Abstract
Carbonatic components of parental melts of the deeper mantle diamonds are inferred from their primary inclusions of (Mg, Fe, Ca, Na)-carbonate minerals trapped at PT conditions of the Earth’s transition zone and lower mantle. PT phase diagrams of MgCO3–FeCO3–CaCO3–Na2CO3 system and its ternary MgCO3–FeCO3–Na2CO3 boundary join were studied at pressures between 12 and 24 GPa and high temperatures. Experimental data point to eutectic solidus phase relations and indicate liquidus boundaries for completely miscible (Mg, Fe, Ca, Na)- and (Mg, Fe, Ca)-carbonate melts. PT fields for partial carbonate melts associated with (Mg, Fe)-, (Ca, Fe, Na)-, and (Na2Ca, Na2Fe)-carbonate solid solution phases are determined. Effective nucleation and mass crystallization of deeper mantle diamonds are realized in multicomponent (Mg, Fe, Ca, Na)-carbonatite–carbon melts at 18 and 26 GPa. The multicomponent carbonate systems were melted at temperatures that are lower than the geothermal ones. This gives an evidence for generation of diamond-parental carbonatite melts and formation of diamonds at the PT conditions of transition zone and lower mantle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deeper mantle diamonds of the transition zone and lower-mantle origin are rare but important “strangers” at the Earth’s surface carrying crucial information about deep interiors. The diamonds contain primary inclusions of Mg–Fe–Al- and Ca–Fe-silicates as well as Mg–Fe- and Si-oxides which are expected in the Earth’s lower mantle due to experimental estimates (Akaogi 2007; Harte 2010). Moreover, the diamonds have primary mineral inclusions of different (Mg, Fe, Ca, Na)-carbonates (McCammon et al. 1997; Brenker et al. 2007; Kaminsky 2012). This indicates that carbonate components are presented in the diamond-parental media of the Earth’s transition zone and lower mantle and can be inferred from primary inclusions.
Previous experimental works showed that solid carbonates of magnesium, calcium, iron, and sodium are stable at a wide interval of PT conditions along the mantle geotherm (Fiquet et al. 2002; Santillan and Williams 2004a, b; Isshiki et al. 2004; Skorodumova et al. 2005; Dasgupta and Hirschmann 2010; Stagno et al. 2011; Merlini et al. 2012). It is assumed that carbonates could be a significant compounds at the conditions of the Earth’s deep interiors (Fiquet et al. 2002; Isshiki et al. 2004; Dasgupta and Hirschmann 2010; Stagno et al. 2011; Boulard et al. 2011).
Mineralogical data on primary inclusions in deeper mantle diamonds provide information about a general chemical composition of diamond-parental medium but could not reveal the substance responsible for diamonds origin. The chemical composition of parental medium and mechanism of diamonds formation may be understood based on results of physicochemical experiments and a syngenesis criterion of diamonds and inclusions (Litvin 2007). The criterion demands parental medium to provide conditions for joint formation of diamonds and complete set of paragenetic inclusions as well as make the possibility for the presence of xenogenetic phases.
The main objectives of this work are experimental studies: (1) melting phase relations of multicomponent carbonate MgCO3–FeCO3–CaCO3–Na2CO3 system and its ternary MgCO3–FeCO3–Na2CO3 boundary join (with representative carbonate components of primary inclusions in deeper mantle diamond), in particular to determine the solidus and liquidus boundaries, (2) a diamond-forming efficiency of the multicomponent carbonate melts, mechanisms of formation of diamonds and paragenetic carbonate minerals in carbonate–carbon melt solutions under conditions of partial and complete melting, and correspondence of results to the syngenesis criterion.
Methods
Experimental study on melting of multicomponent carbonate systems was carried out using high-pressure, high-temperature 1200 t (Sumitomo) multianvil hydraulic press at pressures between 12 and 26 GPa and temperatures from 800 to 1800 °C at BGI (Bayerisches Geoinstitute, Bayreuth, Germany). The conditions and result of the experiments are summarized in Tables 1 and 2. Standard 14/8, 10/5, 10/4, and 7/3 cell assemblages for pressures of 12, 18, 23, and 26 GPa, respectively, were used. The experimental assemblages and procedures have been earlier described by Frost et al. (2004). We used synthetic magnesite MgCO3 (99.9 % purity), calcite CaCO3 (99.9 %), Na2CO3 (99.9 %), and natural siderite with composition (Fe0.98 Mn0.02)CO3 as starting materials. All starting carbonates have been dried for at least 24 h at temperature 250 °C. A starting mixture was placed into a capsule made of a rhenium foil. High temperature was generated using a LaCrO3 heater. Immediate quenching was applied after heating regime. Accuracies of pressure and temperature determination were estimated as ±0.5 GPa and ±50 °C, respectively.
