mayak PA workers are significant for any lag period (0, 5, 10, 15, 20 years) and accounted for 1.1–1.3 % per 100 mGy of external gamma radiation.
With respect to the lag period similar findings were observed in a study for male workers at British Nuclear Fuels where it was reported “that higher levels of significance were attached to trends when calculated with cumulative doses lagged between 10 and 20 years” (McGeoghegan et al. 2008). Studies of DCS mortality in female survivors of breast cancer (Darby et al. 2003) also indicated that excess death cases were mainly observed more than 10 years after exposure.
The present study—as any other study—has its own advantages and limitations. An important aspect of the TRC is the fact that analysis of radiogenic health effects can be performed among members of an unselected population, including men and women of all ages and different ethnic identity. Different baseline health status characteristics of these population groups allows avoiding healthy worker effects typical of worker cohorts, healthy survivor effects in the LSS cohort, and effects of diseases among individuals and patients exposed to radiation due to diagnostic and therapeutic procedures. An increased period of follow-up (1950–2003 in the present study) provided the opportunity to increase the statistical power of the study over the years. Individual residence histories available for all cohort members allow accurate estimation of individual doses and person years at risk. Another advantage is the use of individualized doses to muscle tissues accumulated over the whole period of the follow-up, including external and internal exposures and calculated on the basis of the improved dosimetry system (TRDS-2009).
It should also be stressed that the situation of the residents living along the Techa River is unique, which distinguishes the TRC from other cohorts used in the studies on chronic radiation effects. The distinguishing features of the TRC are: (a) a combined exposure (both external and internal irradiation), which implies that the dose response may differ for external and internal exposure in terms of both quality and quantity (Report of the CERRIE 2004); (b) an exposure that was non-uniform over time (continuous, but with highest-dose rates during a number of initial years followed by a sharp decrease and a gradual decline in the subsequent years); (c) an exposure to short-lived and long-lived radionuclides with different target organs; and (d) an aging cohort (decreasing portion of young population with calendar period of follow-up).
Along with the advantages already mentioned above, the present study has also some limitations. In particular, any long-term follow-up usually involves considerable migration processes in the study population. Here, by the end of the follow-up period, 15.8 % of TRC members had migrated, and for 6.1 % of the TRC members the vital status was unknown, which has led to a certain decrease in the statistical power of the study. Moreover, dose uncertainties which are difficult to quantify may contribute to risk uncertainties.
As noted above, information on non-radiation risk factors for circulatory diseases such as smoking or alcohol consumption, hypodynamia, heredity, concurrent diseases, hyperlipidemia, etc., is currently not available for TRC members and, accordingly, it could not been taken into account in the present risk estimations. However, the fact that an internal control group was used is an advantage in this case: the relative risk of DCS mortality is estimated here by comparing the rates obtained for members of the same cohort assigned to different dose groups but living in the same localities, during the same time period, under similar living conditions with similar dietary habits, with the same level of medical services, and facing the same social problems. That is why the authors believe that the influence of the non-radiation factors on the DCS death rate will be the same in different dose groups, and it should not cause any bias in the risk value per unit dose.
Some non-radiation factors were investigated for the LSS and Mayak worker cohorts (Shimizu et al. 2010, Azizova et al. 2010, 2011), but no connection with dose was found. Additionally, the same trend of decreasing ERR values for IHD with increasing attained age obtained in the present study was also found in the Mayak worker cohort (associated with external dose) (Azizova et al. 2010).
While it is well documented that high radiation exposures affect human health, the consequences of exposures to low radiation doses for long-time periods have not yet been thoroughly elucidated. Therefore, studies of health effects associated with a long-term exposure at low to medium doses received by the residents of the Techa riverside population provide an important source of information, in particular with respect to non-cancer effects.
It should also be pointed out that there are a number of studies showing that the key biological mechanism to develop circulatory diseases is the high level of cell killing, and ensuing pro-inflammatory effects and micro-vascular damage (Little et al. 2010). A better understanding of the nature of the biological low/moderate dose effects is, however, needed that will require further epidemiological and radiobiological data. The data obtained in current study may provide some useful contribution.
Conclusions
From the current TRC study, the following conclusions can be drawn:
-
1.
Chronic radiation exposure of TRC members who received a cumulative dose of up to 500 mGy in the period 1950–2003 resulted in a radiation-related excess risk of death from DCS and from IHD in particular.
-
2.
Estimation of mortality risk from CVD did not reveal any statistically significant linear and non-linear dependence on dose.
-
3.
Radiation risk of death from all DCS and from IHD showed a linear trend with dose, for muscular doses up to 500 mGy.
-
4.
