Abstract
Minerals of olivine–melilite and olivine–monticellite rocks from the Krestovskiy massif contain primary silicate-salt, carbonate-salt, and salt melt inclusions. Silicate-salt inclusions are present in perovskite I and melilite. Thermometric experiments conducted on these inclusions at 1,230–1,250°C showed silicate–carbonate liquid immiscibility. Globules of composite carbonate-salt melt rich in alkalies, P, S, and Cl separated in silicate melt. Carbonate salt globules in some inclusions from perovskite II at 1,190–1,200°C separated into immiscible liquid phases of simpler composition. Carbonate-salt and salt inclusions occur in monticellite, melilite, and garnet and homogenize at close temperatures (980–780°C). They contain alkalies, Ca, P, SO3, Cl, and CO2. According to the ratio of these components and predominance of one of them, melt inclusions are divided into 6 types: I—hyperalkaline (CaO/(Na2O+K2O)≤1) carbonate melts; II—moderately alkaline (CaO/(Na2O+K2O)>1) carbonate melts; III—sulfate-alkaline melts; IV—phosphate-alkaline melts; V—alkali-chloridic melts, and VI—calc-carbonate melts. Joint occurrence of all the above types and their syngenetic character were established. Some inclusions demonstrated carbonate-salt immiscibility phenomena at 840–800°C. A conclusion in made that the origin of carbonate melts during the formation of intrusion rocks is related to silicate–carbonate immiscibility in parental alkali-ultrabasic magma. The separated carbonate melt had a complex alkaline composition. Under unstable conditions the melt began to decompose into simpler immiscible fractions. Different types of carbonate-salt and salt inclusions seem to reflect the composition of these spatially isolated immiscible fractions. Liquid carbonate-salt immiscibility took place in a wide temperature range from 1,200–1,190°C to 800°C. The occurrence of this kind of processes under macroconditions might, most likely, cause the appearance of different types of immiscible carbonate-salt melts and lead to the formation of different types of carbonatites: alkali-phosphatic, alkali-sulfatic, alkali-chloridic, and, most widespread, calcitic ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays most carbonatites are believed to be formed at the magmatic stage from carbonate melts that separated from parental alkaline magma as a result of liquid immiscibility. The probability of carbonate-silicate immiscibility in alkaline systems was supported experimentally (Kjarsgaard and Hamilton 1989; Brooker and Hamilton 1990; Brooker 1998; Minarik 1998) and established by petrographic and geochemical studies of natural objects (Shastry and Kumar 1995; Le Bas 1977). The process of decomposition of silicate melt into immiscible silicate and carbonate fractions was observed directly in thermometric experiments used for melt inclusion studies (Solovova et al. 1996; Veksler et al. 1998; Panina and Usol’tseva 2000).
In this paper we present new results of studies of the chemical composition and homogenization temperatures of silicate-salt and six types of carbonate-salt and salt melt inclusions found in the minerals of olivine–melilite and olivine–monticellite rocks from the Krestovskiy massif. During our thermometric experiments we established the phenomena of liquid carbonate-silicate and, for the first time, multiphase carbonate-salt immiscibility. Unfortunately, carbonate-salt immiscibility is poorly studied (Dawson et al. 1992; Solovova et al. 1996; Mitchell 1997; Andreeva et al. 1998, 1999; Ripp et al. 2000; Panina and Usol’tseva 2000), though its role in the origin of carbonatites seems to be very significant. Most likely, this is due to the fact that carbonate-salt immiscibility is not evident from the mineral and chemical composition of carbonate rocks. It is also hard to detect it during thermometric experiments in melt inclusion studies. For example, when studying carbonate and salt inclusions in minerals of carbonatite-bearing intrusions, carbonate minerals were found to occur together with phosphate, sulfate, and halide minerals (Solovova et al. 1996; Andreeva et al. 1998, 1999). However, it was unreasonable to relate their origin to carbonate-salt immiscibility because of the lack of indisputable direct evidence of liquation. There are also few works devoted to experimental studies of multiphase silicate–carbonate-salt immiscibility in artificial and natural systems. The most interesting recent paper among them is authored by Suk (2001, 2003).
In our paper the emphasis is placed on the evolution of carbonate-salt melts that separated from parental silicate magma and underwent multiphase carbonate-salt immiscibility. The occurrence of immiscibility was many times directly observed in inclusions during thermometric experiments. It was established that in the course of multiphase liquid immiscibility the complex initial carbonate melt decomposes into immiscible liquid fractions of simpler composition. The problem of the origin of calcitic carbonatites and various types of rare-earth and rare-metal carbonatites enriched in P, S, halides, and alkalies is discussed in the light of obtained data.
Brief geological description of the Krestovskiy massif
The alkali-ultrabasic Krestovskiy massif occurs in the north of the Siberian Platform in the zone of contact of the Yenisei-Khatanga accumulative plain and the Middle Siberian Plateau. The widespread occurrence of alkali-ultramafic magmatism in the region is related to continental rifting processes and development of abyssal mantle plume in the area where the central spreading zone of the Pyasino-Khatanga rift system intersects the Kotui transform faults. Alkali-ultrabasic rocks are abundant within a vast area (about 80,000 km2) both in extrusive and intrusive varieties and belong to the Maimecha-Kotui province of alkali-ultrabasic rocks and carbonatites. The intrusive varieties constitute about 50 independent intricate massifs of the central type and have a number of common structural and petrologic features. They are represented by a combination of three contrasting rock series: ultramafic, alkaline, and carbonatite (Egorov 1985). Most of the massifs are localized in the basins of the rivers Maimecha and Kotui and are a submeridionally directed belt up to 170 km wide and to 350 km long. Many researchers (Egorov 1991; Kogarko et al. 1991; Sazonov et al. 2001) think that the massif rocks are comagmatic and formed from the same abyssal magma which was drastically undersaturated with Si and enriched in Ca, alkalies, Ti, CO2, P and other volatile components. The processes of crystallization differentiation and fractionation played the leading part in the formation of rocks. The first to crystallize (and intrude) were ultramafic rocks (olivinites and pyroxenites), whereas carbonatites terminated the process.
The Krestovskiy massif lies in the northwest of the Maimecha-Kotui province, 50 km to the southwest of the giant (about 1,500 km2) Guli pluton of alkali-ultrabasic rocks and carbonatites. According to some geologists (Sazonov et al. 2001), it is a satellite of the Guli pluton. The massif is oval in shape and extended in submeridional direction (Fig. 1). Its northern part is overlapped by Quaternary and Jurassic-Cretaceous deposits and the northern part consists of deluvial-eluvial heaps and rare outcrops of bedrocks.
Scheme of the geologic structures of the Krestovskiy massif (from data of Sazonov et al. 2001). 1 Quaternary deposits; 2 melanoneohelites and their clastolavas; 3,4 dikes of: 3 - alkaline microsyenites; 4 trachybasalts, plagioclase porphyrites, and picrites; 5 melilitolites; 6 olivinites, wehrlites, and pyroxenites; 7 monticellilitolites; 8 geologic borders; 9 supposed tectonic faults; 10 boreholes; 11 trenches, number
The massif occupies the central part of a volcano-plutonic structure and is a layered body of ultramafic rocks consisting of alternating thick (from tens to hundreds of meters) horizons of olivinites, wehrlites, and pyroxenites (Fig. 1). The root part of this body is assumed (Legezina 1999; Sazonov et al. 2001) to be at a depth of 4.5–5 km from the day surface. Ultramafic rocks at the border with enclosing rock is dominated by wehrlites and pyroxenites. The horizons of ultramafic rocks are often deformed by fine kinematic folding and are petrographically nonuniform due to the presence of thin layers and lenses enriched in pyroxene, olivine, and ore minerals. The contacts of basic rocks with layers are sharp. No change of granularity is observed in either of them. The contacts between the horizons of pyroxenites, olivinites, and wehrlites are also distinct but are often complicated by dike bodies of alkaline picrites, trachydolerites, trachybasalts, microsyenites and accompanying boudinage and serpentinization. In the west and east of the massif in the marginal zones of layered series of ultrabasic rocks one can observe small (0.54 and 0.12 km2) bodies of melilitolites and associated monticellitolites. At the contacts with them, ultramafic rocks are recrystallized and injected by the veins and lenses of melilitolite-ultrabasic rocks. According to isotope-geochemical studies (Sazonov et al. 2001), the age of ultrabasic rocks is 250–170 Ma.
The host rocks of the massif are represented by subalkali basalts and melanonephelinites. At the contact zone of intrusion they are hornfelzed and phlogopitized. The intrusion and hosting effusive series contain dikes of dolerites, nepheline and melilite lamprophyres, and alkaline picrites. The dikes group into up to 70 m thick swarms and belts at a distance of 100–120 m from each other and extend through the massif. In the center of the intrusion the dikes form an arching bend parallel to the southern contact of the massif. The only dike of carbonatites was exposed by a borehole at a depth of 15 m in the northwest of the massif among olivine–monticellite–melilite rocks. It is fairly thin (30 cm). In addition to prevailing calcite, carbonatites contain to 3–5% magnetite and less than 1% perovskite and phlogopite. Femic minerals are segregated into separate subparallel alternating bands responsible for the flow-taxitic structure of the rock.