Chemical compositions of starting carbonate systems used for melting phases relation studies were prepared by mixing of: (1) FeCO3—35; MgCO3—35; Na2CO3—30 wt% and (2) FeCO3—26; MgCO3—26; CaCO3—25; Na2CO3—23 wt%. The model compositions take into account mineralogical data of representative carbonate minerals within the primary inclusions in diamonds, especially, from the upper part of the lower mantle (Kaminsky 2012).
Mixtures of the compositions (Mg, Fe, Na-carbonates)60carbon40 and (Mg, Fe, Ca, Na-carbonates)60carbon40 were used for study of diamond crystallization Elemental carbon is graphite that is a metastable phase in experimental conditions of diamond crystallization in carbonate–carbon systems at 18 GPa and 1800 °C (transition zone conditions) as well as 26 GPa and 1900 °C (lower-mantle conditions). Metastability of graphite under these conditions is demonstrated by experimental carbon phase diagram for both stable and metastable solid carbon phases (Bundy et al. 1996). The diagram and corresponding experimental data demonstrate that the metastable graphite is not capable to transform into diamond by mechanism of direct transition in spite of the conditions belong to the diamond thermodynamic stability field. Under experimental PT conditions used in this work, carbonate liquids play a key role in diamond formation because they are capable to dissolve elemental carbon. The parental carbonate + graphite (and/or diamond formed) mixes and resulted melts form strong buffer couples with fO2 close to the FeO/Fe buffer (Litvin et al. 2008).
Compositions of experimental phases and textural relationships were characterized using a scanning electron microscope Tescan Vega II XMU equipped EDS (INCAx-sight), at an accelerating voltage of 20 kV, beam current up to 450 pA, the size of the electron beam 210 nm at the Institute of Experimental Mineralogy of the RAS in Chernogolovka, Moscow region.
Results and discussion
Melting relations in the systems MgCO3–FeCO3–Na2CO3 and MgCO3–FeCO3–CaCO3–Na2CO3 were experimentally studied at 12–23 GPa and 800–1800 °C. Experimental conditions and results are presented in Tables 1 and 2 for the multicomponent carbonate FeCO3–MgCO3–Na2CO3 and MgCO3–FeCO3–CaCO3–Na2CO3 systems, respectively.
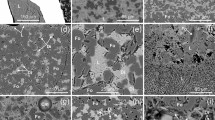
Melting relations of the system MgCO3–FeCO3–Na2CO3 are shown in Fig. 1. The PT phase fields are limited by a lower temperature boundary (1100 °C at 12 GPa) of eutectic melting of the multiphase carbonate system and a higher boundary of complete melting at temperature 1700 °C at 18 GPa. The experimental data are extrapolated within 11–24 GPa. Single-phase field of completely miscible Mg–Fe–Na-carbonate melt is restricted by the liquidus line. The quenched melts (Fig. 2b–c) are composed of relatively high contents of Na (0.64–0.40 mol% Na2CO3), Fe (0.36–0.17 mol% FeCO3), and Mg (0.24–0.19 mol% MgCO3) components. Liquidus phase is presented by solid solution phase of (Mg, Fe)-carbonate (Mg, Fe)CO3 (Fig. 2b) with composition 0.47–0.39 mol% FeCO3 and 0.58–0.24 mol% MgCO3 and admixtured 0.01–0.04 mol% Na2CO3. The quenched melts are characterized by FeCO3 (0.35–0.26 mol%), MgCO3 (0.26–0.11 mol%), and increasing Na2CO3 (0.63–0.54 mol%) contents. At lower temperatures, a solid solution of Na–Mg–Fe-carbonate is formed composed (mol%) of Na2CO3 (0.56–0.77), FeCO3 (0.09–0.32), and MgCO3 (0.41–0.03). Herewith, an invariant eutectic assemblage of (Mg, Fe)-carbonate + Na–Mg–Fe-carbonate + melt (L) is resulted (Fig. 2c). A subsolidus assemblage consists of (Mg, Fe)- and (Na2Mg, Na2Fe)-carbonate phases (Fig. 2d).