For individuals exposed to doses of up to 500 mGy, attributive (related to exposure) risk of death from all DCS is low accounting for 1.5 % and attributive risk of death from IHD is 1 %; for mortality from CVD, there was no statistically significant increase in risk related to dose.
-
5.
The ERR of death from DCS was 3.6 % per 100 mGy (95 % CI: 0.2–7.5 %) while that from IHD was 5.6 % per 100 mGy (95 % CI: 0.1–11.9 %);
-
6.
The minimal lag period where ERR values of death from all DCS and IHD were statistically different from zero was 15 years.
-
7.
Radiation exposure manifested a significant effect modification: (a) for all DCS deaths ethnicity was a statistically significant effect-modifying factor (ERR for Tartars and Bashkirs was higher than that for Slavs, p < 0.05) and (b) for IHD deaths attained age was a statistically significant effect-modifying factor (ERR is decreasing with increasing attained age, p = 0.001), as was age at initial exposure (ERR is decreasing with increasing age of initial exposure by 83 % per decade, p < 0.001).
-
8.
The ERR values obtained in the present study are preliminary and should be viewed critically, because common non-radiation DCS risk factors such as smoking or alcohol consumption, hypodynamia, heredity, concurrent diseases, hyperlipidemia, etc., could not be taken into account in risk estimation, as well as uncertainties associated with dose estimation. The research on the TRC is continuing, however, and a new analysis will be performed with an extended follow-up period, as soon as additional information on non-radiation risk factors and dose uncertainties will become available.
References
Akleyev AV, Kisselyov MF (2002) Medical-biological and ecological impacts of radioactive contamination of the Techa River. FREGAT, Chelyabinsk
Akleyev AV, Krestinina LYu, Preston D, Davis F, Degteva MO, Anspaugh L, Startsev NV, Napier B, Ron E (2008) Radiogenic risk of malignant neoplasms for Techa riverside residents. Medical radiology and radiation safety 53(6):5–26
Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, O’Hagan JA, Zhang W, Haylock RGE, Hunter N (2010) Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res 174:155–168
Azizova TV, Muirhead CR, Moseeva MV, Grigoryeva ES, Sumina MV, O’Hagan JA, Zhang W, Haylock RGE, Hunter N (2011) Cerebrovascular diseases in nuclear workers first employed at the Mayak PA in 1948–1972. Radiat Environ Biophys 50:539–552
Berrington A, Darby SC, Weiss HA, Doll R (2001) 100 years of observation on British radiologists: mortality from cancer and other causes 1897–1997. Br J Radiol 74:507–519
Darby SC, Doll R, Gill SK, Smith PG (1987) Long term mortality after a single treatment course with x-rays in patients treated for ankylosing spondylitis. Br J Cancer 55:179–190
Darby SC, McGale P, Peto R, Granath F, Hall P, Ekbom A (2003) Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90,000 Swedish women. Br Med J 326:256–257
Davis FG, Boice JD Jr, Hrubec Z, Monson RR (1989) Cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients. Cancer Res 49:6130–6136
Degteva MO, Vorobiova MI, Kozheurov VP, Tolstykh EI, Anspaugh LR, Napier BA (2000a) Dose reconstruction system for the exposed population living along the Techa River. Health Phys 78:542–554
Degteva MO, Kozheurov VP, Tolstykh EI, Vorobiova MI, Anspaugh LR, Napier BA, Kovtun AN (2000b) The Techa River dosimetry system: methods for the reconstruction of internal dose. Health Phys 79:24–35
Degteva MO, Tolstykh EI, Vorobiova MI, Shagina NB, Shishkina EA, Bougrov NG, Anspaugh LR, Napier BA (2006a) Techa River dosimetry system: current status and future. Radiat Saf Probl 1:66–80 (in Russian)
Degteva MO, Vorobiova MI, Tolstykh EI, Shagina NB, Shishkina EA, Anspaugh LR, Napier BA, Bougrov NG, Shved VA, Tokareva EE (2006b) Development of an improved dose reconstruction system for the Techa River population affected by the operation of the Mayak Production Association. Radiat Res 166:255–270
Degteva MO, Shagina NB, Tolstykh EI, Vorobiova MI, Anspaugh LR, Napier BA (2009) Individual dose calculations with use of the revised Techa River Dosimetry System TRDS-2009D. Chelyabinsk and Salt Lake City: Urals Research Center for Radiation Medicine and University of Utah; final report for milestone 22
Degteva MO, Shagina NB, Vorobiova MI, Anspaugh LR, Napier BA (2012) Reevaluation of waterborne releases of radioactive materials from the Mayak production association into the Techa River in 1949–1951. Health Phys 102(1):25–38
ICD-9-CM (2009) http://icd9cm.chrisendres.com/index.php?action=contents