At the outcrops of plutonic rocks and their effusive formations 18 boreholes were drilled and three prospecting-test trenches were dug. The most interesting results were obtained when studying melilitolites and monticellitolite samples from trench three and cores from boreholes (BH) 29, 30, 61 (Fig. 1).
Characterization of studied samples
Pyroxenites in which melt inclusions were detected and studied had been found in BH-29 (depths 256 and 306 m) and in BH-30 (depths 9 and 95 m). These inequigranular rocks have pandiomorphic-granular and sideronitic textures. The rocks contain green and light-green clinopyroxene (70–80%), perovskite (~10%), and titanomagnetite (~15%). In fine-grained varieties clinopyroxene is represented by isometric grains with rounded and irregular-polygonal contours. In coarse-grained varieties it acquires a clear elongated-prismatic shape. Perovskite grains are isometric, less often, elongated and irregular with rounded and slightly wavy contours. Occasionally the grains are polysynthetically twinned or zoned. The cores of zoned phenocrysts (perovskite I) are typically dark-brown and the rims (perovskite II) are light-brown to colorless. Titanomagnetiteis strongly xenomorphic with respect to clinopyroxene and perovskite. It sometimes forms aggregates with perovskite.
Olivine–melilite rocks (kugdites) were exposed by BH-30 at depths of 114 and 163 m and in trench no. 3. These medium-grained rocks contain 75–80% melilite, 15–20% olivine, and 5–8% perovskite and titanomagnetite. Occasionally they host (to 5%) tabular grains and aggregates of green clinopyroxene and monticellite. Melilite grains are short-prismatic, a few mm in size, and hypidiomorphic with respect to olivine and clinopyroxene. Olivine has a rounded-isometric and slightly elongated shape and even, but sometimes, resorbed, contours. Perovskite is typically zoned and occurs among melilite grains as elongated accumulations and aggregates of irregular shape. Titanomagnetite is represented by either small isometric grains ingrown in melilite or forms sideronite aggregates of intricate shape between olivine and melilite grains.
When the amount of melilite increases, the rock becomes coarse-grained to pegmatoid and grades into melilitolite. Melilite grains here are of thick-tabular habit with uneven wavy rims. Olivine at the border with melilite is often rimmed by monticellite. The rims around neighboring olivine grains have the same optical orientation. Monticellite, which is occasionally present in the rock, consists of idiomorphic minute (few micron) grains with sites of regular crystallographic contours. In olivine–monticellite–melilite rocks the content of monticellite reaches 10–15% (BH-61).
Olivine–monticellite rocks and monticellitolites were found in trench no. 3 and in BH-61 at depths of 5.5, 63.5, 150, and 511 m. Their texture is transitive from hypidiomorphic-granular to hypidioblastic, in places, poikilitic. The rocks contain (vol%) from 35 to 75 monticellite, 5–40 olivine, 10–15 perovskite, and 10–15 titanomagnetite. The most idiomorphic among minerals is olivine. It is intensely corroded, has an irregular shape, and in places is represented by separate blocks with the same optical orientation. Occasionally, olivine occurs as ingrowths in monticellite. Monticellite forms inequigranular aggregates of granoblastic habit. Perovskite and titanomagnetite in the rocks consists of small grains of isometric rounded and polygonal shape which are either ingrown in monticellite or form accumulations (agreggates) between monticellite grains.
Melilite and monticellite rocks also contain accessory amounts (Panina et al. 2001) of: titanium garnet, phlogopite, wollastonite, pectolite, combeite, larnite (rankinite) and sulfides (djerfisherite, pyrrhotite, pentlandite, chalcopyrite, and arsenopyrite).
The chemical composition of major rock-forming minerals in studied rocks is not permanent (Sazonov et al. 2001; Panina et al. 2001).Olivine in all rocks is represented by forsterite and contains significant amounts of Ca, Mn, and Ni. In kugdites and olivine–monticellite rocks olivine, compared to other rocks, is the highest magnesian (Fo88–90 vs. Fo82–88) and contains more CaO (2.5–1 vs 0.9–0.5 wt%) and NiO (0.20 vs. 0.12 wt%) at the same quantity (0.23–0.32 wt%) of MnO. Clinopyroxene in all rocks belongs to diopside (Morimoto 1988). At the same time, its composition is unstable even within one rock and its major and minor components vary considerably. Compared to light-colored clinopyroxene, the green variety is more ferruginous (#Mg 84–88 and 88–98%, respectively) and is richer in TiO2 (1.5–2 vs. 0–0.9 wt%) and Al2O3 (2–3 vs. 0.02–0.6 wt%). The chemical composition of melilite is dominated by akermanite end member. The mineral demonstrates a tendency toward an increase in the concentrations of FeO (from 1.4–2 wt% to 2.6–4.3 wt%) and decrease in Na2O (from 3 wt% to 1.5 wt%) and alumina (from 5.5 wt% to 2 wt%) from kugdite to melilitolite and olivine–monticellite–melilite rock. The composition of monticellites in most studied rocks is persistent and highly ferruginous: iron replaces Mg by 22–32%; only in monticellitolites #Mg increases to 82–84%. All monticellites contain 0.3–0.4 wt% MnO. Perovskite in all rocks contains the same set of accessory elements: FeO (from 0.1 wt% to 2.0 wt%), SrO (from 0.01 wt% to 0.9 wt%), Na2O (from 0.2 wt% to 0.6 wt%) as well as La, Ce, Sm, Nb (at maximum content of accessory elements 4–5 wt%). Dark-colored perovskite always contains greater amounts of accessory elements than light-colored perovskite II. Titanomagnetitehas a rather uniform composition. Minimum (1.5–5 wt%) concentrations of TiO2 were found in minerals from pyroxenites. With increasing content of melilite and monticellite in the rocks, the concentration of Ti in titanomagnetites increases and reaches its maximum (15–18 wt%) in melilitolites. Titanomagnetites also contained minor MnO (0.2–0.5 wt%), MgO (0.4–6 wt%), and CaO (0–0.5 wt%).
Methods for study of melt and fluid inclusions in minerals
Melt and fluid inclusions in minerals were first studied optically in 0.2–0.3 mm thick polished plates. To heat inclusions and establish their homogenization temperatures, we used a high-temperature stage with a silite heater (Mikhailov and Shatskii 1975). The heating stage was attached to a microscope. Temperatures were measured with the help of a platinum–platinum-rhodium thermocouple.
The heating stage was calibrated by the clearly registered melting points of chemically pure salts (K2Cr2O7, KCl, NaCl) and noble metals (Ag, Au), which were placed in the immediate vicinity of the soldered joint of thermocouple. The accuracy of measurements was ±10–15°C. The experiments were conducted in the air. To avoid decrepitation of salt and silicate-salt inclusions, they were heated at a rather slow rate (10–20°C/min) from the beginning to the end of melting of daughter phases. The holding time of salt and carbonate-salt inclusions at predetermined temperature was 10–30 min and that of silicate-salt inclusions, about 1.5–2 h. Homogenization temperatures reproduced not less than three times were considered reliable. After homogenization, all inclusions were quenched. The cooling rate was 70°C/s in the temperature range from 1,200°C to 850°C and 20°C/s in the range of 650–450°C. To reveal the chemical composition of silicate and carbonate-salt components in the inclusions demonstrating liquid immiscibility, the inclusions were heated to temperatures ensuring complete melting of contained silicate and salt crystal phases and further immiscibility of melt. After that, the inclusions were quenched.
The chemical composition of inclusions and minerals was determined on a “Camebax-micro” analyzer. As standards, we used international standards of minerals (diopside, orthoclase, albite, chlorapatite, garnets, ilmenite, anhydride) and glasses. The photographs were obtained with accelerating voltage of 20 kV, beam current of 40 nA, counting time of 10 s, and a 2–3 μm spot beam. The accuracy of analysis was 1–1.5 wt%. Salt fine-crystallized inclusions were analyzed by scanning the surface with a focused beam.
The most serious difficulties emerged during preparation of heated salt and, especially, silicate-salt inclusions for microprobe analysis: the salt microcrystalline quenched aggregate often flaked when brought to the surface and polished. Difficulties also arose in analyzing salt globules in layered inclusions because of their small sizes and entrapment of silicate component by probe beam. Therefore, of a few tens of analyzed globules we could use only single, most qualitative analyses.
To determine the approximate amounts of CO2 and H2O involved in the crystalline lattice of daughter minerals in salt and carbonate-salt inclusions, the chemical analyses were calculated to normative composition. The calculation was based on molar rations of components in the analysis. The following assumptions were made: (1) All silicon refers to silicate minerals, mainly, to the host-mineral (matrix) of inclusions; (2) SO3, Cl, and PO4-anions predominantly form iron pairs with alkalies and, if alkalies are lacking, with Ca; (3) In case the anionic component is minor, excess cations are bound to CO2; (4) to balance the total to 100%, we used the water that was, most likely, present in crystallization or constitution form (with sulfates, carbonates, phosphates, and silicates), less often, in a free state as thin films enveloping salts in inclusions.
To rule out possible overestimation of the amount of water in salt inclusions, the normative compositions were calculated only for those inclusions whose total of oxides was not evidently underestimated for technical reasons (owing to small sizes of inclusions, burn-up of salts under electron beam, inhomogeneity of inclusion content, etc.).
