Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive and irreversible pulmonary interstitial disease that seriously affects the patient’s quality of life and lifespan. The pathogenesis of IPF has not been clarified, and its treatment is limited to pirfenidone and nintedanib, which only delays the decline of lung function. Alveolar epithelial type 2 (AT2) cells are indispensable in the regeneration and lung surfactant secretion of alveolar epithelial cells. Studies have shown that AT2 cell dysfunction initiates the occurrence and progression of IPF. This review expounds on the AT2 cell dysfunction in IPF, involving senescence, apoptosis, endoplasmic reticulum stress, mitochondrial damage, metabolic reprogramming, and the transitional state of AT2 cells. This article also briefly summarizes potential treatments targeting AT2 cell dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common and fatal idiopathic interstitial pneumonia. The median survival time from diagnosis is 2–4 years [1]. Because of the unclear pathogenesis, limited treatment drugs are available. Pirfenidone and nintedanib, antifibrotic medicines approved by FDA, were shown to slow down the forced vital capacity (FVC) reduction rate, but they do not improve the survival rate of patients [2]. Better understanding of the pathogenesis of IPF will lead to the development of new treatment strategies and medications.
In Europe and North America, the incidence of IPF is between 2.8 and 19 cases per 100,000 people per year, and the prevalence of IPF increases with age. Disease onset is usually at 60 years old, and the peak of disease occurs between 60 and 70 years of age [3]. IPF is an aging-related disease, and AT2 cell aging participates in the occurrence of IPF [4]. AT2 cells in IPF patients show mitochondrial stress, endoplasmic reticulum stress, and gene mutation. Recent studies have also identified metabolic reprogramming and paracrine changes in AT2 cells of IPF patients [5, 6]. Compared with AT2 cells directly transforming into mesenchymal cells, the transformation of fibroblasts into muscle fibroblasts caused by the paracrine signals of AT2 cells is more important to IPF [6]. This article summarizes the role of AT2 cells in IPF from two aspects: the dysfunction of AT2 cells in IPF and the change of signaling between AT2 cells and surrounding cells (mesenchymal cells, macrophages) during IPF (Table 1).
AT2 Cell Senescence or Apoptosis is Dominant in IPF
Mutations in telomerase and telomere genes can lead to abnormal telomere shortening. Clinically, this molecular abnormality is manifested as telomere syndromes characterized by aging. IPF is the most common clinical manifestation of human telomere syndrome [31]. In IPF patients, AT2 cells have the shortest telomere length compared with club cells and myofibroblasts; AT2 cells also show the most serious DNA damage, which is mainly caused by telomere shortening [32]. AT2 cells in mice with telomeric repeat-binding factor 2 (Trf2) gene knockout exhibit decreased proliferation and differentiation ability, but apoptosis was not increased, suggesting that telomere damage in AT2 cells preferentially initiates the cell aging process and damages cell renewal and differentiation ability. AT2 cells with Trf2 gene deletion show upregulated p53 expression through the paracrine pathway, induce mesenchymal cell apoptosis, and hinder normal lung development in mice [7]. Bmi-1 deficiency leads to the accumulation of ROS and cytoplasmic p16, which induces AT2 cell senescence and senescence-associated phenotype (SASP) via the TGF-β1/IL-11/MEK-ERK (TIME) pathway [8]. Sin3a gene deletion also induces cell cycle arrest and aging of AT2 cells by activating the p53-p21 axis, which promotes the occurrence of pulmonary fibrosis [9].
AT2 cell apoptosis in IPF is also a research hotspot. Studies have found that specific depletion of AT2 cells induces pulmonary fibrosis in mice. At present, there are three models of AT2 cell-specific injury: the murine surfactant protein C (SPC) promoter and the diphtheria toxin receptor gene (SPC-DTR) mouse model induced by diphtheria toxin; the tamoxifen-inducible SPC-CreER mouse model, which drives the expression of diphtheria toxin A in AT2 cells; and the ganciclovir-induced transgenic mouse model, in which the SPC promoter drives expression of mutant SR39TK herpes simplex virus-1 thymidine kinase in AT2 cells [33,34,35]. TSPAN1 is a member of the tetraspanins family and is downregulated in lung tissue of patients with IPF and bleomycin-induced pulmonary fibrosis mice. TSPAN1 inhibited AT2 cell apoptosis by inhibiting p-IκBα, which attenuated nuclear NF-κB translocation and activation [10]. The apoptotic sensitivity of AT2 cells increases, while the apoptotic sensitivity of fibroblasts decreases in the lung of aged mice, which is related to the fact that plasma activator inhibitor 1 (PAI-1) can positively or negatively regulate the phosphorylation and expression of p53 according to cell type [11].