Melting relations of the multicomponent carbonate system MgCO3–FeCO3–CaCO3–Na2CO3 are demonstrated in Fig. 3. A partial melting field is located between the boundaries of low-temperature eutectics (900 °C at 12 GPa) and complete melting (1700 °C at 23 GPa) at higher temperature. One-phase field of completely miscible multicomponent carbonate melt is next to the liquidus line (Fig. 4a). In partial melting fields, the compositions (mol%) of quenched melts are variable as 0.26–0.19 FeCO3, 0.26–0.24 MgCO3, 0.27 CaCO3, and 0.29–0.24 Na2CO3. Mg–Fe-carbonate solid solutions is the liquidus phase of compositions (mol%) 0.33–0.28 FeCO3 and 0.67–0.63 MgCO3 with admixtured CaCO3 up to 0.4 mol% and Na2CO3 up to 0.1 mol% (Fig. 4b). At lower temperatures, the assemblage (Mg, Fe)-carbonate + Ca–Na–Fe–Mg-carbonate + melt appears (Fig. 4c). In this case, Ca–Na–Fe–Mg-carbonates are characterized by contents (mol%) of CaCO3 (0.66–0.61), Na2CO3 (0.22–0.20), and FeCO3 (0.12–0.10) and admixtured MgCO3 (less than 0.5). An invariant eutectic assemblage (Mg, Fe)-carbonate + (Ca, Na, Fe, Mg)-carbonate + (Na2Ca, Na2Fe, Na2Mg)-carbonate + melt (Fig. 4d) which is determining for subsolidus assemblage (Mg, Fe)CO3 + (Ca, Na2, Fe, Mg) CO3 + Na2 (Ca, Fe, Mg)(CO3)2 is formed (Fig. 4e). Compositions (mol%) of Na2 (Ca, Fe, Mg)(CO3)2-carbonates are variable within 0.62–0.60 for Na2CO3, 0.21–0.20 for CaCO3, 0.12–0.11 for FeCO3 with about 0.07 for MgCO3.
Crystallization of the deeper mantle diamonds in partially and completely melted carbonate systems MgCO3–FeCO3–Na2CO3–C and MgCO3–FeCO3–CaCO3–Na2CO3–C (with dissolved elementary carbon) was experimentally realized at 18–26 GPa and 1800–1900 °C (Fig. 5). The dissolution of metastable graphite leads to the formation of carbon oversaturated in respect to diamond carbonate–carbon melt solution. The carbon-oversaturated state of carbonate–carbon melt is responsible for nucleation and mass crystallization of diamond (Litvin 2007). Experimental samples were liked as light gray solid aggregates consisting of diamond crystals (up to 34–40 vol %) in quenching carbonatic array. It should be noted that metastable graphite was sometime formed by similar mechanism as laminar single crystals together with diamonds (by SEM and Raman-spectroscopy data). Diamond crystals usually are bright, translucent octahedral individuals with flat faces and have a size of up to 30 µm. Size of diamond crystals is reduced to first microns with increasing pressure. Clusters of diamond crystals up to 10 individuals were observed. In some cases, spinel twins and splices were found.
Growth of diamonds occurs in sectors of the octahedron faces (like natural diamonds). The rate of spontaneous crystallization of diamond is essential and sensitive to changes in pressure and temperature. The maximum rate of diamond spontaneous crystallization is observed at very high pressures. The rate is decreased with a pressure decreasing; it is accompanied by a decrease in both the nucleation density and growth rates of single crystals, increasing their size.
Carbonate–carbon parental melts provide a simultaneous formation of deeper mantle diamonds and paragenetic carbonate minerals. Diamond nucleation and crystal growth are conditioned by generation of completely miscible carbonate melts oversaturated with dissolved carbon in respect of diamond. Hence, the oversaturated carbon is source for diamond, whereas the paragenetic carbonate minerals are crystallized from the melted carbonate solvents.
Carbonatitic (carbonate–silicate) composition of the upper-mantle parental media for majority of diamonds and their primary inclusions was proven based on experimental studies and field observations (Litvin 2007, 2009; Litvin et al. 2012). Based on the syngenesis criterium of diamond and inclusions, parental (growth) media of deeper mantle diamonds are postulated as carbonate–oxide–silicate–carbon systems in partially molten state at conditions of the lower mantle (Litvin et al. 2014). This is compatible with the mantle-carbonatite version of the upper-mantle diamond genesis.
