Abstract
Concerns on health effects from uranium (U) mining still represent a major issue of debate. Any typology of active job in U mines is associated with exposure to U and its decay products, such as radon (Rn), thorium (Th), and radium (Ra) and its decay products with alpha-emission and gamma radiation. Health effects in U miners have been investigated in several cohort studies in the USA, Canada, Germany, the Czech Republic, and France. While public opinion is particularly addressed to pay attention to the safety of nuclear facilities, health hazard associated with mining is poorly debated. According to the many findings from cohort studies, the most significant positive dose-response relationship was found between occupational U exposure and lung cancer. Other types of tumors associated with occupational U exposure are leukemia and lymphoid cancers. Furthermore, it was found increased but not statistically significant death risk in U miners due to cancers in the liver, stomach, and kidneys. So far, there has not been found a significant association between U exposure and increased cardiovascular mortality in U miners. This review tries to address the current state of the art of these studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium (U) is a well-known naturally occurring radioactive element that is positioned along with thorium (Th) and other elements such as plutonium (Pu) in the actinide group of the periodic table. The element has an atomic number of 92, and an atomic mass of 238,02891 U. Out of all naturally occurring chemical elements, U has the highest atomic weight, and according to past reports, it should be formed due to supernova explosions (Plant et al. 1999). Uranium is rather common in rocks and soils with the average concentration in the earth’s crust about 2–3 mg/kg. Igneous rocks have particularly high contents of U. Alkaline granites are the richest in U, where its concentration can reach 100 g/t. Out of sedimentary rocks, clayey rocks have the highest concentrations of U equal to 3–4 mg/kg. In contrast, carbonate and clastic rocks are poor in U with the average values of 0.45–0.59 mg/kg for clastic rocks and of 2.2–2.5 mg/kg for carbonate rocks (Missimer et al. 2019).
Since U is common in the earth’s crust, the challenge is to identify those places where U ore concentration is high enough to establish economically viable mining. To date, U is primarily utilized for the production of nuclear fuel, but it could also be used for the manufacture of nuclear weapons. The commercial mining of U for such purposes started in the early 1940s, while before U was mostly mined for radium extraction, which was utilized in therapeutic or scientific purposes (Zoellner 2009). The largest explored U fields are located in Australia, Kazakhstan, and Canada; these three countries are the leading U producers accounting for about 64% of the global production (Markabayeva et al. 2018). Table 1 presents data on the top 20 U mining countries in the world with tons of U produced from 1948 to 2018 (OECD-NEA and IAEA 2018).
Both U mining and processing are mostly associated with exposure to low-soluble U compounds that are claimed to exert low toxicity (Bjørklund et al. 2017). Still, U undergoes radioactive decay by alpha, beta, and gamma emission and imposes health risks on the workers. Radon (Rn) is a common decay product of U and is a natural radioactive gas, which is found in appreciable levels in U mines. There are several isotopes of Rn, but 222Rn is the only one to originate from 238U. However, daughter nuclides of Rn—isotopes of polonium (218Po, 214Po), lead (214Pb), and bismuth (214Bi)—produce around 50% of the Rn induced damage to humans. Apart from U and Rn, miners and processors are also exposed to other radionuclides like 230Th, 226Ra, and their decay products with alpha-emission and gamma radiation (Paredes et al. 2016). The leakage of Rn from rocks is strongly associated with a set of factors such as density and porosity of the specific material and moisture content (Mudd 2008).
There are several methods of U extraction and in situ leaching is currently being the most common, followed by underground mining, open-pit mining, and heap leaching (Brown 2019). Underground mining is still practiced in almost a quarter of world U mines and is related to the highest occupational radiation exposure (Cinelli et al. 2017). Apart from U mining, phosphate mining is also connected with elevated radiation exposure as phosphorite deposits often contain U in a sufficient amount to be cost effective when the price of U is high (Louw 2020). Deep coal mining sites also may have elevated Rn concentration, which is detained in natural geological structures (Wysocka et al. 2019), and gold mining is also associated with U exposure (Winde et al. 2019).
Type of ventilation and dust control determines the rate of exposure in deep underground mines. Poorly designed and maintained ventilation and dust control systems are among the major causes of U miners’ exposure to excessive levels of Rn daughters and other contaminants. It is unclear what an optimal ventilation rate is to enable Rn control since its levels could differ between the mines and even between different sites of the same mine, but natural ventilation is believed to be unacceptable and secondary ventilation should be preferred. Nowadays, it is a common expectation for all air control systems to enable flexibility, rapid air transit times, high air distribution efficiency, zero recirculation, and no short-circuiting (Mironenkova and Magomet 2017).
In general, the hazards of U mining for miners have been well documented for over past decades. However, it has to be stated that the conditions of worker radiation exposure in U mines of the past (the 1950s–early 1970s) are minimized in modern mines. The early miners worked in conditions that, by today’s standards, would be considered unacceptable and were exposed to very high levels of Rn progeny in poorly ventilated underground mines (Navaranjan et al. 2019). Moreover, many of these miners also had severe smoking habits, which enhanced the ability of the Rn decay products to deliver radiation dose (Grosche et al. 2006). Nowadays, the risk of radiation exposure could be very low if occupational standards are followed carefully, but non-compliance with standards increases the risk of excess exposure. This review aims to describe radiation-related occupational exposures resulting from U mining with a focus on the health effects of underground mining, considering the legacy of past decades as well as contemporary findings. We did not consider occupational exposures at the more distal end of the U fuel cycle resulting from nuclear weapons production and testing as well as nuclear power generation.
Methods
A literature review of the available publications was conducted to elucidate the radiation-induced effects of U mining on the health of underground miners. As a first step, we performed the keyword searches in PubMed and Google Scholar with the help of a series of relevant search terms. Search results covered both papers published in English and Russian languages with no date restrictions. We selected just the first 50 results out of each search when we conducted a search in Google Scholar and sorted them by relevance. This strategy was applied since Google Scholar lacks specificity as compared to other search engines. On the next step, we selected the publications by scanning the titles and abstracts trying to evaluate the relevance to U mining and radiation-related health effects. We excluded studies of U exposure in the general environment and removed all duplicates. Publications eliminated, including those specific to the general population residing around U mines, non-human studies. Also, publications that appeared to be outside the scope of this review, and for which the full-text papers could not be obtained, as well as articles on other languages apart from English and Russian were excluded. Overall, 141 publications were accepted for this review. All accepted publications were carefully reviewed and classified by the following study type: cohort studies of disease incidence and/or mortality and cross-sectional studies. Finally, we constructed the table to highlight the main findings related to associations between lung cancer and U mining (Table 2). The research group, study design, study location, and duration of follow-up, as well as key findings, were presented in this table.
Radon-related radiation exposure
Radon radioactivity can be widespread in the whole body from inhalation in small, restricted environments, such as mines. Still, Rn has limited retention as the biggest part of it is exhaled within the period of its half-life. However, after its formation in the aeriform environment of mines, the immediate Rn progeny quickly attach to surrounding solid surfaces, including dust particles (Chen 2017; Santos et al. 2014; Fan et al. 2016). As soon as these dust particles carrying Rn progeny are inhaled, they begin to deliver radiation (mostly due to alpha-emission) to nasal cavity and sinuses, laryngopharynx, trachea, and bronchi with subsequent deposition. It was estimated that the radiation dose delivered by these particles to the lungs of U miners is about 20 times higher than that delivered by inhaled Rn (Skubacz et al. 2019; Kang et al. 2019).
According to ICRP, the applicable radiation dose standard for workers is 20 mSv per year, while the annual individual doses vary within the range of 3–20 mSv for underground miners and within the range of 1–5 mSv for open-pit miners (ICRP 2017). At the same time, the average annual outdoors concentration of Rn is usually about 10 Bq/m3 but could vary within the range of 1–20 Bq/m3. As for indoors concentration, the WHO proposed the reference level of 100 Bq/m3 and emphasized that it should not exceed 300 Bq/m3 (WHO 2009). Most countries that perceive Rn problem, adopted 400 Bq/m3 for existing dwellings and 200 Bq/m3 for new dwellings. In underground mines, the reference levels for Rn concentrations are set in the range 400–1000 Bq/m3, while the concentrations above 1000 Bq/m3 require protective measures for the miners (Brown and Chambers 2017).
Besides inhalation, there are several other routes on how radionuclides can enter the body of U miners, including pollutants ingestion, absorption, and entry through a cut or other violation of the skin integrity. All of these items represent the internal exposure in humans, and individuals’ doses from it are usually evaluated indirectly via mathematical models describing absorption, distribution, metabolism, and excretion (ADME) of radionuclides (Skubacz et al. 2019). Being constantly improved and updated, these models enable better estimates of internal exposure (ICRP 2017). The key issue of mathematical modeling is to gain an accurate understanding of how radionuclides are transported from lungs to the blood, as this might affect the internal doses assessed (Marsh and Bailey 2013). In order to achieve high reliability of internal dose assessment, the samples of bioassay (usually urinalysis) or of mine air have to be obtained. Still, even with constant improvements in mathematical modeling and laboratory techniques employed for radionuclides measurement, there is a need to apply expert judgment. And thus, internal doses assessed in one epidemiological study may not be comparable with those assessed in another study. To make this issue even more complex, it has to be noted that modeling is also frequently used for estimating the environmental transport of radionuclides, which makes a comparison between the studies even more difficult. As for external dose assessment, it is subjected to lesser uncertainty, but external exposure in U mining is less pronounced (Tirmarche et al. 2012).