Endoplasmic Reticulum Stress of AT2 Cells Promotes IPF
GRP78 is one of the important modifiers of controlling protein quality by activating the unfolded protein response (UPR) in the endoplasmic reticulum. Mice with Grp78 gene knockout develop spontaneous pulmonary fibrosis, and AT2 cells with Grp78 gene knockout are characterized by endoplasmic reticulum stress, apoptosis, senescence, and impaired stem cell ability [12]. Endoplasmic reticulum stress inhibitors decrease the expression of markers of cell aging, apoptosis, and mesenchymal cells [12]. The UPR maintains protein homeostasis by enhancing the ability of the ER to refold proteins and reducing the translation of abnormal proteins [36]. Fkbp13 (13-kD FK506-binding protein) level positively correlated with the UPR marker GRP78 and total XBP1. Fkbp13 knockout mice were more sensitive to bleomycin and showed increased early inflammatory cells (macrophages, neutrophils, lymphocytes) and proinflammatory factors (IL-6, TGFβ-1) [13]. In mice harboring L188Q mutation in SFTPC, which encodes SPC, ER stress is induced but the mice do not show spontaneous fibrosis; upon exposure to bleomycin, much greater lung fibrosis was observed compared with fibrosis in WT mice [37]. In the SFTPC BRICHOS mouse model with C121G mutation in SFTPC, mice show high levels of ER stress in AT2 cells and spontaneous pulmonary fibrosis [38]. These models indicate ER stress as a key driver of lung fibrosis.
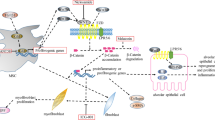
Excessive ER stress damages the signal network with mitochondria and causes mitochondrial dysfunction (Fig. 1). ER stress of AT2 cells impairs mitophagy by activating transcription factor 3 (ATF3) and repressing transcription of the PTEN-induced putative kinase 1 (PINK1) gene [14]. Mitophagy dysfunction promotes AT2 cell aging and IPF [39]. When UPR cannot be compensated due to persistent ER stress, ER-mitochondrial tethering will decrease, and the expression of transient receptor potential cation channel subfamily V member 1 (TRPV1) and phosphofulin acid cluster sorting protein 2 (PACS2) are downregulated. The deletion of Pacs2 has been proven to induce the cleavage of BAP31 to lead to mitochondrial-dependent apoptosis. In vitro and ex vivo experiments showed that ER-mitochondrial tethering induced by ER stress was related to the PACS2-TRPV1 axis [40]. The TRPV1-modulating drug capsaicin (CPS) inhibited the degradation of TRPV1, increased the level of PACS2 protein, and reduced the apoptosis of alveolar epithelial cells and collagen expression [15].
Mitochondrial Damage and Metabolism Reprogramming of AT2 Cells Participate in IPF
Mitochondrial DNA (mtDNA) and mitophagy damage are critical factors in the process of IPF (Fig. 1). Cigarette smoke (CS) may cause mitochondrial reactive oxygen species (mitoROS), mitoROS-induced DNA damage activates poly ADP-ribose polymerase (PARP1), which competitively depletes nicotinamide adenine dinucleotide (NAD+) with SIRT1. Suppressed SIRT1 leads to a lack of regulation of mitophagy, resulting in DNA damage and AT2 cell senescence. Mitophagy damage in turn leads to mitoROS, resulting in a positive feedback between mitophagy and SIRT1 [16]. SIRT1 activator and mitoROS scavenger can inhibit CS-induced AT2 cell senescence and pulmonary fibrosis [16].