Study of melting phase relations of the multicomponent carbonates trapped into deeper mantle diamonds is of fundamental importance for characterization of their genesis. Congruent melting of simple carbonates CaCO3, MgCO3, and Na2CO3 and stability of their melts over a wide PT range of the transition zone and lower mantle was earlier revealed by experimental investigations (Spivak et al. 2011, 2012, 2013; Solopova et al. 2013; Litvin et al. 2014). It was also found that molten carbonate–carbon systems are very effective as diamond-forming media. At the same time, the carbonate melts are effective solvents and growth media for oxide and silicate minerals of the transition zone and lower mantle. Multicomponent carbonate melts studied in this work are composed of components of all the carbonate minerals included into deeper mantle diamonds and thus may be considered as representatives of carbonatitic constituents of parental media for the transition zone and lower-mantle diamonds. This is supported by preliminary experiments on diamond crystallization in multicomponent oxide–silicate–carbonate–carbon melts within lower-mantle pressures of 23–26 GPa (Litvin et al. 2014).
Concluding remarks
High-pressure high-temperature experiments on the MgCO3–FeCO3–Na2CO3 and MgCO3–FeCO3–CaCO3–Na2CO3 systems demonstrate (Figs. 1, 3) that multicomponent carbonate melts are completely miscible and stable under the transition zone and lower-mantle conditions. The PT parameters of the partial melting of the system are compatible with the transition zone conditions: The melts starts to form at temperatures noticeably lower than the geothermal ones. The positive superposition of geothermal and primary melting conditions is determining in the formation of the transition zone and lower-mantle diamond-parental melts, nucleation, and mass crystallization of deeper mantle diamonds in the parental carbonatite–silicate–oxide–carbon melts (Litvin et al. 2014).
The multicomponent Mg–Fe–Na- and Mg–Fe–Ca–Na-carbonatitic melts provide perfect environment for deeper mantle diamond formation. As shown in Figs. 1 and 3, carbonates and diamonds may form paragenetic associations at conditions of partial carbonates melting. Thus, it was revealed that temperatures of partial melting of multicomponent systems of carbonate minerals are even lower than the geothermal ones. This may not limit a generation of carbonatite parental media of deeper mantle diamonds. High capability for nucleation and mass crystallization of the multicomponent carbonatite–carbon melts studied for the formation of deeper mantle diamonds is demonstrated in high-pressure high-temperature experiments.
References
Akaogi M (2007) Phase transitions of minerals in the transition zone and upper part of the lower mantle. In: Ohtani E (ed) Advances in high-pressure mineralogy. Geological society of America special paper 421. Geological society of America, Boulder, pp 1–13
Boulard E, Gloter A, Corgne A, Antonangeli D, Auzende AL, Perrillat JP, Guyot F, Fiquet G (2011) New host for carbon in the deep Earth. PNAS 108(13):5184–5187. doi:10.1073/pnas.1016934108
Brenker FE, Vollmer C, Vincze L, Vekemans B, Szymanski A, Janssens K, Szaloki I, Nasdala L, Kaminsky F (2007) Carbonates from the lower part of transition zone or even the lower mantle. EPSL 260:1–9. doi:10.1016/j.epsl.2007.02.038
Bundy FP, Basset WA, Weathers MS, Hemley RJ, Mao H-K, Goncharov AF (1996) The pressure–temperature phase and transformation diagram for carbon updated through 1994. Carbon 34:141–153
Dasgupta R, Hirschmann MM (2010) The deep carbon cycle and melting in Earth’s interior. EPSL 298:1–13. doi:10.1016/j.epsl.2010.06.039
Fiquet G, Guyot F, Kunz M, Matas J, Andrault D, Hanfland M (2002) Structural refinements of magnesite at very high pressure. Am Miner 87:1261–1265
Frost DJ, Poe BT, Tronnes RG, Libske C, Duba F, Rubie DC (2004) A new large-volume multianvil system. PEPI 143:507–514. doi:10.1016/j.pepi.2004.03.003
Harte B (2010) Diamond formation in the deep mantle: the record of mineral inclusions and their distribution in relation to mantle dehydration zones. Mineral Mag 74(2):189–215. doi:10.1180/minmag.2010.074.2.189