Although miners are exposed to radiation mainly due to Rn nuclear decay products, they also face certain risks typical for the mining sector in general, i.e., inhalation of dust particles and diesel exhausts, vibration, exposure to physical trauma, etc. (Laurence 2011). Nevertheless, the assessment of such multiple combined exposures is not always possible since there is a lack of detailed studies that might provide a better understanding of these risks.
Several strategies were implemented to decrease Rn exposure in underground U miners. First, broad dissemination of in situ leaching technology helps to avoid the health risks posed by underground mining. Second, introduction of strict occupational standards, which envisaged certain measures like monitoring of Rn levels, implementation of wet drilling, installation of forced ventilation, and proper dust control system, was useful in mitigation of health hazards to U miners. In general, the ICRP’s recommendations to keep a worker’s annual effective dose below 20 mSv helps to minimize the mortality rates, in particular, from lung cancer (ICRP 2017).
However, non-compliance with occupational standards may lead to unexpected radiation exposure that might happen accidentally. There are several treatment strategies that address different stages of sickness, which vary from intake of stable iodine, administration of granulocyte-colony stimulating factor, and antimicrobial agents to hematopoietic stem cell transplantation (López and Martín 2011). Besides, a range of radioprotective agents is currently available. Still, these agents have not yet been appropriately tested in a clinical setting to quantify the desirable clinical benefits although they were demonstrated in decreasing the radiation-related damage on a molecular level. Basically, such agents either increase natural antioxidants’ defense or act as the direct free radical scavenge (Smith et al. 2017). Conducting such studies in the field of underground U mining could be of interest.
Overview of the studies on health effects of uranium miners
Because U is a radioactive element, its effects on human health have been widely researched. First reports on the so-called Schneeberg lung disease, which is a lung carcinoma in U miners of Saxon Ore Mountains, are dated as early as 1879. Harting and Hesse developed a method for measuring dust inhaled by U miners that led to the subsequent discovery of Rn and its decay products (Greenberg and Selikoff 1993). However, the first suggestion that radioactivity is responsible for Schneeberg lung disease was made only in 1932 (Pirchan and Sikl 1932). The effective radiation dose received by U miners is determined by the exposure to Rn, gamma radiation photons, and radiation from long-lived alpha emitters. Nowadays, personal dosimeters are commonly used for individual monitoring of radiation dose coming from all these sources (Otahal et al. 2014). The excess relative risk (ERR) related to Rn exposure is usually expressed in working level months (WLM) per year (yr), which is used to quantify the cumulative exposure to Rn progeny (Cucoş Dinu et al. 2017, Tchorz-Trzeciakiewicz and Parkitny 2015, Burghele et al. 2018).
The basis for epidemiological research among U miners is the identification of the fact of exposure. Air sampling and contamination surveys are the primary methods used to ascertain worker internal exposure to 238U. Bioassay, e.g., urinalysis, is used as a “verification” of the robustness of internal exposure control programs and to identify if follow-up actions are necessary. Such, the 238U content is measured by high-resolution inductively coupled plasma mass spectrometry. Daily values of 238U in the urine are calculated according to the expected daily excretion of creatinine of each miner individually (Malátová et al. 2013; Malátová et al. 2016; Kotík et al. 2017). According to recommendations of the International Commission on Radiological Protection (ICRP), the excess relative risk of lung cancer in U miners should be calculated in the case if the duration of chronic exposure exceeds 10 years. This calculation has to be grounded on time from exposure, the age of the miner, or age at the start of exposure (Tirmarche et al. 2012).
Like in the case of all causative studies, exposure to U production has to be identified before any assumptions could be made, and cohort studies fit this purpose better than other types of trials. Cohort study represents a particular type of a longitudinal study, which follows up a “cohort” (a group of exposed individuals) over a specified period (Euser et al. 2009). Although being inferior, the data from case-control studies and case series can also be used to supplement the findings of cohort studies (Oh et al. 2016; Scott 2019).
Lung cancer in uranium miners
Lung cancer in U miners can be triggered by ancillary factors aside from U contamination (Mielke et al. 2018). This may be a fundamental confounder in tracing up correct epidemiology of U health effects. Increased incidence of lung cancer in U miners can be mainly attributed to ionizing radiation, with alpha radiation being perhaps the most important for the carcinogenic effect. Alpha particles are far more destructive than beta or gamma particles when hitting cells, but have a very short range of tissue penetration (Messier and Serre 2017, Baur and Woitowitz 2016; Kreuzer et al. 2015, b, Kelly-Reif et al. 2019). Epidemiological surveys are also difficult because 222Rn can be even captured in home environments. (Hassfjell et al. 2017): This raises concerns and criticism on the U-contaminant power as a leading factor in occupational medicine. Finally, small dust particles also play their role as they can be accumulative in the macrophages, which put potentially chemical toxicity. It has to be noted that the bulk of radiation dose is delivered not through Rn, but through short-lived decay products, 218Po and 214Po. Cancer origin in radiation exposure has monoclonal nature rather than polyclonal, i.e., it results from DNA damage to a single cell (Molaie et al. 2012).
There are a number of retrospective cohort studies, which established an association between exposure to Rn decay products and lung cancer mortality (Lubin 2010; Ramkissoon et al. 2018; Navaranjan et al. 2016). The largest of these studies are coming from Germany, the USA, Canada, the Czech Republic, and France (Kido 2019; Kreuzer et al. 2010; Navaranjan et al. 2016; Rage et al. 2015; Tomásek 2012). Most of these studies presented a common challenge since the retrospective evaluation of Rn exposure was not based on the personal dosimetry data but rather on the periodic measurements of the working area. Another restraint of such studies is that the assessment of other factors contributing to lung cancer, like cigarette smoking, exposure to dust, diesel, etc. was not always carried out (Committee on Uranium Mining in Virginia 2011). Still, a positive dose-response relationship between employment in U mines and respiratory neoplasms was confirmed by the vast majority of studies even despite the limitations mentioned above. Table 1 gives an overview of the generalized characteristics of the relevant cohort studies.
The first cohort study demonstrating an association between employment in U mines and increased risk of lung cancer came from the 1960s (miners from Colorado Plateau, USA). The first publication reported on lung cancer incidence (Archer et al. 1962), while the second study (Wagoner et al. 1964) added updated data and established relations between respiratory neoplasms and cumulative exposure to Rn decay products in terms of WLM. The third study conducted on this cohort provided revised exposure estimates in the analysis of mortality from respiratory neoplasms based on a new statistical model. This study helped to establish a stronger overall relationship between mortality from respiratory neoplasms and exposure to Rn decay products in comparison with estimates of the earlier studies (Stram et al. 1999).
Another group of reports on the incidence of and mortality from lung cancer in U mine workers is based on cohorts from the former Czechoslovakia, where first epidemiological studies were initiated in the 1960s. Increased incidence of lung cancer in U miners was reported (Sevc et al. 1976) as well as raised mortality significantly exceeding the rates of the general population (RR = 5.08, 95% CI 4.71–5.47) (Tomásek et al. 1994). Like in the case of the Colorado Plateau miners, the pooled analysis of a combined cohort of Czechian U miners is also available, providing reliable estimates for the exposure-response relationship, which helped to clarify the effects of uncertainties in radiation exposure (Tomásek2012).
There is also a French cohort consisting of more than 5000 U miners with a follow-up period from the late 1940s to the late 2000s. Like in the case with other retrospective epidemiological studies, cancer incidence or mortality were assessed many years after initial exposure. A statistically significant excess mortality from lung cancer was established, and the cumulative effects of Rn had a clear association with the risk of lung cancer, while hard physical work and the period of exposure were proven to be the major modifying factors (Vacquier et al. 2009; Rage et al. 2015).
Probably, the largest cohort of U miners known to date is a German cohort, including nearly 59,000 men working at the Wismut Company, Germany (Kreuzer et al. 2002; Grosche et al. 2006). The International Commission on Radiological Protection conducted a study on this cohort, and the main finding was a statistically significant increase in mortality from lung cancer with increased cumulative exposure to Rn decay products. No additive effect of smoking was found (Kreuzer et al. 2015, b; Kreuzer et al. 2018). Recently this cohort also was used to study the genetic modifiers of lung cancer risk related to Rn exposure and established a strong association between the genomic region 15q25 and lung cancer. Analysis of genomic loci, genes, or sets of genes that could modify such susceptibility showed to be significant for the entire genome interaction of the rs12440014 marker in the CHRNB4 gene of the members of the German U miners’ cohort. The interaction of non-equilibrium adhesion blocks was found in chromosomal regions 18q21.23, 5q23.2, 1q21.3, 10p13, and 12p12.1. It was concluded that increased susceptibility to lung cancer is due to the functional ability of DNA damage signaling through the process of ubiquitination and restoration induced by double-stranded radiation breaks (Rosenberger et al. 2018).