Results of studies of melt inclusions in minerals
The rock-forming minerals from the Krestovskiy massif were found to contain carbonate, carbonate-salt, silicate-salt, and silicate inclusions. Silicate-salt and silicate inclusions were found mainly in perovskite, less often, in melilite from olivine–melilite and monticellite-melilite rocks. These inclusions had a complex mineral composition and a complex phase transformation on heating. The chemical analysis of their carbonate-salt compound was technically difficult and had errors. Carbonate and carbonate-salt inclusions were less complex and more abundant than silicate-salt inclusions.
Silicate-salt inclusions
Silicate-salt inclusions in perovskite
Perovskite contains a great number of inclusions but their distribution is irregular. In large zoned phenocrysts, inclusions segregate in the cores composed of dark-colored perovskite I and in peripheral light-colored zones represented by perovskite II (Fig. 2). The intermediate zone between them is typically sterile from inclusions. Generally, perovskite II contains more inclusions than perovskite I. Iinclusions in perovskite I are isometric and rounded, whereas in perovskite II, mainly elongate-rounded and, less often, irregular (Fig. 3). The sizes on inclusions are from 20–30 μm to150×30 μm. At room temperature the inclusions are black with rare small light spots (Fig. 3-2a, c). The phase composition of unheated inclusions in transmitted light is poorly observable, especially in perovskite I, but in reflected light in decrepitated inclusions one can clearly see daughter phases and a fine-crystallized carbonate-salt aggregate between them. Microprobe analysis of inclusions in perovskite showed (Panina et al. 2001) the following minerals among daughter phases: pyroxene, kalsilite, apatite, phlogopite, combeite, rankinite, magnetite, djerfisherite, pyrrhotite, and sphene. In all inclusions from perovskite II the daughter minerals were represented by: phlogopite, kalsilite, hauyne, pectolite, magnetite, and rutile. Most daughter minerals in inclusions have xenomoprhic contours but, very seldom, pyroxene and combeite show some elements of faceting. Magnetite and sulfides often have a rounded shape.
Silicate-salt inclusions in perovskite. 1 Inclusion with silicate–carbonate-immiscibility in perovskite I from monticellite, heated to 1,170°C. The chemical composition of carbonate-salt phase (globules) is in Table 1, an. 1. 2 Inclusions of silicate-salt melts in perovskite II from olivine–monticellite–melilite rocks: a, c unheated inclusions; b,d inclusions heated to 1,100 and 1,050°C.In heated inclusions one clearly observe that the isolated carbonate-salt fraction (globules) is nonuniform and is separated into immiscible phases. 3 Inclusion with three-phase silicate–carbonate-salt imiiscibility, heated to 1,030°C, in perovskite II. Immiscible carbonate-salt phases (globules) are spatially isolated in inclusion glass. The chemical composition of salt phases is given in Table 1, ans. 12 and 13. 5 Inclusions with miltiphase silicate–carbonate-salt immiscibility, heated to 1,050°C, in perovskite II. It is seen that spatially separated immiscible carbonate-salt phases (globules) are nonuniform in composition
Phase transformations in inclusions from perovskite I. On heating the air, the content of inclusions in perovskite I gradually lightens and becomes grayish-brown. At 700–980°C, salt aggregates in inclusions and, at about 1,000°C, silicate phases, start to melt. At 1,130–1,150°C, in many inclusions one can observe spherical isolation (globules) of salt liquid in silicate melt with clear interphases between them. The ratio of immiscible phases is predominantly 10:90 but, occasionally, salt globule occupies to 20 vol% of inclusion. This fact suggests that at some conservation stage of inclusions the melt was already heterogeneous owing to the partial isolation of salt phase from silicate melt.
At 1,160–1,180°C, all daughter phases in inclusions melt completely and the content of inclusions consists of two immiscible phases. On cooling, sometimes a gas bubble appears in salt globules at 900°C, which disappears again at a slight increase in temperature (at 910–950°C). At 1,200°C, the salt globule gradually decreases and, at 1,230–1,250°C, in some inclusions it disappears and the melt becomes homogeneous. The inclusions that remain heterogeneous during further increase in temperature decrepitate, which is, most likely, related to the abnormal ratios of their silicate and salt components owing to the heterogeneity of conserved melt. Nevertheless, we believe that the temperature 1,250–1,230°C can be recognized as the temperature of silicate–carbonate liquid immiscibility. After quenching, inclusions clearly exhibited silicate–carbonate immiscibility. Here the silicate component is represented by glass and the carbonate globule, by a fine-crystallized aggregate (Fig. 3-1).
The chemical composition of homogenized inclusions with normal ratios of silicate and carbonate phases included (wt%): 32.0–34.0 SiO2, 4–5 TiO2, 5–6 Al2O3, about 12.0 FeO, 6–8 MgO, 19–20 CaO, to 11 alkalies (with predominance of K over Na), 0.2 BaO, 0.2 SrO, 1.4 P2O5, 1–3 SO3, and, most likely, to 5–6 CO2 (Panina et al. 2001). It is comparable with the composition of melilitite melts found in inclusions from melilite and perovskite of melilitolites from the Gardiner complex in Greenland (Nielsen et al. 1997). It is also similar to the composition of melilitite ring dikes of the same complex but, unlike them, K in it predominates over Na. Under some assumption, here petrochemical parallels can be drawn with katungites from the Eastern rift zone of Africa (Knorring and Dubois 1961; Le Bas 1977) and the melilitolites from the Pian di Celli province in Italy (Gallo et al. 1984).
The chemical composition of silicate component from layered inclusions corresponds to alkali-basic melts (Panina et al. 2001) and contains on average (wt%): 34.50 SiO2, 6.4 TiO2, 7.70 Al2O3, 9.4 FeO, 6.10 MgO, 15.10 CaO, 4.2 Na2O, 4.9 K2O, 0.4 BaO, 0.4 SrO, 2.4 P2O5, 0.6 SO3, 0.2 Cl. This composition is rather close to that of melanonephelinites hosting the Krestovskiy massif (Sazonov et al. 2001).
The chemical composition of salt globules (Table 1, an. 1) is dominated by CaO (to 23 wt%), K predominates in the total of alkalies (15–18 wt%), and the content of SiO2 is significant (9–10 wt%). The globules also contain appreciable amounts (3–5 wt%) of TiO2, FeO, SO3 and lesser (0.2–1.5 wt%) BaO, SrO, and Al2O3. According to the calculated normative composition, the amount of bound CO2 might reach 20–24 wt%, whereas the content of bound water is no higher than few percent. The composition by its characteristics differs significantly from that of carbonatite lavas of volcano Oldoinyo Lengai in Tanzania (1960 eruption) owing to the presence of considerable amounts of SiO and CaO and lower contents of alkalies. However, to a certain extent it is comparable with the products (ashes) of the 1960 eruption of the above volcano (Dawson et al. 1992). We think that the composition of carbonate-salt globules in silicate-salt inclusions, obviously, characterizes the composition of initial carbonate melts separated from primordial silicate magma as a result of liquid immiscibility during the formation of the Krestovskiy massif rocks.
The normative composition of salt phase (Table 2) is dominated by alkaline carbonates (gregoryite, natron to 40 wt%) and calcite (to 30 wt%) and includes minor contents (1–3 wt%) of carbonates Sr, Ba, Mg. In addition, the salt phase contains appreciable (9–15 wt%) amounts of alkali sulfates (arcanite, mirabilite) and phosphates (apatite) and 6–15 wt% silicate (melilite, monticellite, foids, combeite) and ore (magnetite, perovskite or rutile) minerals.
Phase transformationss in inclusions from perovskite II. In light-colored perovskite II, melting of salt phases in inclusions becomes noticeable already at 660°C, and at 1,000°C silicate phases start to melt. At 1,030–1,050°C, isolation of several salt globules takes place in silicate melt. The ratio of salt and silicate fractions varies from 15:85 to 25:75. During further increase in temperature, salt globules in some inclusions decrease in size and come closer to each other and at 1,190–1,200°C merge into one bubble. When temperatures decreases by 30–50°C, the bubble again disintegrates into 2 or even 3 globules. Repeated increase in temperature turns them into one bubble again. We failed to homogenize salt fraction in silicate melt as all inclusions decrepitated, most likely, owing to the abnormal ratios of coexisting silicate and salt phases. After cooling to 1,150–1,100°C and quenching, the silicate glass of heated inclusions typically contain nonuniform salt globules represented by two spatially superposed immiscible phases (Fig. 3-2b, d). Probably, immiscible salt phases existed also in liquid phase. In some inclusions in silicate melt one can observe two salt globules isolated from each other (Fig. 3-3), one or both being inhomogeneous and represented by two immiscible phases (Fig. 3-4, −5). This suggests that at 1,190–1,200°C the initial carbonate melt, which separated from the parental silicate magma at 1,250–1,230°C, started to disintegrate into immiscible fractions.