Isshiki M, Irifune T, Hirose K, Ono S, Ohishi Y, Watanuki T, Nishibori E, Takata M, Sakata M (2004) Stability of magnesite and its high-pressure form in the lowermost mantle. Nature 427:60–63. doi:10.1038/nature02181
Kaminsky F (2012) Mineralogy of the lower mantle: a review of ‘super-deep’ mineral inclusions in diamond. Earth Sci Rev 110:127–147. doi:10.1016/j.earscirev.2011.10.005
Litvin YuA (2009) The physicochemical conditions of diamond formation in the mantle matter: experimental studies. Russian Geol Geophys 50(12):1188–1200. doi:10.1016/j.rgg.2009.11.017
Litvin YuA (2007) High-pressure mineralogy of diamondgenesis. In: Ohtani E (ed) Advances in high-pressure mineralogy. Geological society of America special paper 241. Geological society of America, Boulder, pp 83–103
Litvin YuA, Litvin VYu, Kadik AA (2008) Experimental characterization of diamond crystallization in melts of mantle silicate–carbonate–carbon systems at 7.0–8.5 GPa. Geochem Intern 46(6):531–553. doi:10.1134/S0016702908060013
Litvin YuA, Vasiliev PG, Bobrov AV, Okoemova VYu, Kuzyura AV (2012) Parental media of natural diamonds and primary mineral inclusions in them: evidence from physicochemical experiment. Geochem Int 50(9):726–759. doi:10.1134/S0016702912070051
Litvin YuA, Spivak AV, Solopova NA, Dubrovinsky LS (2014) On origin of lower-mantle diamonds and their primary inclusions. PEPI 228:176–185. doi:10.1016/j.pepi.2013.12.007
McCammon C, Hutchison MT, Harris JW (1997) Ferric iron content of mineral inclusions in diamonds from São Luiz: a view into the lower mantle. Science 278:434–436. doi:10.1126/science.278.5337.434
Merlini M, Crichton W, Hanfland M, Gemmi M, Mueller H, Kupenko I, Dubrovinsky L (2012) Structures of dolomite at ultrahigh pressure and their influence on the deep carbon cycle. Proc Natl Acad Sci USA 109(34):13509–13514. doi:10.1073/pnas.1201336109
Santillan J, Williams Q (2004a) A high pressure X-ray diffraction study of aragonite and the post-aragonite phase transition in CaCO3. Am Miner 89:1348–1352
Santillan J, Williams Q (2004b) A high-pressure infrared and X-ray study of FeCO3 and MnCO3: comparison with CaMg(CO3)2-dolomite. PEPI 143:291–304. doi:10.1016/j.pepi.2003.06.007
Skorodumova NV, Belonoshko AB, Huang L, Ahuja R, Johansson B (2005) Stability of the MgCO3 structures under lower mantle conditions. Am Miner 90:1008–1011. doi:10.2138/am.2005.1685
Solopova NA, Litvin YuA, Spivak AV, Dubrovinskaia NA, Dubrovinsky LS, Urusov VS (2013) Phase diagram of Na-carbonate, the alkaline component of growth media of the super-deep diamond. Dokl Earth Sci 453(1):1106–1109. doi:10.1134/S1028334X13110068
Spivak AV, Dubrovinskii LS, Litvin YuA (2011) Congruent melting of calcium carbonate in a static experiment at 3500 K and 10–22 GPa: its role in the genesis of ultra-deep diamonds. Dokl Earth Sci 439(2):1171–1174. doi:10.1134/S1028334X11080319
Spivak AV, Litvin YuA, Ovsyannikov SV, Dubrovinskaia N, Dubrovinsky LS (2012) Stability and breakdown of Ca13CO3 melt associated with formation of 13C-diamond in high-pressure static experiments up to 43 GPa and 3900 K. J Solid State Chem 19:102–106. doi:10.1016/j.jssc.2012.02.041
Spivak AV, Solopova NA, Litvin YuA, Dubrovinsky LS (2013) Carbonate melts at lower mantle conditions: to superdeep diamonds genesis. Mineral J (Ukraine) 35(2):73–80 in Russian
Stacey FD (1992) Physics of the earth, 3rd edn. Brookfield Press, Brisbane
Stagno V, Tange Y, Miyajima N, McCammon CA, Irifune T, Frost DJ (2011) The stability of magnesite in the transition zone and the lower mantle as function of oxygen fugacity. Geophys Res Lett 38:L19309. doi:10.1029/2011GL049560
Acknowledgments
This work was funded by Program 12P/2 of Russian Academy of Sciences and Grants RFBR 13-05-00835, 14-05-31142.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spivak, A., Solopova, N., Dubrovinsky, L. et al. Melting relations of multicomponent carbonate MgCO3–FeCO3–CaCO3–Na2CO3 system at 12–26 GPa: application to deeper mantle diamond formation. Phys Chem Minerals 42, 817–824 (2015). https://doi.org/10.1007/s00269-015-0765-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-015-0765-6