Mutations in the ataxia-telangiectasia gene are considered to be among the predictors for lung cancer in people exposed to radiation. Ataxia-telangiectasia is an autosomal recessive disease characterized by neurological and immunological symptoms, radio-sensitivity, and predisposition to cancer. A study conducted on U miners from the Wismut cohort showed that only three of nine mutations in the ATM gene had changed (S707P, S49C, or IVS10-6). Eight percent of the patients with lung cancer were heterozygous compared with 1.9% in the control group (Schneider et al. 2007).
Being the second leading U-producing country in the world, Canada reported a large cohort of miners in the Eldorado mine, Ontario. The association between low-cumulative effects of Rn exposure and mortality from lung cancer was established. So, with WLM of more than 100, the risk of lung cancer almost doubled (RR = 1.89, 95% CI 1.43–2.50) (Navaranjan et al. 2016). This cohort also contributed to establishing a relationship between the cumulative effects of Rn exposure and the histological structure of lung cancer. The most common form of lung cancer found was squamous cell carcinoma (31%), followed by adenocarcinoma (20%), large cell lung carcinoma (18%), and small cell lung carcinoma (14%). The most significant association was between radiation dose and small cell lung carcinoma (RR = 2.76; 95% CI 1.67–4.57) (Ramkissoon et al. 2018).
Some pooled analyses of cohort studies were performed to increase statistical power and to enable a more comprehensive assessment of Rn exposure, including the contribution of other risk factors. A large pooled dataset laid the basis for reports prepared by the Committee on the Biological Effects of Ionizing Radiation (BEIR) within the National Academy of Sciences, combining 11 cohorts of underground miners from the USA, Canada, Australia, France, the Czech Republic, Sweden, and China. The BEIR-VI report is useful to clarify the relationship between smoking and Rn exposure and demonstrated a more than additive joint effects with the ratio of excess relative risk per WLM between non-smokers and smokers being 3.0 (Lubin 2010). Another sample of pooled analysis could be made of a combined cohort of French and Czechian miners, which showed like the BEIR-VI study an association between decreased lung cancer risk, the age of exposure, and the time since exposure (Tomasek et al. 2008). The most recent pooled analysis was published by Rage and co-authors in 2020 and is based on the largest to date combined cohort of 124,507 U miners from North America and Europe (Rage et al. 2020).
Such, the available evidence presented in cohort studies seems to leave no doubt that Rn decay products are the cause of lung cancer in humans. The very concern of 222Rn pollutant in lung cancer regards the possibility that radon could be captured by further sources aside from mines (Vogeltanz-Holm and Schwartz 2018). For example, Rn can be present in dwelling houses made with granite stones (Abbasi 2017). It is particularly difficult to ascertain Rn effects on miners if these also live in polluted houses. Therefore, new modeling and statistic tools and new occupational safety guidelines are needed, pending these critical issues (Garcia-Rodriguez 2018; Boice Jr et al. 2018; McColl et al. 2015; Little et al. 2007).
Anyway, U miners were the first occupational group with the established association between Rn exposure and excess lung cancer risk. In general, the discovery of carcinogenic properties of Rn progeny helped to introduce strict occupational standards that were targeted on the reduction of Rn levels in U mines. According to the current occupational exposure guideline, Rn concentrations in underground mines should not exceed 2 WLM/yr, and actual Rn levels in ventilated mines are well below this standard (IARC 2009). These measures helped to protect the modern generations of U miners from increased risk of lung cancer.
Leukemia and lymphoid tumors in uranium miners
Although the role of occupational Rn exposure in the induction of respiratory neoplasms has been well-documented for many decades of years, its association with other types of tumors has been less clear. However, this issue requires clarification as apart from the respiratory epithelium, U decay products can also deliver radiation to other body tissues, including bone marrow. Still, a difficulty arises when trying to establish an association between U exposure and non-lung cancers as generally they tend to have more extended survival. Since most retrospective mortality studies rely on death certificates, long-lasting health conditions may not be detected with adequate accuracy and are likely to be underestimated.
Many studies addressed in the past the issue of leukemia and lymphoid tumors associated with U mining. In a pooled statistical analysis combining 11 epidemiological studies on underground U miners, Darby and co-authors established increased leukemia mortality only in the period less than 10 years after beginning work at mine (Darby et al. 1995). Few studies investigating the effects of Rn progeny exposure on leukemia incidence and mortality were done on a cohort of Czechian miners. The first report demonstrated an increased mortality trend from multiple myeloma with cumulative exposure to Rn and an increasing trend of leukemia mortality with long-lasting employment in U mines (Tomásek et al. 1993). Later, Řeřicha and co-authors conducted a case-cohort study that focused on leukemia incidence rather than mortality. The increased incidence of all types of leukemia, along with chronic lymphocytic leukemia, was observed in relation to cumulative Rn exposure. Although it was found an increased Hodgkin lymphoma incidence in association with Rn exposure, it was not statistically significant (Řeřicha et al. 2006).
A part of the analyses done on the largest to date cohort of German U miners was also dedicated to establishing associations between exposure to Rn decay products and the risk of leukemia and lymphoid tumors. An individually matched case-control study failed to confirm a dose-response relationship between leukemia risk and exposure to Rn decay products. However, a significantly elevated leukemia incidence was found among the highest U exposed miners (Möhner et al. 2006). The earlier reports could not confirm the association between mortality from leukemia and exposure to Rn progeny (Kreuzer et al. 2008; Walsh et al. 2015). However, a recent study identified a positive insignificant dose-response for mortality from non-chronic lymphatic leukemia related to both external gamma radiation and Rn progeny. The subgroup of chronic myeloid leukemia had significant excess related to gamma radiation exposure, while the subgroup of myeloid leukemia had that in relation to Rn progeny. But no association of mortality from chronic lymphatic leukemia was established with either type of radiation exposure (Kreuzer et al. 2017).
In a large cohort of U mining workers in the Eldorado mines in Canada, the radiation-related risks of hematologic cancers were analyzed. The study showed a consistent, but non-statistically significant association between exposure to low gamma-ray doses and increased mortality risk of non-Hodgkin lymphoma, chronic lymphocytic leukemia, and increased Hodgkin lymphoma was reported (Zablotska et al. 2014). Although elevated mortality rates from multiple myeloma and non-Hodgkin lymphoma were observed in a Colorado Plateau cohort of miners, they were not associated with increasing exposure to Rn progeny (Schubauer-Berigan et al. 2009). The extended analysis of the French cohort failed to confirm the excess risk of death from leukemia, hematopoietic, and lymphoid malignancies (Vacquier et al. 2008; Rage et al. 2015).
Apart from lung cancer, hematopoietic lesions were investigated in a number of epidemiological studies on U miners. Although some observations failed to confirm the association between U mining and leukemia or lymphoid tumors, collectively, these studies provide sufficient evidence that U mining increases the risk of hematopoietic lesions. Still, it might be difficult to differentiate between exposure to Rn progeny and external gamma radiation, which is another known risk factor for leukemia (Kreuzer et al. 2017). There is a need to conduct more analyses to clarify the existing associations and to rule out other contributing factors.
As with lung cancer, retrospective cohort studies may hide confounders due to domestic exposure to radon (Ha et al. 2017). In many countries, indoor air pollution with Rn is still a big concern, though many data must be taken with caution (Chen and Xie 2019). The majority of studies are obviously of the occupational type, as surveys on the general, commonest population are much more cumbersome and time expensive that the mandatory controls on U miners (Seo et al. 2019; Kang et al. 2019).
Uranium mining and non-lung cancers other than leukemia
Besides respiratory epithelium and bone marrow, other body sites targeted by Rn decay products are skin, kidney, and gastrointestinal tract. The intake of radionuclides through ingestion may be occasionally seen in U mining and processing when occupational standards are not strictly followed, and excretion of nuclear decay products with urine puts the urinary system under threat (Vermeulen et al. 2019; Skubacz et al. 2019; Aßenmacher et al. 2019). Intact skin is affected by radionuclides through direct contact with contaminated surfaces or precipitation of radioactive dust (Sakoda et al. 2016; Vienneau et al. 2017; Bräuner et al. 2015; Barbosa-Lorenzo et al. 2016). Although it is considered to be insignificant, dermal absorption of soluble U compounds through intact skin may also occur occasionally (Committee on Uranium Mining in Virginia 2011).
Several studies raised the issue of increased mortality from extra-pulmonary cancers other than leukemia in a cohort of U miners. The first pooled analysis of 11 cohort trials conducted by Darby and co-authors failed to confirm the excess risk of mortality from non-lung cancers (Darby et al. 1995). One of the early publications on the Wismut cohort established a significant association between exposure to Rn progeny and increased mortality from all non-lung cancers combined, and this effect preserved even after adjustment for other potential risk factors, like exposure to silica dust, fine dust, and arsenic. This study demonstrated the significant increase of excess relative risk for such cancer sites as liver cancer, stomach cancer, and larynx cancer. However, after adjustment for other confounders, increased cancer risks became insignificant (Kreuzer et al. 2008). These findings were later supported by further analysis of the Wismut cohort. After adjustment for potential risk factors, the excess relative risk of extra-pulmonary solid cancers was also found to be insignificant (Walsh et al. 2010).