SIRT3 also inhibits ROS-induced damage to mitochondrial DNA by preventing acetylation of 8-oxoguanine DNA glycosylase (OGG1). Deacetylation of OGG1 is essential for maintaining DNA integrity by preventing mitophagy from being damaged [17]. OGG1 can prevent mitophagy dysfunction caused by PINK1 deficiency to avoid AT2 cell aging and alleviate IPF [39]. SIRT7 participates in the mitochondrial UPR, which is important for AT2 cell homeostasis [41]. Klotho is an anti-aging gene and Klotho indirectly prevents lung fibrosis through lessening mtDNA damage and apoptosis of AT2 cells [18, 42, 43]. Klotho promotes FGFR1 binding to FGF23 and actives AKT signaling to prevent apoptosis of AT2 cells caused by mtDNA damage [44, 45].
Studies have shown that MLE12 exhibits mitochondrial respiratory inhibition after bleomycin induction for 3 h, specifically manifested as the decline in oxidative phosphorylation. Mitochondrial respiratory inhibition is accompanied by DNA damage (γ- H2AX is upregulated). The glycolysis of cells induced by bleomycin is also inhibited, which involves the significant decrease of the expression of glucose uptake and transport 1 (GLUT1), the decrease of the exchange rate of intermediate products of the tricarboxylic acid cycle, and the decrease of extracellular acidification rate (ECAR) [46]. Another study showed that iAEC2s with SFTPCI73T gene mutation exhibit damaged oxidative phosphorylation and higher glycolysis [5].
In addition, inhibiting glutamine metabolism can inhibit the proliferation and differentiation of AT2 cells. The specific mechanism may be that the inhibition of glutamine metabolism reduces α-KG production, which is the most important intermediate product in the tricarboxylic acid cycle. The impaired TCA cycle and limited ATP are not conducive to the regeneration of damaged cells [47]. Glutamine can maintain various intermediates of the TCA cycle and pentose phosphate pathway, glycolytic intermediates, and almost all amino acids. Bleomycin-damaged MLE12 can restore cell mitochondrial respiration and ECRA after supplementing glutamine [46]. Glutamine can also reduce the cytotoxicity of bleomycin, because glutamine and α-KG play important roles in nucleotide synthesis and DNA repair, respectively [48, 49].
Transitional State of AT2 Cells in IPF
A previous study identified the presence of aberrant basaloid cells, which is a previously unidentified epithelial cell population, that coexpress basal epithelial markers, mesenchymal markers, aging markers, developmental transcription factors, and known IPF markers [50]. AT2 cells in the IPF lung can also differentiate into KRT5+ basal cells in response to fibrotic signaling. TGFβ-1 and anti-bone morphogenic protein (anti-BMP) promote this transdifferentiation [51]. In models of progressive lung fibrosis and human IPF patients, AT2 cells can differentiate into Krt8+ alveolar differentiation intermediate (ADI), which can reprogram into AT1 cells [52]. RhoGTPase Cdc42 depletion leads to an accumulation of cells in the AT2 intermediate cell state, which prevents AT2 cells from differentiating into AT1 cells. This impaired regenerative ability causes AT2 cells to be exposed to persistently elevated mechanical tension, activates the TGF-β signal in AT2 cells, and promotes periphery-to-center progression of lung fibrosis [19]. Another study showed that there was accumulation of pre-alveolar type-1 transitional cell state (PATS), an intermediate cell state during the differentiation of AT2 cells into AT1 cells, in the pulmonary fibrosis area of IPF patients. PATS cells in human lungs exhibit enrichment of genes associated with cellular senescence, TP53 signaling, and TGF-β-regulated genes. Moreover, PATS cells are vulnerable to DNA damage during the process of differentiation from AT2 cells to AT1 cells (Fig. 2). Long-term aging and stress regulatory signals in transitional cells will promote the occurrence of fibrosis [20].
AT2 cells without Nedd4-2 can also lead to epithelial remodeling of the peripheral airway, mainly manifested as decreased club cells, increased ciliated cells and goblet cells in local and terminal airways. The expression of MUC5B in proximal and distal airways is also increased. Deletion of Nedd4-2 in AT2 cells increases the activity of ENaC, which leads to the consumption of airway surface fluid and decreases mucociliary clearance [21]. The lncRNA mir-100-let-7a-2-mir-125b-1 cluster host gene (MIR100HG) modulates TGF-β1-induced fibrotic changes in AT2 cells. It can increase the expression of TGF-β-activated kinase 1/MAP3K7 binding protein 1 (Tab1) by inhibiting microRNA-29a-3p (mir-29a-3p), promote the production of TIMP-1, and inhibit the degradation of collagen by MMP-1 [22, 23]. Mir-29 can also activate the Wnt/β-catenin pathway to promote TGF-β1-induced extracellular matrix synthesis [53].