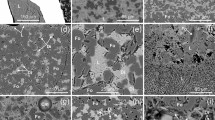
Various types of carbonate and carbonate-salt inclusions in minerals from the rocks of the Krestovskiy massif. 1 Type I. Unheated inclusions of hyperalkaline carbonate melts in monticellite of olivine–monticellite rocks. Chemical composition in Table 1, an. 7. 2 Type II. Inclusions of low-alkaline carbonate melts: (a) unheated coarse-crystallized inclusions in melilite of olivine–melilite rocks. Calculated chemical composition in Table 1, an. 10. (b) heated inclusion in monticellite of olivine–monticellite rocks. Chemical composition in Table I, an. 9. 3 Type III. Unheated alkaline-sulfate inclusion in monticellite of olivine–monticellite rocks. Averaged chemical composition Table 1, an. 11. 4 Type IV. Unheated alkaline-phosphate inclusion in monticellite of olivine–monticellite rocks. Chemical composition in Table 1, an. 17. 5 Type V. Heated inclusion of alkali-chloride melts in melilite of olivine–monticellite–melilite rocks. Chemical composition in Table 1, an. 19. 6 Type VI. Inclusions of essentially calc-carbonatite melts in clinopyroxene of pyroxenites. Chemical composition in Table 1, an. 24
Because of the small sizes of salt globules, only twice we managed to qualitatively analyze both salt globules in silicate-salt inclusions by microprobe. It was found that one of them had an alkaline-sulfate composition (Table 1, an. 12), whereas the other (Table 1, an. 13) contained CaO in amounts comparable to alkalies (15–16 vs. 6–18 wt%) and appreciable (7–8 wt%) concentrations of SO3 and P2O5. The chemical composition of single inhomogeneous salt globules from inclusions in perovskite II was also close to that of alkaline sulfate melts but noticeably enriched in P (Table 1, ans. 14, 15). Hence, the crystallization stage of perovskite II was marked not only by the silicate-salt immiscibility of initial melt but also by heterogenization of salt melt and separation into simple immiscible fractions and their spatial isolation. The composition of silicate melt, however, remained alkali-basic.
Silicate-salt inclusions in melilite
Melilite contains few inclusions and their shape is rounded and irregular. Their size is 20–30 micron and the content is completely crystallized. Fluid bubble is normally not observable. Among daughter phases we established (Panina et al. 2001): pyroxene, phlogopite, rankinite (?), larnite (?), magnetite, djerfisherite, pyrrhotite, and brown and greenish salt aggregates. On heating, the first to melt at 640–680°C were salt phases. At 1,000–1,050°C, some inclusions exhibited two liquid phases: a green salt phase in the center rimmed by a brown silicate one. Their ratios vary from 10:90 to 20:80, which suggests conservation of initially heterogeneous melt by inclusions. An increase in temperature above 1,100°C led to decrepitation of inclusions.
The chemical composition of heated layered inclusions is very close to that of similar inclusions in perovskite I. Here the silicate fraction is low-siliceous, enriched in Ca and alkalies and contains 7–12 wt% MgO (Panina et al. 2001). But again, the salt part of inclusions corresponds to carbonate-salt globules of inclusions in perovskite I (Table 1, an. 2): K predominates over Na, with approximately equal amounts of alkalies and CaO (17–18 and 20–22 wt%), 9–10 wt% SiO2,and 3–4 wt% SO3 and Cl.
Carbonate and carbonate-salt inclusions
These occur mainly in melilite and monticellite, less often, in apatite and garnet of olivine–melilite and monticellite rocks, in which they are primary. In older minerals, olivine of monticellite and melilite rocks and in pyroxene of pyroxenites, they were found to be secondary inclusions and to develop in the cracks of host minerals. Primary inclusions are arranged azonally, often as large accumulations. In chemical composition and homogenization temperatures, primary and secondary inclusions of the same type are comparable with each other. Their sizes vary from few to 20–40 and even 100–120 μm. The shape is rounded, tetragonal and hexagonal, prismatic, partially faceted, and, occasionally, irregular. The content of inclusions is fine- and coarse-crystallized, fluid phase is often not observed, sometimes flattened (Fig. 4). The main compounds are K2O, Na2O, CaO, P2O5, Cl, SO3, and CO2. Depending on the ratio of these components and predominance of one of them, the inclusions are divided into six groups (types): I—hyperalkaline carbonate melts with CaO/(Na2O+K2O)⩽1; II—low-alkaline carbonate melts with CaO/(Na2O+K2O)≥1; III—alkali-sulfate melts containing 10–15 wt% SO3; IV—alkali-phosphate melts containing 15–35 wt% P2O5; V—alkali-chloride melts containing 10–30 wt% Cl, and VI—essentially Ca-carbonate melts. Carbonate and carbonate-salt inclusions with different compositions often occur in the same growth zones of host minerals, which suggests their syngenetic character. The content of all types of inclusions after their homogenization at rapid quenching, crystallizes finely.
Inclusions of type I
This type of inclusions exists mainly in monticellite of olivine–monticellite rocks, less often, in melilite and apatite of olivine–melilite rocks. They are primary and are spatially associated with microlites of phlogopite, apatite, nepheline, and carbonate-salt inclusions of types II–V. The inclusions consist of the prevailing amount of transparent colorless cubes and aggregates, brown segregations and greenish platelets among which one can observe ore minerals and deformed gas bubbles (Fig. 4-1). On heating, the first to melt at 450°C are brown and then, at 550–570°C, colorless phases. At these temperatures, the salt aggregate disintegrates into a great number of crystals, the gas bubble gradually becomes spherical. At about 780°C, green phases melt and gas moves in inclusion vacuole. At about 800°C, some inclusions exhibit a clear separation of liquid into brown and greenish phases. During further heating, most inclusions decrepitate. Homogenization of individual inclusions occurs at ~980°C in melilite, at 870–890°C in monticellite, and at ~820°C in apatite into a salt melt (Table 2).
The chemical composition of heated and unheated inclusions from different minerals is rather similar (Table 1, ans. 3–7): the content of alkalies either predominates over CaO or is in equal ratios. The total amount of alkalies and CaO varies from 19 wt% to 27–30 wt%. The inclusions also contain 2–4 wt% SiO2, 1–2 wt% MgO and FeO, and to 1–4 wt% SO3. In the inclusions exhibiting liquid immiscibility the content of SO3 increases to 5–7 wt% and that of P2O3 or Cl also grows to 6–8 wt% (Table 1, ans. 4, 6). The chemical composition of unheated fine-crystallized inclusions in monticellite differs from heated ones only in slightly lesser concentrations of SrO, P2O5, and SO3 (Table 1, an. 7). In general, the composition is rather close to that of carbonate-salt globules from perovskite I. Owing to this, we united them into one group. However, unlike the globules, type I inclusions contain much less Si, whereas K can both prevail and vice versa. The composition of carbonate-salt inclusions of this type is also close to that of Na-carbonate melts found in the inclusions in melilite and perovskite of melilitolites from the Gardiner complex in Eastern Greenland (Nielsen et al. 1997). They also contained 19–25 wt% CaO and 28–35 wt% alkalies and homogenization temperature of inclusions was 1,030–900°C.
The normative composition of inclusions is represented (Table 2) mainly (35–52 wt%) by alkaline carbonates (predominantly gregoryite and trona as well as nyerereite and natrite) and calcite (from 10 wt% to 50 wt%). It also involves ~11–13% alkaline sulfates (arcanite, thenardite, mirabilite) and to 6% alkaline and calcium phosphates (mainly apatite and nahpoite). Typically, small amounts (1–3%) of magnetite, halite, carbonates of Mg, Fe, Ba, Sr and silicate minerals (melilite, monticellite, foids) are also present. According to calculations, the content of bound water varies from 0 wt% to 12 wt% and that of bound CO2, from 25 wt% to 40 wt%.
Inclusions of type II
This type of inclusions was found in melilite and monticellite of olivine–monticellite rocks. They are primary, normally occur in the same zone together with other types of carbonate-salt inclusions, most often with type IV. The inclusions consist of colorless, brown, and greenish salt phases, among which there occur magnetite and sulfide minerals of square and irregular shape as well as a deformed gas bubble. Rarely, fine-crystallized salt aggregates coexist with large (5–10 μm) daughter phases (Fig. 4-2a) which, according to microprobe analysis belong to patite, sylvite, and djerfisherite. On heating, the first (~500°C) to melt are brown phases followed by colorless ones and at 710°C, greenish salt crystals melt (Fig, 4-2b). At these temperatures, the gas bubble’s shape becomes spherical and it easily moves in inclusion vacuole. At 800°C, the content of monticellite and melilite in some inclusions is divided into two phases: greenish in the center and colorless around it, i. e. two-phase carbonate-salt liquid immiscibility takes place. During further heating, most inclusions decrepitate and explode. In rare cases, the inclusions lacking immiscibility homogenize at 810–890 and 970°C (Table 2).
The chemical composition of heated homogeneous inclusions (Table 1, ans. 8, 9) is dominated (~33 wt%) by CaO and 13–20 wt% are alkalies . The amount of SiO2 is in general lower than in the composition of type I inclusions, whereas SO3 accounts for 5–6 wt%. In the inclusions exhibiting liquid immiscibility the contents of P2O5 and Cl reach 5–6 wt%. According to the calculations of normative composition of inclusions, the amount of bound CO2 is 27–37 wt% and water is from 0 wt% to 7 wt%.