Later on, a series of publications on this extensively studied cohort of U miners appeared that looked into more details on mortality from such cancer sites as prostate cancer, stomach cancer, and liver cancer. Underground works on U mines were reported to have a statistically significant small protective effect on prostate cancer mortality, which authors explained by “the melatonin hypothesis” (Walsh et al. 2012). In an attempt to investigate the association between exposure to radiation, fine dust, arsenic dust, and mortality from stomach cancer, Kreuzer and co-authors conducted a detailed analysis on a subset of the Wismut cohort comprising stomach cancer deaths exclusively. Slightly elevated but statistically insignificant relationships between stomach cancer death and exposure to alpha and low linear energy transfer radiation dose, silica dust, and arsenic dust were established (Kreuzer et al. 2012). An increased but not statistically significant risk of death from primary liver cancer with high linear energy transfer radiation dose was confirmed, and adjustment for arsenic dust appeared to be of little importance (Dufey et al. 2013).
Nephrotoxicity is a known health effect of U toxicity, and “uranium nephritis” was first described in 1915 (Oliver 2015). As U inhaled during the mining process undergoes kidney clearance, it causes kidney exposure (Rage et al. 2015). Still, kidney cancer is attributed to the radiological effects of Rn decay products rather than to U chemical toxicity. The analysis of the French cohort of U miners confirmed a significant excess of kidney cancer mortality but failed to relate it with cumulative radon exposure (Vacquier et al. 2008). However, the excess mortality from kidney cancer was not persistent in the post-1955 sub-cohort (Rage et al. 2015). The pooled analysis performed on a combined cohort of French (n = 3377) and German (n = 58,986) U miners did not establish the significant excess of kidney cancer mortality. Furthermore, there was no significant association between exposure to Rn progeny or kidney equivalent dose and mortality from kidney cancer (Drubay et al. 2014).
Skin cancer is believed to be one of the commonest types of cancer in humans and is mostly attributed to exposure to ultraviolet radiation (Charles 2007). The elevated risk of basal cell carcinoma 2–12 times exceeding that in the general male population was reported in one of the early papers on the Czech cohort, and the mean equivalent dose in the epidermis was found to range from 0.6 to 5.0 Sv. Most tumors observed had facial localization and were diagnosed in miners with more than 10 years of experience (Sevcová et al. 1978). In a later study with 12 additional years of follow-up, a standardized incidence ratio of skin cancer was found to be 5.7 (90% CI = 4.1, 7.8) and the cumulative average dose equivalent to the basal skin layer was 5.0 Sv for those U miners who had more than 10 years of experience. Still, it has to be noted that increased concentration of arsenic—a known carcinogen for skin—was present in one of the two major mines but was not accounted for in the incidence analysis (Sevcová and Sevc 1989).
The paucity of publications on the association between skin cancer and employment in U mines could be explained by the fact that most studies rely on mortality data while non-melanoma skin cancers have a low death rate. In a cohort of Colorado Plateau, six skin cancer-related deaths were reported as compared with 3.19 expected deaths, although this difference was not statistically significant (Hornung and Meinhardt 1987). No significant excess of skin cancer deaths was demonstrated neither in BEIR-IV (NAS 1988) nor in BEIR-VI (NRC 1999) reports.
As for cancers of the brain or central nervous system (CNS), a borderline significant excess mortality rate was established in the updated 1946–2007 follow-up of the French cohort of U miners as compared with the reference population (Rage 2015). Nevertheless, no evidence of excess mortality from brain and CNS cancers was identified in the Wismut cohort, which is best explained by the fact that only malignant tumors were taken into account (Kreuzer et al. 2008). Because the vast majority of workers employed in U mines are men, little is known about Rn-induced cancers of female reproductive organs, which dictates the need for further epidemiological studies focusing on female workers (Field 2010).
So, during their work, U miners are chronically exposed to Rn and its decay products, which put them under elevated risks of lung cancer. Although the risks of non-lung cancers other than leukemia have sometimes been significantly associated with U mining, these findings across the studies are rather inconsistent. Moreover, until recently, the studies of U miners have been mostly focused on exposure to Rn progeny and paid no attention to the fact that miners were also exposed to external gamma radiation and long-lived radionuclides. Even now, such studies are rather scarce. Another limitation of these studies is that they commonly lack the information related to other potential risk factors, including smoking and alcohol consumption habits, as well as other occupational exposures, like silica or diesel exhaust, all of which are known to have carcinogenic properties.
Non-cancer adverse health effects of uranium mining
Silicosis is a common non-malignant respiratory disease among workers employed in the mining industry, and U mining is not the exception as many of the known U ores are deposited in rocks that contain silica. Because silicosis is a life-threatening disorder, the elevated standardized mortality ratio was reported in a number of retrospective U miners’ cohorts (Schubauer-Berigan et al. 2009; Vacquier et al. 2008; Schröder et al. 2002). However, the issue to which extent it was influenced by Rn progeny or other sources of radiation related to U mining remains very doubtful. The study performed on a German cohort of U miners showed that exposures to Rn progeny and crystalline silica were highly correlated (Sogl et al. 2012). As silica exposure is likely to be a confounding factor, it would not be appropriate to attribute an increased risk of silicosis to Rn exposure.
Although overall incidence and mortality rates in occupational cohorts are generally lower than those observed in reference populations (Baillargeon 2001), the known to date cohorts of U miners are not subject to this “healthy worker effect” (HWE). This is probably because the excess of lung cancer deaths, as well as mortality from other causes, counterbalance any potential HWE.
Uranium toxicity
Uranium is known to possess both a chemo-toxicological and a radiological activity. Although various U isotopes have different radiological profile, their chemical action is identical, and thus, natural, depleted, and enriched uranium possess identical chemical toxicity (Bjørklund et al. 2020a, b). No observed adverse effect level and the lowest observed no adverse effect level along with uncertainty factors are the approaches used to derive tolerable effects from such substances as U. However, these are mostly used for exposure estimates in general population and not in such occupational groups as miners, for whom data on workplace exposures are often available and who do not represent vulnerable population groups like children, the sick, and the elderly (Bjørklund et al. 2019).
Chemical toxicity of various U isotopes depends on their biological solubility and the ability to interact with body tissues. The most bioavailable are water-soluble U componds (uranyl nitrate, fluoride, uranium hexafluoride, and tetrachloride) and the least bioavailable are the insoluble U compounds (uranium dioxide, trioxide, peroxide, and triuranium octaoxide) (Bjørklund et al. 2020a, b). If inhalation is the major route of intake, short-time exposure to high concentrations of U hexafluoride was reported to cause acute respiratory illness, although this could be explained by liberation of the hydrogen fluoride upon hydrolysis. As it is amply discussed in other parts of this review, an increased risk of lung cancer among U miners is mostly associated with radiological effects produced by Rn decay products. At the same time, these miners might also have an increased risk of other respiratory disease, which may be attributed to the presence of some other toxicants in mines, including inhaled dust particles of U (Zychowski et al. 2018).
As for nephrotoxicity—the hallmark of U toxicity—an increased mortality rate from chronic nephritis was observed in a cohort of 2514 U workers, although this effect was statistically insignificant (Dupree-Ellis et al. 2000). Meanwhile, smaller studies on associations between U exposure and the risks of renal disease provided controversial results (Kathren and Moore 1986; Lu and Zhao 1990). Renal tubular dysfunction was revealed in U workers chronically exposed to insoluble U compounds as compared with a reference group of cement workers. The duration of exposure to U correlated with both the incidence and severity of these nephrotoxic effects (Saccomanno 1982; Thun et al. 1985).
In addition to nephrotoxicity, oral intake of U results in acute nausea, vomiting, and diarrhea, and might even induce the development of paralytic ileus. Although it is impossible to attribute anemia and other hematological effects solely to U toxicity, they also follow U intake (Pavlakis et al. 1996). Besides, oral administration of U induces hepatotoxic changes that are manifested as increased serum concentrations of alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transpeptidase (Domingo et al. 1992). Decreased concentrations of sex hormones and an associated decrease in fertility are also the signs of U toxicity (Wang et al. 2019). Figure 1 summarizes the radiation-related health problems of U miners.
Political opinions about U miners, ecology, methods, and guidelines on mining and domestic Rn pollution
There exists much data about the association between 222Rn and U-derived radionuclides and different types of cancer. However, it is due to confounders difficult to evaluate the radiation burden and the risk correctly for other sources than occupational ones (Sarkar et al. 2017; Sheen et al. 2016). A lot of uncertainty may bias a proper evaluation of U-radioactive risk among the general population and workers. The ability to discriminate between indoor Rn and Rn assumed with occupational exposure is particularly hard (Friedmann et al. 2017a; Friedmann et al. 2017b). Therefore, the best policy is to create scientific updates and technical task forces to distinguish these two issues, particularly from geological exposure to Rn respect to the occupational one (McLaughlin 2019; Kavasi et al. 2019).