Ivanov VK (2007) Late cancer and non cancer risks among Chernobyl emergency workers of Russia. Health Phys 93:470–479
Kossenko MM, Thomas TL, Akleyev AV, Krestinina LYu, Startsev NV, Zhidkova CM, Vyushkova OV, Hoffman DA, Preston DL, Davis F, Ron E (2005) The Techa River cohort: study design and follow-up methods. Radiat Res 164:591–601
Krestinina LYu, Preston DL, Ostroumova EV, Degteva MO, Ron E, Vyushkova OV, Startsev NV, Kossenko MM, Akleyev AV (2005) Protracted radiation exposure and cancer mortality in the Techa River cohort. Radiat Res 164:602–611
Krestinina LYu, Davis F, Ostroumova EV, Epifanova SB, Degteva MO, Preston DL, Akleyev AV (2007) Solid cancer incidence and low-dose-rate radiation exposures in the Techa River cohort: 1956–2002. Int J Epidemiol 36(5):1038–1046
Krestinina L, Preston DL, Davis FG, Epifanova S, Ostroumova E, Ron E, Akleyev A (2010) Leukemia incidence among people exposed to chronic radiation from the contaminated Techa River 1953–2005. Radiat Environ Biophys 49:195–201
Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, Tapio S, Elliot P (2010) Review and meta-analysis of epidemiological associations between low or moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat Environ Biophys 49(2):139–153
McGale P, Darby SC (2005) Low doses of ionizing radiation and circulatory diseases: a systematic review of the published epidemiological evidence. Radiat Res 163:247–257
McGeoghegan D, Binks K, Gillies M, Jones S, Whaley S (2008) The non-cancer mortality experience of male workers at British nuclear fuels plc 1946–2005. Int J Epidemiol 37:506–518
Preston DL, Lubin J, Pierce D, McConney M (1993) Epicure users guide. Hirosoft international company, Seattle, Washington
Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K (2003) Studies of the mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 160(4):381–407
Report of the committee examining radiation risks of internal emitters (CERRIE) (2004) London: Crown Copyright
Shagina NB, Vorobiova MI, Degteva MO, Peremyslova LM, Shishkina EA, Anspaugh LR, Napier BA (2012) Reconstruction of the Techa River contamination in 1949-1951 as a result of releases from the “Mayak” Production Association. Radiat Environ Biophys 51(4):349–366
Shimizu Yu, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, Grant EJ, Sugiyama H, Sakata R, Moriwaki H, Hayashi M, Konda M, Shore RE (2010) Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data 1950–2003. Br Med J 340:b5349
Tolstykh EI, Degteva MO, Vorobiova MI, Peremyslova LM, Shagina NB, Anspaugh LR, Napier BA (2006) Reconstruction of long-lived radionuclide intakes for Techa Riverside residents. Cesium-137. Radiat Saf Probl 1(Special issue):68–79 (in Russian)
Tolstykh EI, Degteva MO, Peremyslova LM, Shagina NB, Shishkina EA, Krivoshchapov VA, Anspaugh LR, Napier BA (2011) Reconstruction of long-lived radionuclide intakes for Techa Riverside residents: strontium-90. Health Phys 101(1):28–47
UNSCEAR (2006) Report Vol. 1 Effects of ionizing radiation. Annex B: Epidemiological evaluation of cardiovascular disease and other non-cancer diseases following radiation exposure. United Nations: New York
Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G (2004) Non cancer disease incidence in the atomic bomb survivors 1958–1998. Radiat Res 161:622–632
Acknowledgments
This study was conducted with financial support from the European Commission in the framework of the 6th Programme Euroatom under the project “Southern Urals Radiation Risk Research (SOUL).” This work has also been supported by the US National Cancer Institute, NIH (collection of vital status information), the US Department of Energy (dosimetry study), and the Federal Medical Biological Agency of Russia (all aspects of this study). The authors would like to thank members of the Chelyabinsk Regional Center of the National Radio-Epidemiological Register (Head: Nikolai Startsev) which represents an important source of information for the URCRM core database, and professor Per Hall from the Department of Medical Epidemiology and Biostatistics of Karolinska Institute who worked with the URCRM researchers in the framework of the SOUL Project and helped to develop the design of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krestinina, L.Y., Epifanova, S., Silkin, S. et al. Chronic low-dose exposure in the Techa River Cohort: risk of mortality from circulatory diseases. Radiat Environ Biophys 52, 47–57 (2013). https://doi.org/10.1007/s00411-012-0438-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-012-0438-5