We made an attempt to compare the obtained results with calculated data of bulk composition of unheated inclusion (Fig. 4-2a) which, in addition to the predominant (70 vol%) fine-crystallized carbonate component, included large daughter phases—apatite (5.4 vol%), KCl (15 vol%), magnetite (7.6 vol%), and djerfisherite (2 vol%). The calculation was performed for two 10 μm deep cuts with due regard for the volume fractions of the inclusion phases. The calculations showed that conserved melts contained (wt%): 36 CaO, 15–16 alkalies, ~~1 SrO, ~3 P2O5, 4.6 Cl, <1 SO3 (Table 1, an. 10), i. e., calculated compositions were close to the averaged compositions of heated inclusions. Carbonate inclusions of such low-alkaline composition were found in perovskite and melilite of melilitolites from the Gardiner complex in Greenland (Veksler et al. 1998). They also contained (wt%): 28–45 CaO, 8–16 Na2O, and 1.3–2.7 K2O. Inclusions homogenized at 1,060–1,030°C.
The normative composition of type II inclusions (Table 2) is dominated by calcite (more than 60 wt%) and alkaline carbonates—nyerereite and gregoryite (about 20 wt%). The content of strontianite, magnesite, apatite, arcanite, and silicates is 2 to 5–6 wt% and that of magnetite, alkaline chlorides and sulfides is less than 1–2 wt%.
Inclusions of type III
This type of inclusions occur in melilite of olivine–melilite and monticellite of olivine–monticellite rocks. They are primary and associated with types I, II, IV, V of carbonate-salt inclusions as well as with microlites of nepheline and phlogopite. Their shape is rounded, prismatic, and irregular. Sizes range from 1–2 to 20–30 micron. The inclusions consist of uniform colorless and brown salt aggregates and appreciable number of greenish crystals. The first to melt at 500°C are greenish crystals, at 630–650°C gas bubble acquires a regular spherical shape (but most salt phases still remain (Fig. 4-3). At about 800°C, all daughter salt phases melt completely, the content of inclusions becomes colorless, and, at 860–890°C, homogeneous (Table 2).
The chemical composition of inclusions (Table 1, an. 11) is characterized by high (7–10 to 17 wt%) contents of SO3 and considerable (30– 35 wt%) amount of alkalies with significant predominance of Na over K. It also includes mainly 7–8, rarely to 15 wt% CaO, 2–7 wt% P2O5, ~1% SrO, and 0 to 2–3 wt% Cl.
The normative composition of inclusions (Table 2) is dominated (20–35 wt%) by alkaline sulfates (15–20 wt% arcanite, to 10% thenardite, and 1–20 wt% mirabilite). The inclusions also contain 20–30 wt% alkaline carbonates (mainly gregoryite), 5–8 wt% carbonates (mainly calcite), ~9 wt% phosphates (mainly apatite) and few percent silicate (monticellite, foids, combeite) minerals, sylvite, and magnetite. The calculations show that the concentrations of bound CO2 and H2O in these inclusions are 7–12 and from 0 wt% to 7 wt%, respectively.
In the chemical composition this type is close to carbonate-salt globules from inclusions in perovskite II. For this reason we referred these globules to the type under discussion (Table 1, ans. 12–15). Inclusions of similar high-alkaline sulfate composition were found earlier (Panina and Usoltseva 2000) in monticellite from the rocks of the Malyi Murun massif (Aldan, Russia). Their homogenization temperatures (1,040–1,170°C), however, appeared to be higher. A similar composition was also found in inclusions from bastnesite of rare-earth carbonatites from Western Transbaikalia (Ripp et al. 2000). High-sulfate (28–35 wt% SO3) but mainly high-calcium (26–35 wt% CaO) melt composition was found in the inclusions from minerals of fluorite-celestine veins of the carbonatite-bearing Mushugai-Khuduk complex in Southern Mongolia (Andreeva et al. 1999). Homogenization temperatures of these inclusions did not exceed 670°C. This seems to be related to the presence of high concentrations of F and Cl in conserved melt.
Inclusions of type IV
These inclusions occur mainly in monticellite and, less often, in olivine of olivine–monticellite rocks. The inclusions in monticellite are primary and associated with all other types of salt inclusions and microlites of nepheline. In olivine they are secondary and are spatially superposed with inclusions of types V and VI. The shape of inclusions is rounded, irregular, prismatic, square, and rhomboid. Sizes range from few microns to a few tens of micron (Fig. 4-4). The inclusions are fine-crystallized, colorless, occasionally, brown or contain colorless phases with individual greenish prisms as well as cubes and irregularly shaped segregations of ore minerals. One inclusion was found to contain daughter phlogopite.
On heating, the first to melt (~470°C) are greenish phases, which at about 700°C melt completely. At these temperatures one can observe a spherical gas bubble and minute colorless and black crystals. Inclusions homogenize in a rather wide temperature range from 850°C to 980°C and, most likely, to a certain degree depend on the ratios of major components in conserved melt.
In general, the melt (Table 1, ans. 16–18) is markedly enriched in P2O5 (15 to 35–39 wt%), CaO (20–30 wt%), and alkalies (totalling 17–30 wt% at significant predominance of Na over K). An increase in P2O5 concentrations in salt melt is accompanied by a slight increase in the amount of CaO and SO3 and decrease in alkalies. The concentrations of SrO and BaO (1.5–2 and 0.2–0.5 wt%, respectively) remain the same. Melts with low concentrations of phosphorus might contain to 10–15 wt% bound CO2 and in fractions extremely enriched in P2O5 its concentration is no more than 1–2 wt%. Calculations show that the content of bound water increases with increasing phosphorus content and might reach 1.5–4 wt%.
The normative composition of inclusions (Table 2) consists mainly (>60%) of phosphates (nahpoit, apatite, brianite, buchwaldite), >10% alkaline carbonates (gregoryite, nyerereite), 5–7% alkaline sulfates (arcanite, thenardite), 5–8 wt% calcite and from 0 wt% to 15 wt% portlandite. Constantly present are small amounts (1–2 wt%) of carbonates of Sr and Ba as well as magnetite. Halite and silicates (monticellite, combeite, nepheline) might also occur.
The inclusions of phosphate-carbonate composition were earlier found in apatite of teralites and apatite from magnetite-apatite rocks from the carbonatite-bearing Mushugai-Khuduk complex (Andreeva et al. 1999). The chemical composition was dominated (54 wt%) by CaO, included ~18% P2O5 and minor (1–2 wt%) rare earths, F, and Cl. Alkalies were virtually absent.
Homogenization temperatures are ≥1,200°C. Inclusions with a similar compositions but much lesser (11.5 wt%) P2O5 were found in apatite and clinopyroxene from the rocks of the Palabora carbonatite complex (S. Africa). Homogenization temperature of these inclusions was 850–870°C, P=4–4.5 kbar (Solovova et al. 1998).
Inclusions of type V
These inclusions occur in melilite of olivine–melilite rocks and monticellite and olivine of olivine–monticellite rocks and in diopside of pyroxenites. In melilite and monticellite they are primary and associated with other types of carbonate and carbonate-salt inclusions. In diopside and olivine, the inclusions are secondary and associated mainly with secondary type VI inclusions and with gas-liquid inclusions. The latter homogenize at 470°C. The shape of type V inclusions is prismatic, often irregular, occasionally close to isometric. The inclusions contain colorless cubes, shapeless brown and plate-like greenish phases as well as cubic ore phases (magnetite). Occasionally, a deformed gas bubble is observed. On heating, the first to melt at 290°C are brown phases, then colorless ones. At 510–580°C, the green plates melt intensely and the gas bubble becomes spherical (Fig. 4-5). In some inclusions, at 750°C, salt melt boils. Homogenization temperatures range from 840°C to 790°C in melilite and monticellite and to 720–670°C in pyroxene (Table 2). Most likely, a wide spread in homogenization temperatures reflects the wide range of chemical composition of conserved melt (Table 1, ans. 19–21), which varies from high-calcium with moderate contents of Cl and alkalies (Table 1, an. 19) to high-alkaline with very high (27.5–31 wt%) concentrations of Cl. The normative composition of inclusions (Table 2) is dominated mainly (>50 wt%) by alkaline chlorides (halite, sylvite) and involves to 35 wt% carbonates (calcite, nyerereite, gregoryite, strontianite). The inclusions also contain 3–4 wt% alkaline sulfates (thenardite), to 5 wt% silicates, and few percent magnetite and portlandite. The amount of bound water in salt melt might reach 5–10 wt% and that of CO2 from 5 wt% to 20 wt% in inclusions with high Ca contents.
Location of composition of silicate-salt and various types of carbonate-salt and salt inclusions on the presudoternary diagram (Na2O+K2O)–(CaO+MgO+FeO)–(SiO2+Al2O3+TiO2) (Freestone and Hamilton 1980) 1 homogenized silicate-salt melts in inclusions. 2 line of coexisting immiscible silicate and carbonate melts in inclusions. Connonds 1–1′ and 1–1′′ reflect separation of carbonate-salt globules in silicate-salt inclusions contained on perovskite into SO3- and P2O5-rich immiscible salt fractions. 3–8 various types of carbonate-salt and salt inclusions: 3 type I of hyperalkaline carbonate melts; 4 type II of low-alkaline carbonate melts; 5 type III of alkali-sulfate melts; 6 type IV of alkali-phosphate melts; 7 type V of alkali-chloride melts; 8 type VI of essentially Ca carbonate melts. Location of fields of silicate–carbonate immiscibility at 2 and 5 kbar, 1,250°C for conditions of CO2 saturation are giver according to (Kjarsgaard and Hamilton 1989)
Inclusions of type VI
Inclusions of this type were found in diopside of pyroxenites, olivine, and monticellite of olivine–monticellite rocks, and in garnet of olivine–melilite rocks. In olivine and pyroxene, the inclusions are secondary, whereas in garnet and monticellite, primary. The inclusions consist mainly of colorless salt phases among which one can observe brown segregations (Fig. 4-6). On heating, the first to melt at 320°C are brown phases and at 500–550°C, colorless crystals start to melt and the gas bubble acquires a spherical shape. Homogenization takes place at 810–780°C.