Furthermore, in a huge number of cases, collecting and retrieving data from U-pollution is becoming particularly difficult, due to local policies and global agreements (Dawson and Madsen 2011; Brugge 2016). This may create some difficulty in testing U-derived radionuclides in vitro studies, although various approaches were attempted, with encouraging results (Asic et al. 2017; Gritsaenko et al. 2018; Paredes et al. 2019). Finally, the methodic must be updated, also in the light of these new concerns (Meisenberg et al. 2017, Meisenberg and Tschiersch 2011, Petersell et al. 2017, Ooe et al. 2019, Feng et al. 2019).
Until recent times, the social costs of U mining remained unknown. However, due to global climate change concerns, there is a renewed interest in U as a source of low-carbon, sustainable energy. Thus, Jones estimated economic costs to society of premature mortality due to U mining on the basis of Colorado Plateau cohort study. The main finding was that U mining resulted in USD 43.1 million in annual health costs over 1960–2005, which is equivalent to USD 10,418 per miner per year. The actual costs are likely to be underestimated as uncertain medical costs were not included in the analysis (Jones 2017).
Therefore, to give a thorough and exhaustive overview and description of the occupational risk of U miners, much wider involvement of different expertise is needed to make the most correct statistics and environmental evaluations.
Conclusions
In the twentieth century, U mining became a massive global industry imposing the earlier generations of U miners to various health hazards as occupational standards were not strictly monitored. Although Rn is a common decay product of U, miners are also exposed to other radionuclides like 230Th, 226Ra, and its decay products with alpha-emission and gamma radiation. Focusing on older cohorts (pre-1980s) of U miners, the following risks are well documented: lung cancer and chronic lung disease, particularly silicosis. Leukemia and lymphoid tumors are the other types of tumors associated with occupational U exposure.
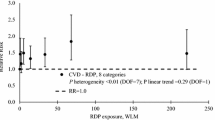
Despite ample evidence that exposure to Rn progeny leads to lung cancer, the information related to other cancer sites remains rather controversial. Increased but not statistically significant death risk in U miners due to cancers in the liver, stomach, and kidneys was found. As for non-cancer effects associated with U mining, there is some evidence for an increase in the risk of cardiovascular disease with increasing exposure to Rn progeny, but consistency between the studies could not be ensured. More prospective studies are needed to clarify the relationship between U mining and adverse health effects with an emphasis on leading U producers, most of which belong to the developing countries.
References
Abbasi A (2017) Modeling of lung cancer risk due to radon exhalation of granite stone in dwelling houses. J Cancer Res Ther 13(2):208–212
Archer VE, Magnuson HJ, Holaday DA, Lawrence PA (1962) Hazards to health in uranium mining and milling. J Occup Med 4:55–60
Asic A, Kurtovic-Kozaric A, Besic L, Mehinovic L, Hasic A, Kozaric M, Hukic M, Marjanovic D (2017) Chemical toxicity and radioactivity of depleted uranium: the evidence from in vivo and in vitro studies. Environ Res 156:665–673
Aßenmacher M, Kaiser JC, Zaballa I, Gasparrini A, Küchenhoff H (2019) Exposure-lag-response associations between lung cancer mortality and radon exposure in German uranium miners. Radiat Environ Biophys 58(3):321–336
Baillargeon J (2001) Characteristics of the healthy worker effect. J Occup Med 16(2):359–366
Barbosa-Lorenzo R, Barros-Dios JM, Raíces Aldrey M, Cerdeira Caramés S, Ruano-Ravina A (2016) Residential radon and cancers other than lung cancer: a cohort study in Galicia, a Spanish radon-prone area. Eur J Epidemiol 31(4):437–441
Baur X, Woitowitz HJ (2016) Lung Cancer as an occupational disease. Pneumologie. 70(8):510–513
Bjørklund G, Christophersen OA, Chirumbolo S, Selinus O, Aaseth J (2017) Recent aspects of uranium toxicology in medical geology. Environ Res 156:526–533. https://doi.org/10.1016/j.envres.2017.04.010
Bjørklund G, Pivina L, Dadar M, Semenova Y, Chirumbolo S, Aaseth J (2019) Mercury exposure, epigenetic alterations, and brain tumorigenesis: a possible relationship? Curr Ed Chem. https://doi.org/10.2174/0929867326666190930150159
Bjørklund G, Pivina L, Dadar M, Semenova Y, Rahman MM, Aaseth J, Chirumbolo S (2020a) Depleted uranium and gulf war illness: updates and comments on possible mechanisms behind the syndrome. Environ Res 181:108927. https://doi.org/10.1016/j.envres.2019.108927
Bjørklund G, Semenova Y, Pivina L, Dadar M, Rahman MM, Aaseth J, Chirumbolo S (2020b) Uranium in drinking water: a public health threat. Arch Toxicol 94:1551–1560. https://doi.org/10.1007/s00204-020-02676-8
Boice JD Jr, Ellis ED, Golden AP, Girardi DJ, Cohen SS, Chen H, Mumma MT, Shore RE, Leggett RW (2018) The past informs the future: an overview of the million worker study and the Mallinckrodt chemical works cohort. Health Phys 114(4):381–385
Bräuner EV, Loft S, Sørensen M, Jensen A, Andersen CE, Ulbak K, Hertel O, Pedersen C, Tjønneland A, Krüger Kjær S, Raaschou-Nielsen O (2015) Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS One 10(8):e0135642
Brown SH (2019) Occupational radiation protection aspects of alkaline leach uranium in situ recovery (ISR) facilities in the United States. Health Phys 117(1):106–113
Brown SH, Chambers DB (2017) Uranium mining and NORM in North America – some perspectives on occupational radiation exposure. Health Phys 113(1):13–22
Brugge D (2016) Why has it taken so long to address the problems created by uranium mining in the Navajo nation? New Solut 25(4):436–439
Burghele BD, Cucos A, Papp B, Stetca FA, Mirea I, Constantin S (2018) Distribution of radon gas in Romanian show caves and radiation safety. Radiat Prot Dosim 181(1):1–5
Charles MW (2007) Radon exposure of the skin: II. Estimation of the attributable risk for skin cancer incidence. J Radiol Prot 27(3):253–274
Chen J (2017) Comparative study of radon exposure in Canadian homes and uranium mines-a discussion on the importance of national radon program. Radiat Prot Dosim 177(1–2):83–86
Chen J, Xie L (2019) Domestic radon exposure and childhood leukaemia and lymphoma: a population-based study in Сanada. Radiat Prot Dosim 184:486–492. https://doi.org/10.1093/rpd/ncz068
Cinelli G, Tondeur F, Dehandschutter B, Bossew P, Tollefsen T, De Cort M (2017) Mapping uranium concentration in soil: Belgian experience towards a European map. J Environ Radioact 166:220–234
Committee on Uranium Mining in Virginia; Committee on Earth Resources; National Research Council (2011) Uranium mining in Virginia: scientific, technical, environmental, human health and safety, and regulatory aspects of uranium mining and processing in Virginia. Washington (DC): National Academies Press (US) Available at https://www.ncbi.nlm.nih.gov/books/NBK201047/. Accessed 16 February 2019
Cucoş Dinu A, Călugăr MI, Burghele BD, Dumitru OA, Cosma C, Onac BP (2017) Radon levels in Romanian caves: an occupational exposure survey. Environ Geochem Health 39(5):1085–1099
Darby SC, Whitley E, Howe GR, Hutchings SJ, Kusiak RA, Lubin JH, Morrison HI, Tirmarche M, Tomásek L, Radford EP (1995) Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 87(5):378–384
Dawson SE, Madsen GE (2011) Psychosocial and health impacts of uranium mining and milling on Navajo lands. Health Phys 101(5):618–625
Domingo JL, Colomina MT, Llobet JM, Jones MM, Singh PK, Campbell RA (1992) The action of chelating agents in experimental uranium intoxication in mice: variations with structure and time of administration. Fundam Appl Toxicol 19(3):350–357. https://doi.org/10.1016/0272-0590(92)90173-f
Drubay D, Ancelet S, Acker A, Kreuzer M, Laurier D, Rage E (2014) Kidney cancer mortality and ionizing radiation among French and German uranium miners. Radiat Environ Biophys 53(3):505–513. https://doi.org/10.1007/s00411-014-0547-4
Dufey F, Walsh L, Sogl M, Tschense A, Schnelzer M, Kreuzer M (2013) Radiation dose dependent risk of liver cancer mortality in the German uranium miners cohort 1946-2003. J Radiol Prot 33(1):175–185. https://doi.org/10.1088/0952-4746/33/1/175
Dupree-Ellis E, Watkins J, Ingle JN, Phillips J (2000) External radiation exposure and mortality in a cohort of uranium processing workers. Am J Epidemiol 152(1):91–95. https://doi.org/10.1093/aje/152.1.91
Euser AM, Zoccali C, Jager KJ, Dekker FW (2009) Cohort studies: prospective versus retrospective. Nephron Clin Pract 113(3):c214–c217. https://doi.org/10.1159/000235241
Fan D, Zhuo W, Zhang Y (2016) Occupational exposure to radon in different kinds of non-uranium mines. Radiat Prot Dosimetry 170(1-4):311–314
Feng B, Tang Q, Zhan H, Chen B, Qiu S, Zhuo W (2019) Measurement of the potential alpha energy concentration of radon progeny by using liquid scintillation counting method. Radiat Prot Dosim 184:440–443. https://doi.org/10.1093/rpd/ncz069
Field RW (2010) Environmental factors in cancer: radon. Rev Environ Health—Spec Ed (Part 2) 25(1):25–31
Friedmann H, Baumgartner A, Gruber V, Kaineder H, Maringer FJ, Ringer W, Seidel C (2017a) The uncertainty in the radon hazard classification of areas as a function of the number of measurements. J Environ Radioact 173:6–10
Friedmann H, Baumgartner A, Bernreiter M, Gräser J, Gruber V, Kabrt F, Kaineder H, Maringer FJ, Ringer W, Seidel C, Wurm G (2017b) Indoor radon, geogenic radon surrogates and geology - investigations on their correlation. J Environ Radioact 166:382–389
Garcia-Rodriguez JA (2018) Radon gas-the hidden killer: what is the role of family doctors? Can Fam Physician 64(7):496–501
Greenberg M, Selikoff IJ (1993) Lung cancer in the Schneeberg mines: a reappraisal of the data reported by Harting and Hesse in 1879. Ann Occup Hyg 37(1):5–14
Gritsaenko T, Pierrefite-Carle V, Creff G, Vidaud C, Carle G, Santucci-Darmanin S (2018) Methods for analyzing the impacts of natural uranium on in vitro osteoclastogenesis. J Vis Exp 131:14–23
Grosche B, Kreuzer M, Kreisheimer M, Schnelzer M, Tschense A (2006) Lung cancer risk among German male uranium miners: a cohort study, 1946–1998. Br J Cancer 95(9):1280–1277. https://doi.org/10.1038/sj.bjc.6603403
Ha M, Hwang SS, Kang S, Park NW, Chang BU, Kim Y (2017) Geographical correlations between indoor radon concentration and risks of lung cancer, non-Hodgkin's lymphoma, and leukemia during 1999–2008 in Korea. Int J Environ Res Public Health 14(4):344
Hassfjell CS, Grimsrud TK, Standring WJF, Tretli S (2017) Lung cancer incidence associated with radon exposure in Norwegian homes. Tidsskr Nor Laegeforen 137:14–15
Hornung R, Meinhardt T (1987) Quantitative risk assessment of lung cancer in US uranium miners. Health Phys 52:417–430
IARC (2009) Working group on the evaluation of carcinogenic risks to humans (2009) a review of human carcinogens. Part D: radiation. Lyon, France. Available at https://www.ncbi.nlm.nih.gov/books/NBK304362/. Accessed 31 Aug 2019
ICRP (2017) Occupational intakes of radionuclides: Part 3. ICRP Publication 137. Ann. ICRP 46(3/4). Available at http://www.icrp.org/publication.asp?id=ICRP%20Publication%20137. Accessed 30 Jan 2019
Jones BA (2017) The social costs of uranium mining in the US Colorado plateau cohort, 1960-2005. Int J Public Health 62(4):471–478. https://doi.org/10.1007/s00038-017-0943-z
Kang JK, Seo S, Jin YW (2019) Health effects of radon exposure. Yonsei Med J 60(7):597–603
Kathren RL, Moore RH (1986) Acute accidental inhalation of U: a 38-year follow-up. Health Phys 51(5):609–619. https://doi.org/10.1097/00004032-198611000-00004
Kavasi N, Csordas A, Nagy K, Beltran S, Kikaj D, Vaupotič J, Kovacs T (2019) Occupational exposure assessment at a therapeutic radon spa facility in Hungary. Radiat Prot Dosim 184:470–473. https://doi.org/10.1093/rpd/ncz077
Kelly-Reif K, Sandler DP, Shore D, Schubauer-Berigan M, Troester MA, Nylander-French L, Richardson DB (2019) Mortality and cancer incidence among underground uranium miners in the Czech Republic 1977-1992. Occup Environ Med 76(8):511–518
Kido E (2019) The legacies of the uranium mining company “Wismut” in East Germany. Asian J Peacebuilding 7(1):55–72
Kotík L, Becková V, Malátová I, Tomasek L (2017) 238U content in urine of uranium miners and its modeled values. Radiat Prot Dosim 177(4):424–439. https://doi.org/10.1093/rpd/ncx061
Kreuzer M, Brachner A, Lehmann F, Martignoni K, Wichmann HE, Grosche B (2002) Characteristics of the German uranium miners cohort study. Health Phys 83(1):26–34
Kreuzer M, Walsh L, Schnelzer M, Tschense A, Grosche B (2008) Radon and risk of extrapulmonary cancers: results of the German uranium miners' cohort study, 1960-2003. Br J Cancer 99(11):1946–1953. https://doi.org/10.1038/sj.bjc.6604776
Kreuzer M, Grosche B, Schnelzer M, Tschense A, Dufey F, Walsh L (2010) Radon and risk of death from cancer and cardiovascular diseases in the German uranium miners cohort study: follow-up 1946–2003. Radiat Environ Biophys 49(2):177–185. https://doi.org/10.1007/s00411-009-0249-5
Kreuzer M, Straif K, Marsh JW, Dufey F, Grosche B, Nosske D, Sogl M (2012) Occupational dust and radiation exposure and mortality from stomach cancer among German uranium miners, 1946-2003. Occup Environ Med 69(3):217–223. https://doi.org/10.1136/oemed-2011-100051
Kreuzer M, Fenske N, Schnelzer M, Walsh L (2015) Lung cancer risk at low radon exposure rates in German uranium miners. Br J Cancer 113(9):1367–1369. https://doi.org/10.1038/bjc.2015.324
Kreuzer M, Sobotzki C, Fenske N, Marsh JW, Schnelzer M (2017) Leukaemia mortality and low-dose ionising radiation in the WISMUT uranium miner cohort (1946–2013). Occup Environ Med 74(4):252–258. https://doi.org/10.1136/oemed-2016-103795
Kreuzer M, Sobotzki C, Schnelzer M, Fenske N (2018) Factors modifying the radon-related lung Cancer risk at low exposures and exposure rates among German uranium miners. Radiat Res 189(2):165–176. https://doi.org/10.1667/RR14889.1
Laurence D (2011) Mine safety. In: Darling P (ed) SME mining engineering handbook, vol 2, 3rd edn. Society for Mining, metallurgy, and exploration, Inc, Englewood
Little MP, Hall P, Charles MW (2007) Are cancer risks associated with exposures to ionising radiation from internal emitters greater than those in the Japanese A-bomb survivors? Radiat Environ Biophys 46(4):299–310
López M, Martín M (2011) Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother 16(4):138–146. https://doi.org/10.1016/j.rpor.2011.05.001
Louw I (2020) Potential radiological impact of the phosphate industry in South Africa on the public and the environment (paper 1). J Environ Radioact 217. https://doi.org/10.1016/j.jenvrad.2020.106214
Lu S, Zhao FY (1990) Nephrotoxic limit and annual limit on intake for natural U. Health Phys 58(5):619–623. https://doi.org/10.1097/00004032-199005000-00007
Lubin JH (2010) Environmental factors in cancer: radon. Rev Environ Health 25(1):33–38
Malátová I, Becková V, Tomásek L, Slezáková-Marusiaková M, Hůlka J (2013) Reassessment of individual dosimetry of long-lived alpha radionuclides of uranium miners through experimental determination of urinary excretion of uranium. Radiat Prot Dosim 154(2):198–206. https://doi.org/10.1093/rpd/ncs208
Malátová I, Bečková V, Kotík L (2016) Urinary excretion of uranium in adult inhabitants of the Czech Republic. J Environ Radioact 152:92–96. https://doi.org/10.1016/j.jenvrad.2015.11.011
Markabayeva A, Bauer S, Pivina L, Bjørklund G, Chirumbolo S, Kerimkulova A, Semenova Y, Belikhina Т (2018) Increased prevalence of essential hypertension in areas previously exposed to fallout due to nuclear weapons testing at the Semipalatinsk test site, Kazakhstan. Environ Res 167:129–135. https://doi.org/10.1016/j.envres.2018.07.016
Marsh JW, Bailey MR (2013) A review of lung-to-blood absorption rates for radon progeny. Radiat Prot Dosimetry 157(4):499–514
McColl N, Auvinen A, Kesminiene A, Espina C, Erdmann F, de Vries E, Greinert R, Harrison J, Schüz J (2015) European code against Cancer 4th edition: Ionising and non-ionising radiation and cancer. Cancer Epidemiol 39(Suppl 1):S93–S100