Location of compositions type IV alkali-phosphate melt inclusions on the diagram (Na2O+K2O)–(CaO+MgO)–(SiO2+Al2O3) by Suk (2001). 1 carbonate-silicate systems; 2 line of coexisting immiscible silicate and carbonate melts in inclusions (this work); 3, 4 phosphate-carbonate-silicate systems containing 10.4 wt% P2O5; 5 type IV of alkali-phosphate melt inclusions (this work). Location of liquid immiscibility fields is given for dry conditions at 1,250°C, P=2 kbar
The chemical composition of inclusions (Table 1, ans. 22–26) is simple. The cation part is represented mainly (45–54 wt%) by CaO. The inclusions also contain 0.2–1.6 FeO and MgO, to 2–8 wt% alkalies, to 2 wt% SrO, and 0.1–0.5 wt% P2O5. One of inclusions in diopside contained about 68 wt% CaO (Table 1, an. 26). Excess concentrations of CaO seem to be due to the presence of portlandite. According to calculations, the normative composition of this inclusion involves about 60 wt% portlandite and 40 wt% calcite. The normative composition of other inclusions of this type is essentially calcitic. Occasionally it includes to 8 wt% alkaline carbonates (nyerereite, gregoryite) and minor (~1 wt%) apatite and silicate minerals. Carbonate melts conserved in inclusions contain from 20 wt% to 40 wt% bound CO2 and from 2 wt% to 14 wt% bound water.
The chemical and normative composition of this type of inclusions is rather similar to the chemical and modal composition of intrusive calcite carbonatites widespread in nature.
Discussion of results
Our thermometric studies showed that carbonatite melts during formation of the Krestovskiy massif appeared at the stage of perovskite crystallization as a result of the occurrence of silicate–carbonate liquid immiscibility of parental alkali-ultrabasic magma. This magma was enriched in Ca, Mg, K, Na, and S, which is suggested not only by the chemical and mineral composition of rocks but also by the modal composition of perovskite inclusions containing phlogopite, diopside, combeite, rankinite, larnite, pectolite, apatite, kalsilite, djerfisherite, and pyrrhotite (Panina et al. 2001). Considerable amounts of Ca, K, and S in the melt might be related to the mantle conditions of melting of parental magma at high pressure of CO2. It is known (Ryabchikov 1987) that under high-pressure conditions the concentration of CO2 attains high values, which results in increased solubility of CaO. With increasing pressure and depth, the K/Na values in fluids and melts also grow. Experimental data (Luhr 1990; Poulson and Ohmoto 1990) show that high pressures in magma systems also increase the solubility of sulfur in melts. Sulfur solubility is influenced by oxygen fugacity: at its high potential, part of S passes into a sulfate form, which is more soluble in silicate melt compared to sulfide sulfur (Carrol and Rutherford 1985). The high potential of oxygen in the initial melt is suggested by the presence of daughter magnetite in silicate-salt inclusions, detected by us.
Carbonate-silicate immiscibility
The occurrence of carbonate-silicate liquid immiscibility in the initial melt during the formation of the Krestovskiy massif rocks is suggested by the fact that perovskite and melilite contain carbonate-silicate inclusions in which we observed separation of the melt into two equilibrium coexisting silicate and carbonate liquids directly during our thermometric experiments at 1,250–1,230°C. Immiscibility is also supported by the presence of coexisting silicate and carbonate inclusions in melilite in the same growth zones of host mineral (Panina et al. 2001). The presence of carbonate-silicate inclusions in the cores of perovskite grains and their abundance in the rims are evidence that the onset of melt immiscibility coincided with the beginning of perovskite crystallization and occurred most vigorously at the final stages of formation of this mineral.
The phenomena of carbonate-silicate liquid immiscibility are widespread in nature and were supported by other researchers when studying inclusions in some alkaline carbonatite-bearing complexes. However, in most cases the conclusions about the temperaturesof occurrence of immiscibility were based on homogenization temperatures of coexisting syngenetic silicate and carbonate inclusions. For example, in the alkaline-carbonatite Alno complex, Sweden (Morogan and Lindlom 1995) the homogenization temperature of silicate inclusions in Al-diopside coexisting with the inclusions of Mg-bearing calcite melts was taken as the temperature of carbonate-silicate liquid immiscibility (1,175°C at P=5–6 kbar). In melilitolites of the ultramafic alkaline Gardiner complex, the homogenization temperatures of silicate and low-alkaline carbonate inclusions (1,060–1,030°C) were regarded as the temperature of liquid immiscibility (Nielsen et al. 1997). In carbonatite-bearing alkaline Mushugai-Khuduk complex the temperature of carbonate-silicate liquid immiscibility (~1,200°C) was again based on the homogenization temperatures of coexisting silicate and carbonate inclusions (Samoilov et al. 1988).
Thus, various authors report rather close but, compared to ours, lower temperatures of the occurrence of carbonate-silicate liquid immiscibility. This is quite explicable because our data reflect the onset of immiscibility, while other workers report the temperatures of later stage, i. e., the temperatures of spatially separated coexisting silicate–carbonate immiscible liquids.
Our temperatures of the occurrence of carbonate-silicate liquid immiscibility obtained in inclusion studies agree with the data of experimental investigations of synthetic and natural systems. According to them (Freestone and Hamilton 1980; Kjarsgaard and Hamilton 1988, 1989; Brooker and Hamilton 1990; Brooker 1998), liquid immiscibility occurs in a wide range of temperatures and pressures, depends on the bulk composition of initial silicate melt, its fluid saturation, and oxygen fugacity and increase in alkalies and CO2 pressure broadens its field.
Liquid immiscibility is normally accompanied by redistribution of major and secondary components between coexisting phases. According to our data, the composition of separated silicate phase resulting from immiscibility was, compared to the composition of initial melt, somewhat enriched in Si, Al and depleted in Ca and alkalies. In general, it was rather close to the composition of melanonephelinites. The composition of coexisting carbonate-salt melt was highly alkaline, enriched in Ca, with Ca(CaO/(Na2O+K2O)⩽1, and included small amounts of Si, S, and P. We think that it reflected the composition of initial carbonate melts separated from parental silicate magma during the occurrence of carbonate-silicate immiscibility. This composition differs markedly from the chemical composition of carbonatite lavas of the Oldoinyo Lengai volcano in Tanzania of 1960 and 1988 eruptions (Dawson et al. 1990) in the increased contents of Si and Ca. Incidentally, according to the petrographic observations of Mitchell (1997), who studied lavas erupted in 1995 from the above volcano, sodium carbonatites are highly fractionated compared to primitive magma. At the same time, our analysis of the chemical composition of carbonate-salt fractions (globules) is similar to that of low-alkaline carbonate melts from inclusions in perovskite and melilite of melilitolites from the Gardiner complex in Greenland, which are also considered (Nielsen et al. 1997) liquate of silicate (melilitolite) magma. The composition of primordial carbonatite magma that participated in the formation of Ca-carbonatites of the Guli massif in East Siberia, according to studies of carbonate inclusions in perovskite and calzirtite (Kogarko et al. 1991), was also enriched in lime and alkalies.
On the pseudoternary diagram (Na2O+K2O)–(CaO+MgO+FeO)–(SiO2+Al2O3+TiO2) reflecting silicate–carbonate immiscibility under the conditions of CO2 saturation at T=1,250°C and P=5 kbar (Kjarsgaard and Hamilton 1988, 1989) the compositions of homogenized silicate-salt inclusions fall both into the region of silicate melts and immiscibility field and tend to the boundary between them (Fig. 5). The compositions of coexisting silicate and carbonate fractions from inclusions are located in the immiscibility field (connected by connods). With a certain degree of assumption, we can suggest that the carbonate-silicate immiscibility detected by us at 1,250–1,230°C took place at pressure no lower than 5 kbar. On a similar diagram (Suk 2001) determining the position of carbonate-silicate immiscibility field at T=1,250°C and P=2 kbar the compositions of silicate liquates analyzed by us are arranged beyond the immiscibility field (Fig. 6). We think the reason for this is that the experiments by Suk (2001) were performed under dry conditions and at rather low pressures, which significantly reduce the immiscibility field. According to Brooker (1998), the size of double-liquidus field considerably increases under elevated contents of CO2and high total pressures.