McLaughlin JP (2019) Dosimetric and epidemiological approaches to radon lung cancer risk assessment. Radiat Prot Dosim 184(3–4):285–289. https://doi.org/10.1093/rpd/ncz082
Meisenberg O, Tschiersch J (2011) Thoron in indoor air: modeling for a better exposure estimate. Indoor Air 21(3):240–252
Meisenberg O, Mishra R, Joshi M, Gierl S, Rout R, Guo L, Agarwal T, Kanse S, Irlinger J, Sapra BK, Tschiersch J (2017) Radon and thoron inhalation doses in dwellings with earthen architecture: comparison of measurement methods. Sci Total Environ 579:1855–1862
Messier KP, Serre ML (2017) Lung and stomach cancer associations with groundwater radon in North Carolina, USA. Int J Epidemiol 46(2):676–685
Mielke S, Taeger D, Weitmann K, Brüning T, Hoffmann W (2018) Influence of quartz exposure on lung cancer types in cases of lymph node-only silicosis and lung silicosis in German uranium miners. Arch Environ Occup Health 73(3):140–153
Mironenkova NA, Magomet RD (2017) Hygienic assessment of working conditions on a radiation-hazardous factor in mines. Ecol Environ Conserv 23(2):1017–1021
Missimer TM, Teaf C, Maliva RG, Danley-Thomson A, Covert D, Hegy M (2019) Natural radiation in the rocks, soils, and groundwater of southern Florida with a discussion on potential health impacts. Int J Environ Res Public Health 16(10):1793. https://doi.org/10.3390/ijerph16101793
Möhner M, Lindtner M, Otten H, Gille HG (2006) Leukemia and exposure to ionizing radiation among German uranium miners. Am J Ind Med 49(4):238–248. https://doi.org/10.1002/ajim.20289
Molaie Y, Latifynia A, Kalamzadeh A, Abofazeli T, Nuraie M, Khansarii N (2012) Phagocyte functions of human subjects living in high level of natural radiation areas in Iran. J Ayub Med Coll Abbottabad 24(3–4):177–179
Mudd G (2008) Radon releases from Australian uranium mining and milling projects: assessing the UNSCEAR approach. J Environ Radioact 99(2):288–315. https://doi.org/10.1016/j.jenvrad.2007.08.001
National Academy of Sciences (NAS) (1988) Health risks of radon and other internally deposited alpha emitters. BEIR IV report of the National Academy of Sciences, National Research Council. National Academy, Washington Available at https://www.nap.edu/read/1026/chapter/1#v. Accessed 25 February 2019
Navaranjan G, Berriault C, Do M, Villeneuve PJ, Demers PA (2016) Cancer incidence and mortality from exposure to radon progeny among Ontario uranium miners. Occup Environ Med 73(12):838–845. https://doi.org/10.1136/oemed-2016-103836
Navaranjan G, Chambers D, Thompson PA, Do M, Berriault C, Villeneuve PJ, Demers PA (2019) Uncertainties associated with assessing Ontario uranium miners' exposure to radon daughters. J Radiol Prot 39(1):136–149. https://doi.org/10.1088/1361-6498/aaf1eb
NRC - National Research Council (1999) Health effects of exposure to radon: BEIR VI. National Academies Press, Washington. https://doi.org/10.17226/5499. Accessed 30 January 2019
Nuclear Energy Agency (2006) Forty years of uranium resources, production and demand in perspective: the red book retrospective. OECD, Paris. Available at https://www.oecd-nea.org/ndd/pubs/2006/6096-40-years-uranium.pdf Accessed 26 April 2020
OECD-NEA & IAEA, Uranium (2018) Resources, production and demand (‘Red Book’) World Nuclear Association, The Nuclear Fuel Report 2015, 2017 & 2019
Oh SS, Koh S, Kang H, Lee J (2016) Radon exposure and lung cancer: risk in nonsmokers among cohort studies. Ann Occup Environ Med 9(28):11
Oliver J (2015) The histogenesis of chronic uranium nephritis with especial reference to epithelial regeneration. J Exp Med 21:425–451
Ooe K, Watabe T, Kamiya T, Yoshimura T, Hosono M, Shinohara A, Hatazawa J (2019) Quantitative measurement of (219)Rn radioactivity in exhaled breath from patients with bone metastasis of castration-resistant prostate cancer treated with (223)RaCl(2). EJNMMI Phys 6(1):13. https://doi.org/10.1186/s40658-019-0249-8
Otahal P, Burian I, Nasir MM, Gregor Z (2014) Radon contribution to the total effective dose of uranium miners. Radiat Prot Dosim 160(1–3):117–119. https://doi.org/10.1093/rpd/ncu065
Paredes E, Avazeri E, Malard V, Vidaud C, Reiller PE, Ortega R, Nonell A, Isnard H, Chartier F, Bresson C (2016) Evidence of isotopic fractionation of natural uranium in cultured human cells. Proc Natl Acad Sci U S A 113(49):14007–14012
Paredes E, Malard V, Vidaud C, Avazeri E, Ortega R, Nonell A, Isnard H, Chartier F, Bresson C (2019) Isotopic variations of copper at the protein fraction level in neuronal human cells exposed in vitro to uranium. Analyst. 144:5928–5933. https://doi.org/10.1039/c9an01081e
Pavlakis N, Pollock CA, McLean G, Bartrop R (1996) Deliberate overdose of uranium: toxicity and treatment. Nephron 72(2):313–317. https://doi.org/10.1159/000188862
Petersell V, Täht-Kok K, Karimov M, Milvek H, Nirgi S, Raha M, Saarik K (2017) Radon in the soil air of Estonia. J Environ Radioact 166:235–241
Pirchan A, Sikl H (1932) Cancer of the lung in miners of Jachymov (Joachimsthal). Report of cases observed in 1929–1930. Am J Cancer 16:681–722. https://doi.org/10.1158/ajc.1932.681
Plant J, Simpson PR, Smith B, Windley BF (1999) “Uranium ore deposits: products of the radioactive earth”, in Burns, P.C., and Finch, R., Reviews in mineralogy, volume 38: uranium: mineralogy, geochemistry and the environment. Washington D.C., USA.: Mineralogical Society of America, 255–320
Rage E, Caër-Lorho S, Drubay D, Ancelet S, Laroche P, Laurier D (2015) Mortality analyses in the updated French cohort of uranium miners (1946–2007). Int Arch Occup Environ Health 88(6):717–730. https://doi.org/10.1007/s00420-014-0998-6
Rage E, Richardson DB, Demers PA, Do M, Fenske N, Kreuzer M, Samet J, Wiggins C, Schubauer-Berigan MK, Kelly-Reif K, Tomasek L, Zablotska LB, Laurier D (2020) PUMA – pooled uranium miners analysis: cohort profile. Occup Environ Med 77(3):194–200. https://doi.org/10.1136/oemed-2019-105981
Ramkissoon A, Navaranjan G, Berriault C, Villeneuve PJ, Demers PA, Do MT (2018) Histopathologic analysis of lung cancer incidence associated with radon exposure among Ontario uranium miners. Int J Environ Res Public Health 15(11):E2413. https://doi.org/10.3390/ijerph15112413
Řeřicha JV, Kulich M, Řeřicha R, Shore DL, Sandler DP (2006) Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 114(6):818–822
Rosenberger A, Hung RJ, Christiani DC, Caporaso NE, Liu G, Bojesen SE, Le Marchand L, Haiman CA, Albanes D, Aldrich MC, Tardon A, Fernández-Tardón G, Rennert G, Field JK, Kiemeney B, Lazarus P, Haugen A, Zienolddiny S, Lam S, Schabath MB, Andrew AS, Brunnsstöm H, Goodman GE, Doherty JA, Chen C, Teare MD, Wichmann HE, Manz J, Risch A, Muley TR, Johansson M, Brennan P, Landi MT, Amos CI, Pesch B, Johnen G, Brüning T, Bickeböller H, Gomolka M (2018) Genetic modifiers of radon-induced lung cancer risk: a genome-wide interaction study in former uranium miners. Int Arch Occup Environ Health 91(8):937–950. https://doi.org/10.1007/s00420-018-1334-3
Saccomanno G (1982) The contribution of uranium miners to lung cancer histogenesis. Recent Results Cancer Res 82:43–52. https://doi.org/10.1007/978-3-642-81768-7_5
Sakoda A, Ishimori Y, Tschiersch J (2016) Evaluation of the intake of radon through skin from thermal water. J Radiat Res 57(4):336–342
Santos TO, Rocha Z, Cruz P, Gouvea VA, Siqueira JB, Oliveira AH (2014) Radon dose assessment in underground mines in Brazil. Radiat Prot Dosim 160(1–3):120–123
Sarkar A, Wilton DH, Fitzgerald E (2017) Indoor radon in micro-geological setting of an indigenous community in Canada: a pilot study for hazard identification. Int J Occup Environ Med 8(2):69–79
Schneider J, Philipp M, Yamini P, Dörk T, Woitowitz HJ (2007) ATM gene mutations in former uranium miners of SDAG Wismut: a pilot study. Oncol Rep 17(2):477–482
Schröder C, Friedrich K, Butz M, Koppisch D, Otten H (2002) Uranium mining in Germany: incidence of occupational disease 1946-1999. Int Arch Occup Environ Health 75:235–242