Multiphase carbonate-salt liquid immiscibility
Complex carbonate melts and silicate liquates separated as a result of liquid immiscibility, most likely, for a long time existed under stable physicochemical conditions and in equilibrium with each other. This can be indirectly inferred from the absence of inclusions in the intermediate zone between the cores and rims of zoned perovskite phenocrysts. At the crystallization stage of the rims of perovskite grains, the physicochemical conditions of the magmatic system strongly changed, which is suggested from the presence of zonality and the abundance of inclusions in perovskite II. The disturbance of the equilibrium of coexisting silicate and carbonate immiscible liquids resulted in their spatial separation and at 1,200–1,190°C,caused decomposition of the separated carbonate melt into fractions of simpler chemical composition. The onset of multiphase carbonate-salt immiscibility was detected in silicate-salt inclusions present in perovskite II. The inclusions in the course of thermometric experiments exhibited separation of salt globules into several immiscible fractions, namely, into SO3-rich and P2O5-enriched ones (Fig. 5, connods 1–1′ and 1–1′′). The evidence of the multiphase carbonate-salt immiscibility is the presence of coexisting syngenetic carbonate-salt inclusions of different chemical compositions (different types) in the one and the same zones of monticellite and melilite. This is also supported by the frequent occurrence of carbonate-salt immiscibility in carbonate-salt inclusions observed during thermometric experiments (Table 1, ans. 4, 6, 8, 9).
Separation of carbonate-salt melt into simpler fractions can be theoretically substantiated in the following way. As carbonate-salt melts are ionic liquids, according to the polarity law, the equilibria of exchange reactions in them are shifted toward the combination of strong acid anions with the strongest alkaline cations. Inhomogeneity and immiscibility in this kind of melts appear when the interaction energy of some ion pairs exceeds that of other pairs (Kogarko 1978). In the carbonate melts under discussion the cations are represented mainly by alkalies and Ca, whereas anions, are represented by Cl, SO3, PO4, and CO3 (reported in decreasing order of their basic and acid properties). The presence of stronger acid than CO3 in the melt allowed alkalies (as stronger bases) to establish relationship first of all namely with them, producing inhomogeneities and widening the immiscibility field when a weaker acidic anion is replaced by a stronger one. As Ca carbonates are the lowest energy pair, they must have terminated decomposition of initial carbonate melt.
The syngenetic carbonate-salt and salt inclusions detected by us were separated into six types with due regard for various properties of acidic bases. The chemical composition of carbonate-salt inclusions of type I is the closest analog of the chemical composition of carbonate globules in perovskite I that we take as the composition of initial carbonate melts separated from parental silicate magma. Therefore, it can be assumed that the remaining five types of carbonate-salt inclusions are derivatives of this initial melt and are the result of its decomposition into simpler immiscible fractions.
The process of decomposition of initial carbonate-salt melt seems to be long and realized in a wide temperature range: immiscibility in inclusions was observed starting from 1,200°C to 1,190°C and continued up to 800°C. The decrease in melt temperature, most likely, increased the immiscibility field.
The presence of the distinguished types of carbonate-salt inclusions agrees well with the results of Suk experiments (2001, 2003), who studied the influence of phosphorus, sulfur, and halides on the silicate–carbonate immiscibility of feldspathoid silicate melt. During the experiments he established nonuniformity of obtained salt (phosphate, fluoride, chloride, sulfate-carbonate) liquids, which suggested the possibility of further decomposition of salt phases after their separation from silicate melt.
On the pseudoternary diagram of Kjarsgaard and Hamilton (1989), the composition of all the types of carbonate-salt inclusions distinguished by us are arranged in the crystallization field of carbonates and close to it in the immiscibility region along its boundary (Fig. 5). The compositions of inclusions of types III, V, and VI form isolated fields. The compositions of type IV inclusions are arranged in the central part of crystallization field of carbonates, whereas the compositions of inclusions of types of I and II form a wide field with gradual transitions into each other. The last fact is explicable because the compositions of type II inclusions reflect fractionated (to a varying degree) compositions of initial carbonate melts after the removal of part of separated liquid of types III–V.
At the same time, the experimental compositions on the diagram (Kjarsgaard and Hamilton 1989) differ strongly from our inclusion compositions owing to some unaccounted factors, including the absence of P, S, and Cl in the system. This might have resulted in the change in configuration and size of the field of carbonate-silicate immiscibility. However, Suk experiments (2001) showed that addition of phosphorus into the system at T=1,250°C and P=2 kbar does not essentially influence the size of silicate–carbonate immiscibility field and only slightly increases the contrast range of compositions of coexisting phases. On Suk’s diagram (2001), plotted on the basis of these experiments, the compositions of P-rich inclusions of type IV are also arranged in the central part of crystallization field of carbonates and often coincide with experimental data (Fig. 6).
Nearly all types of carbonate-salt and salt inclusions distinguished by us exist in natural objects. For example, in the Na-carbonatite lavas of volcano Oldoinyo Lengai in Tanzania some authors describe exotic occurrences of carbonate-salt immiscibility. Namely, in the products of the 1966 eruption in alkaline carbonate Cl- and F-rich cement of lapilli Dawson et al. (1992) describing amebiform and rounded bodies consisting of submicroscopic intergrowths of faceted minerals represented by (a) mixture of alkaline carbonates and alkaline phosphates (analog of IV type inclusions) and (b) mixture of alkaline carbonates and alkaline Fe-sulfides (analog of III type). In contrast to our type III, sulfur here existed in sulfide form, probably, owing to the low oxygen fugacity which prevented transition of S into sulfate form. The rounded aggregates from submicroscopic intergrown minerals are assumed (Dawson et al. 1992) to be a result of rapid quenching of two types of liquids, which separated from primordial magma whose composition is close to that of the magma of the 1960 eruption.
The porphyritic lavas of 1995 eruption were found (Mitchell 1997) to contain globules consisting of intergrown gregoryite, Na-sylvite, neighborite, djerfisherite, and a complex of Ba-, Sr-, and Ca-rich carbonates (analog of type V inclusions). According to Mitchell (1997), this evidences the occurrence of liquid immiscibility of Na-K-Ca-CO2-Cl-rich F-bearing melt with Na-rich Cl-poor carbonate liquid.
Larger-scale occurrences of silicate–carbonate-salt immiscibility seem to be related to the formation of sulfate-bearing Ba-Sr carbonatites in Western Transbaikalia (Ripp et al. 2000). Carbonatites here are associated with high-potassium alkaline-basic silicate rocks. They compose dikes and manto deposits containing isolated sharply outlined lenses and bands of baritocelestite and Sr-barite (analog of type III inclusions). It was established (Ripp et al. 2000) that carbonatites formed at the high-temperature magmatic stage as a result of liquid immiscibility of silicate melt into silicate and carbonate-sulfate phase. The latter in turn liquated into carbonate and sulfate liquids. The high concentrations (2–10 times higher than average level) of sulfate sulfur in carbonatites and its participation in the formation of rocks allowed the researchers to raise a question about recognition of sulfate type of carbonatites.
In East Siberia in the Tomtor massif polycarbonate rocks and breccias of carbonate-phosphate compositions (analog of type IV inclusions) were referred to rare-metal carbonatites. They occur close to the center of calcite-dolomite carbonatite stocks surrounded by the rocks of jacupirangite-urtite series, alkaline and nepheline syenites, and alnoites (Entin et al. 1990). Melt immiscibility is considered to be also responsible for the formation of P-bearing rocks in the alkali-carbonatite Mushugai-Khuduk complex in Mongolia, which refers to the Late Mesozoic volcano-plutonic association of potassium alkaline and subalkaline rocks and calcite carbonatites. The latter are enriched in fluorite and barite and are closely associated by gradual transitions with veined fluorite-barite, celestine-fluorite, fluorite, and calcite-barite-quartz rocks. Veins and stocks of magnetite-apatite and fluorite-celestine-apatite rocks are also abundant here. Based on results of thermometric studies of inclusions in the minerals of this complex, a conclusion is made (Andreeva et al. 1998, 1999) about the reality of separation of phosphate, carbonate, and essentially sulfate melts containing elevated concentrations of Cl and F from silicate magma is real at high temperatures (~1,200°C). These melts were responsible for the crystallization of carbonatites and associated rocks. Carbonatites of similar type were also described in Mountain-Pass in California (Lieber 1997).
The initial carbonate-salt melts isolated from parental silicate magma (corresponding to type I inclusions), most likely, can preserve their primary composition only in rare cases and only in volcanic environment at rapid eruption and quenching on the Earth’s surface. The phenomena of multiphase liquid immiscibility seem to be widely spread in natural carbonate-silicate systems. However, the indications of this seem to be indistinct and hard to detect. Probably, widening the scope of technical resources and application of new research procedures will help to reveal liquid immiscibility in many natural objects even on those not directly connected with the described alkaline basic-ultrabasic magmatism.
Of great interest are recent data (Golovin et al. 2003) about the presence of secondary silicate-salt inclusions in the olivine of unaltered kimberlites from the Udachnaya-Vostochnaya pipe in Yakutia. Daughter mineral phases in these inclusions were found to coexist with silicates, calcareous and alkaline carbonates, alkaline sulfates, chlorides, phosphates, djerfisherite, and magnetite. In other words, their modal composition was close to the normative composition of silicate-salt and carbonate-salt inclusions from rocks of alkali-ultrabasic carbonatite complexes (our data; Nielsen et al. 1997; Andreeva et al. 1998, 1999; Veksler et al. 1998; Ripp et al. 2000). The homogenization temperatures of carbonate-salt inclusions (700–800°C) were also similar to ours. This similarity might suggest: (a) the same way of the formation of carbonatite magmas responsible for kimberlites and rocks of alkaline ultrabasic complexes, (b) similarity of their chemical composition and the same type of evolution in the Earth’s crust, resulting in the immiscibility of carbonate-salt fractions. Some questions rise in connection with the secondary nature of detected inclusions. They are easy to answer with the following taken into account: (a) olivine crystallized at higher temperature than those at which the immiscibility phenomenon might occur, (b) high mobility and volatility of the compounds of alkalies with S, P, and Cl, owing to which carbonate-salt melts could migrate to considerable distances and penetrate along cracks into older high-temperature minerals.
Therefore, study of silicate-salt inclusions in the minerals of olivine–melilite and olivine–monticellite rocks from the Krestovskiy massif allowed us to reveal (at 1,230–1,250°C) the fact of the occurrence of carbonate-silicate liquid immiscibility of alkali-ultrabasic melt and to determine the composition of resulting silicate and carbonate-salt liquates. Analysis of carbonate-salt and salt inclusions contained in the minerals of these rocks helped us to detect multiphase decomposition of separated carbonate-salt liquid into fractions of simpler composition—carbonate-sulfate, carbonate-phosphate, carbonate-chloride, and calc-carbonate. Multiphase liquid carbonate-salt immiscibility took place in a wide temperature range, starting from 1,200–1,190°C to 800°C under unstable physicochemical conditions. The revealed regularities are also found in natural carbonatite complexes.
References
Andreeva IA, Naumov VB, Kovalenko VI, Kononkova NN (1998) Fluoride-sulfate and chloride-sulfate salt melts of carbonate-bearing Mushugai-Khuduk complex, Southern Mongolia. Petrologiya 6:307–315
Andreeva IA, Naumov VB, Kovalenko VI, Kononkova NN (1999) Composition of magmas and genesis of teralites of carbonate bearing Mushugai-Khuduk complex (Southern Mongolia). Geokhimiya 8:826–841
Brooker RA (1998) The effect of CO2 saturation on immiscibility between silicate and carbonate liquids: an experimental Study. Petrology 39:1905–1915
Brooker RA, Hamilton DL (1990) Three-liquid immiscibility and the origin of carbonatites. Nature 346:459–462
Carrol MR, Rutherford MJ (1985) Sulfide and sulfate saturation in hydrous silicate melts. Geophys Res 90:601–612
Dawson JB, Pinkerton H, Norton GE, Pyle DM (1990) Physico-chemical properties of alkali carbonatite lavas: data from the 1988 eruption of Oldoinyo Lengai, Tanzania. Geology 18:260–263
Dawson JB, Smith JV, Stelle IM (1992) 1966 ash eruption of the carbonatite volcano Oldoinyo Lengai: mineralogy of lapilli and mixing of silicate and carbonate magmas. Mineral Mag 56:1–16
Egorov LS (1985) Alkali-ultrabasic magmatism and its mineralogy. Geologiya Rudnykh Mestorozgdenii 4:24–40
Egorov LS (1991) Iiolite-carbonatite plutonism (in Russian). Leningrad
Entin AR, Zaitsev AI, Nenashev NI et al. (1990) On the sequence of geologic events related to the intrusion of the Tomtor Massif of ultrabasic-alkaline rocks and carbonatites. Russian Geology and Geophysics 31:42–51
Freestone IC, Hamilton DL (1980) The role of liquid immiscibility in the genesis of carbonatites: an experimental study. Contrib Mineral Petrol 73:105–117
Gallo F, Giammetti F, Venturelli G, Vernia L (1984) The kamafugitic rocks of San Venanzo and Cupaello, Central Italy. N Jahrb Mineral Monatsch 5:198–210
Golovin AV, Sharygin VV, Pokhilenko NP, Mal’kovets VG, Kolesov BA, Sobolev NV (2003) Secondary melt inclusions in olivine of unaltered kimberlites from the Ugachnaya-Vostochnaya pipe, Yakutia. Dokl Earth Sci 388:369–372
Kjarsgaard BA, Hamilton DL (1988) Liquid immiscibility and the origin of alkali-poor carbonatites. Mineral Mag 52:43–55
Kjarsgaard BA, Hamilton DL (1989) The genesis of carbonatites by immiscibility. In: Bell K (ed) Carbonatites: genesis and evolution. Unwin Hyman, London, pp 388–409
Knorring O von, Dubois CGB (1961) Carbonatitic lava from Fort Portal Area in Western Uganda. Nature 192:1064–1065
Kogarko LN (1978) Principle of polarity of chemical bond and its petrologic significance. Problems of petrology of the Earth’s crust and upper mantle (in Russian). Moscow, pp 222–228
Kogarko LN, Plant DA, Henderson CMB, Kjarsgaard BA (1991) Na-rich carbonate inclusions in perovskite and calzirtite from the Guli intrusive Ca-carbonatite, Polar Siberia. Contrib Mineral Petrol 109:124–129
Le Bas MJ (1977) Carbonatite-nephelinite volcanism. Wiley, New York
Legezina OG (1999) Rocks of the Krestovskaya intrusion (north of the Siberian Platform). Geology and mineral resources of the Krasnoyarsk Territory (in Russian). Krasnoyarsk, pp 189–190
Lieber Werner (1997) Bastnasit from Mountain Pass, California. Aufschluss 48:137–142
Luhr JF (1990) Experimental phase relations of water- and sulfur-saturated arc magmas and the 1982 eruptions of E1 Chichon Volcano. Petrology 31:1071–1114
Mikhailov MY and Shatskii VS (1975) A silicon carbide heating element for a high-temperature microscopic stade. Mineralogy of endogenic rocks (in Russian). Novosibirsk, pp 109–110
Minarik W (1998) Complications to carbonate melt mobility due to the presence of an immiscible silicate melt. Petrology 36:1965–1973
Mitchell R (1997) Carbonate-carbonate immiscibility, neighborite and potassium iron sulphide in Oldoinyo Lengai natrocarbonatite. Mineral Mag 61:779–789
Morimoto N (1988) Nomenclature of pyroxenes. Mineral Petrol 39:55–76
Morogan V, Lindblom S (1995) Volatiles associated with the alkaline-carbonatite magmatism at Alno: a study of fluid and solid inclusions in minerals from the Langazsholmen ring complex. Contrib Mineral Petrol 122:262–274
Nielsen TFD, Solovova IP, Veksler IV (1997) Parental melts of melilitolite and origin of alkaline carbonatite: evidence from crystallized melt inclusions, Gardiner complex. Contrib Mineral Petrol 126:331–344
Panina LI, Usol’tseva LM (2000) Role of liquid immiscibility in the formation of calcitic carbonatites of the Malyi Murun Massif (Aldan). Russian Geology and Geophysics 41:655–670
Panina LI, Sazonov AM, Usol’tseva LM (2001) Melilitic and monticellite-bearing rocks of the Krestovskaya intrusion (north of Siberian Platform) and their genesis. Russian Geology and Geophysics 42:1313–1332
Poulson SR, Ohmoto H (1990) An evalution of the solubility of sulfide sulfar in silicate melts from experimental data and natural samples. Chem Geol 85:57–75
Ripp GS, Kobylkina OV, Doroshkevich AG, Sharakshinov AO (2000) Late-Mezozoic carbonatites of Western Transbaikalia (in Russian). Ulan Ude
Ryabchikov ID (1987) Mantle magma formation. Evolution of magmatism in the Earth’s history (in Russian). Moscow, pp 349–371
Samoilov VS, Kovalenko VI, Naumov VB, Sandimirova GA, Chuvashova LA (1988) Immiscibility of silicate and salt melts during formation of Mushugai-Khuduk alkaline complex (Southern Mongolia). Geokhimiya 10:1447–1460
Sazonov AM, Zvyagina EA, Leontiev SI et al. (2001) Platinum-Bearing alkaline-ultrabasic intrusions of the Polar Siberia (in Russian). Tomsk
Shastry A, Kumar S (1995) Petrogenesis of carbonatite veins and associated melanephelinite from Sarnu-Dandali Igneous Complex, Barmer, Rajasthan. Nat Acad Sci Lett 18:139–143
Solovova IP, Girnis AV, Ryabchikov ID (1996) Inclusions of carbonate and silicate melts in minerals of alkaline basaltoids from Eastern Pamir. Petrologiya 4:339–363
Solovova IP, Pyabchikov ID, Kogarko LN, Kononkova NN (1998) Studies of inclusions in minerals of Palabora carbonatite complex (S Africa). Geokhimiya 5:435–447
Suk NI (2001) Experimental study of silicate–carbonate systems. Petrologiya 9:547–558
Suk NI (2003) Experimental studies of fluid-magmatic differentiation of alkaline systems in connection with the problem of genesis of carbonatites.Plumes and problem of deep sources of alkaline magmatism. Irkutsk-Habarovsk, pp 62–74
Veksler IV, Nielsen TF and Sokolov SV (1998) Mineralogy of crystallized melt inclusions from Gardiner and Kovdor ultramafic alkaline complexes: implications for carbonatite genesis. Petrology 39(11–12):2015–2031
Acknowledgements
Our sincere thanks go to J. Touret for commenting on an early version of the manuscript and for his valuable critical remarks. The work was supported by grant 02–05–64617 and grant 05–05–64361 from the Russian Foundation for Basic Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.L.R. Touret
Rights and permissions
About this article
Cite this article
Panina, L.I. Multiphase carbonate-salt immiscibility in carbonatite melts: data on melt inclusions from the Krestovskiy massif minerals (Polar Siberia). Contrib Mineral Petrol 150, 19–36 (2005). https://doi.org/10.1007/s00410-005-0001-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-005-0001-3