Schubauer-Berigan MK, Daniels RD, Pinkerton LE (2009) Radon exposure and mortality among white and American Indian uranium miners: an update of the Colorado plateau cohort. Am J Epidemiol 169(6):718–730
Scott BR (2019) Epidemiologic studies cannot reveal the true shape of the dose-response relationship for radon-induced lung Cancer. Dose Response 17(1):1559325819828617
Seo S, Ha WH, Kang JK, Lee D, Park S, Kwon TE, Jin YW (2019) Health effects of exposure to radon: implications of the radon bed mattress incident in Korea. Epidemiol Health 41:e2019004
Sevc J, Kunz E, Placek V (1976) Lung cancer in uranium miners and long-term exposure to radon daughter products. Health Phys 30(6):433–437
Sevcová M, Sevc J (1989) Skin basalioma in workers at risk from the daughter products of radon. Pracov Lek 41:398–401
Sevcová M, Sevc J, Thomas J (1978) Alpha irradiation of the skin and the possibility of late effects. Health Phys 35(6):803–806
Sheen S, Lee KS, Chung WY, Nam S, Kang DR (2016) An updated review of case-control studies of lung cancer and indoor radon-is indoor radon the risk factor for lung cancer? Ann Occup Environ Med 28:70
Skubacz K, Wysocka M, Michalik B, Dziurzyński W, Krach A, Krawczyk J, Pałka T (2019) Modelling of radon hazards in underground mine workings. Sci Total Environ 695:133853
Smith TA, Kirkpatrick DR, Smith S, Smith TK, Pearson T, Kailasam A, Herrmann KZ, Schubert J, Agrawal DK (2017) Radioprotective agents to prevent cellular damage due to ionizing radiation. J Transl Med 15(1):232. https://doi.org/10.1186/s12967-017-1338-x
Sogl M, Taeger D, Pallapies D, Brüning T, Dufey F, Schnelzer M, Straif K, Walsh L, Kreuzer M (2012) Quantitative relationship between silica exposure and lung cancer mortality in German uranium miners, 1946–2003. Br J Cancer 107(7):1188–1194. https://doi.org/10.1038/bjc.2012.374
Stram DO, Langholz B, Huberman M, Thomas DC (1999) Correcting for exposure measurement error in a reanalysis of lung cancer mortality for the Colorado plateau uranium miners cohort. Health Phys 77(3):265–275
Tchorz-Trzeciakiewicz DE, Parkitny T (2015) Radon as a tracer of daily, seasonal and spatial air movements in the underground tourist route “coal mine” (SW Poland). J Environ Radioact 149:90–98
Thun MJ, Baker DB, Steenland K, Smith AB, Halperin W, Berl T (1985) Renal toxicity in uranium mill workers. Scand J Work Environ Health 11(2):83–90. https://doi.org/10.5271/sjweh.2249
Tomásek L (2012) Lung cancer mortality among Czech uranium miners-60 years since exposure. J Radiol Prot 32(3):301–314. https://doi.org/10.1088/0952-4746/32/3/301
Tomásek L, Darby SC, Swerdlow AJ, Placek V, Kunz E (1993) Radon exposure and cancers other than lung cancer among uranium miners in West Bohemia. Lancet 341(8850):919–923
Tomásek L, Swerdlow AJ, Darby SC, Placek V, Kunz E (1994) Mortality in uranium miners in West Bohemia: a long-term cohort study. Occup Environ Med 51(5):308–315
Tomasek L, Rogel A, Tirmarche M, Mitton N, Laurier D (2008) Lung cancer in French and Czech uranium miners: radon-associated risk at low exposure rates and modifying effects of time since exposure and age at exposure. Radiat Res 169(2):125–137. https://doi.org/10.1667/RR0848.1
Vacquier B, Caer S, Rogel A, Feurprier M, Tirmarche M, Luccioni C, Quesne B, Acker A, Laurier D (2008) Mortality risk in the French cohort of uranium miners: extended follow-up 1946-1999. Occup Environ Med 65(9):597–604. https://doi.org/10.1136/oem.2007.034959
Vacquier B, Rogel A, Leuraud K, Caer S, Acker A, Laurier D (2009) Radon-associated lung cancer risk among French uranium miners: modifying factors of the exposure-risk relationship. Radiat Environ Biophys 48(1):1–9. https://doi.org/10.1007/s00411-008-0196-6
Vermeulen R, Portengen L, Lubin J, Stewart P, Blair A, Attfield MD, Silverman DT (2019) The impact of alternative historical extrapolations of diesel exhaust exposure and radon in the diesel exhaust in miners study (DEMS). Int J Epidemiol 49:459–466. https://doi.org/10.1093/ije/dyz189
Vienneau D, de Hoogh K, Hauri D, Vicedo-Cabrera AM, Schindler C, Huss A, Röösli M (2017) SNC study group. Effects of radon and UV exposure on skin cancer mortality in Switzerland. Environ Health Perspect 125(6):067009
Vogeltanz-Holm N, Schwartz GG (2018) Radon and lung cancer: what does the public really know? J Environ Radioact 192:26–23
Wagoner JK, Archer VE, Carroll BE, Duncan MA, Holaday DA, Pope A, Lawrence PA (1964) Cancer mortality patterns among US uranium miners and millers, 1950 through 1962. JNCI J Natl Cancer Inst 32(4):787–801. https://doi.org/10.1093/jnci/32.4.787
Walsh L, Dufey F, Tschense A, Schnelzer M, Grosche B, Kreuzer M (2010) Radon and the risk of cancer mortality--internal Poisson models for the German uranium miners cohort. Health Phys 99(3):292–300. https://doi.org/10.1097/HP.0b013e3181cd669d
Walsh L, Dufey F, Tschense A, Schnelzer M, Sogl M, Kreuzer M (2012) Prostate cancer mortality risk in relation to working underground in the Wismut cohort study of German uranium miners, 1970-2003. BMJ Open 8:2–3. https://doi.org/10.1136/bmjopen-2012-001002
Walsh L, Grosche B, Schnelzer M, Tschense A, Sogl M, Kreuzer M (2015) A review of the results from the German Wismut uranium miners cohort. Radiat Prot Dosim 164(1–2):147–153. https://doi.org/10.1093/rpd/ncu281
Wang S, Ran Y, Lu B, Li J, Kuang H, Gong L, Hao Y (2019) A review of uranium-induced reproductive toxicity. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01920-2
WHO (2009) WHO handbook on indoor radon a public health perspective. WHO international radon project. Available at http://whqlibdoc.who.int/publications/2009/9789241547673_eng.pdf. Accessed 24 April 2020
Winde F, Geipel G, Espina C, Schüz J (2019) Human exposure to uranium in south African gold mining areas using barber-based hair sampling. PLoS One 14(6):e0219059
World Nuclear Association (2019) World Uranium Mining Production. Available at http://www.world-nuclear.org/information-library/nuclear-fuel-cycle/mining-of-uranium/world-uranium-mining-production.aspx. Accessed 21 February 2019
Wysocka M, Chałupnik S, Chmielewska I, Janson E, Radziejowski W, Samolej K (2019) Natural radioactivity in polish coal mines: an attempt to assess the trend of radium release into the environment. Mine Water Environ 38:581–589. https://doi.org/10.1007/s10230-019-00626-0
Zablotska LB, Lane RS, Frost SE, Thompson PA (2014) Leukemia, lymphoma and multiple myeloma mortality (1950–1999) and incidence (1969–1999) in the Eldorado uranium workers cohort. Environ Res 130:43–50. https://doi.org/10.1016/j.envres.2014.01.002
Zoellner T (2009) Uranium: war, energy, and the rock that shaped the world. Penguin Group, New York
Zychowski KE, Kodali V, Harmon M, Tyler CR, Sanchez B, Suarez YO, Herbert G, Wheeler A, Avasarala S, Cerrato JM, Kunda NK, Muttil P, Shuey C, Brearley A, Ali A-M, Lin Y, Shoeb M, Erdely A, Campen MJ (2018) Respirable uranyl-vanadate-containing particulate matter derived from a legacy uranium mine site exhibits potentiated cardiopulmonary toxicity. Toxicol Sci 164(1):101–114
Tirmarche M, Harrison J, Laurier D, Blanchardon E, Paquet F, Marsh J (2012) Risk of lung cancer from radon exposure: contribution of recently published studies of uranium miners. Annals of the ICRP 41(3–4):368–377
Author information
Authors and Affiliations
Contributions
All authors confirmed that they have contributed to the intellectual content of this paper and have met the following three requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data, (b) drafting or revising the article for intellectual content, and (c) final approval of the published article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Additional information
Responsible editor: Georg Steinhauser
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Semenova, Y., Pivina, L., Zhunussov, Y. et al. Radiation-related health hazards to uranium miners. Environ Sci Pollut Res 27, 34808–34822 (2020). https://doi.org/10.1007/s11356-020-09590-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09590-7