Paracrine Signals in AT2 Cells Contribute to IPF
Some studies have pointed out that only a small part of fibroblasts in fibrotic lesions come from AT2 cells [6]. Compared with the direct transformation of AT2 cells into mesenchymal cells, the transformation from fibroblasts to myofibroblasts caused by the imbalance of paracrine signals is more important to EMT (Fig. 2). EGFR-RAS-ERK signaling in AT2 cells upregulates transcription regulator zinc finger E-box-binding homeobox 1 (ZEB1) and promotes AT2 cells to secrete tissue plasminogen activator (tPA), which can enhance TGF-β-induced myofibroblast differentiation [24]. AT2 cells can secrete TGF-β2 after injury, which highlights the role of paracrine signals between AT2 cells and fibroblasts in mediating EMT [24]. Fibroblasts activated by TGF-β can secrete secreted protein acidic and rich in cysteine (SPARC), which can activate EGFR-RAS signal transduction in AT2 cells. Thus, the bidirectional epithelial–mesenchymal crosstalk promotes the progression of fibrosis [54]. Studies have shown that AT2 cells in IPF patients have not completely lost the ability to differentiate into AT1 cells. PDGFA ligands activate beneficial feedback of the epithelial–mesenchymal crosstalk by promoting PDGFRα+ fibroblast differentiation into beneficial PDGFRA+ matrix fibroblasts. PDGFRA+ matrix fibroblasts promote the differentiation of AT2 cells into AT1 cells [25]. A specific balance between the activation of the PDGFA ligand and inhibition of the PDGF-B ligand may enhance alveolar repair [25]. These findings suggest that the changes in signal connections between AT2 cells themselves or between AT2 cells and mesenchymal cells are involved in the occurrence of pulmonary fibrosis.
The developmental transcription factor Sine oculis homeobox homolog 1 (SIX1) plays an important role in lung development [55]. The mRNA and protein levels of Six1 and its transcriptional coactivators (EYA1 and EYA2) were increased in AT2 cells of bleomycin-induced pulmonary fibrosis model mice and telomere dysfunction pulmonary fibrosis model mice [26, 56]. The mechanism of Six1 in promoting the progression of pulmonary fibrosis may be related to the downstream macrophage migration inhibitory factor (MIF). SIX1 directly binds to the 5′-TCAGG-3′ consensus sequence of the MIF promoter, and MIF then promotes the proliferation of fibroblasts and the expression of α-SMA and COL1A1 [26]. The treatment of pulmonary fibrosis targeting MIF, mainly in the form of anti-MIF antibody therapy and MIF antagonist, is still under study [57, 58].
Inflammation activated by AT2 cells aggravates IPF
iACE2s with SFTPCI73T mutation activates the NF-κB pathway and promote the secretion of inflammatory mediators (GM-CSF, CXCL5, and MMP-1) [5]. Activated WNT/β-catenin signal in AT2 cells also induces downstream IL-1β and IL-6, which activates TGF-β or STAT3 signal transduction, respectively [59]. Macrophage colony-stimulating factor (M-CSF) stimulates monocyte macrophages to produce CC chemokine ligand 2 (CCL2), which is involved in the occurrence of IPF [60]. The number of macrophages and neutrophils in alveolar lavage fluid and the secretion of IL-6 and monocyte chemoattractant protein 1 (MCP-1) are increased in pulmonary fibrosis mice induced by GRP78 knockout [12].
The interaction between AT2 cells and immune cells such as pulmonary macrophages is also involved in pulmonary fibrosis. Lrrk2 deletion can not only damage autophagy through ERK and JNK signaling pathways of AT2 cells, but also leads to activation of the CCL2/CCR2 axis and recruitment of monocyte-derived macrophages [27]. Fibrosis induced by the MCP-1/CCR2/TGF-β axis was also present between injured AT2 cells and macrophages [28]. AT2 cells can also secrete Sonic hedgehog (Shh), initiate the Shh/Gli signal cascade, induce macrophages to secrete osteopontin, activate the JAK2/STAT3 pathway, mediate M2 polarization of macrophages, and promote pulmonary fibrosis [29]. Apoptosis resistance of pulmonary macrophages promotes the occurrence of pulmonary fibrosis. The mitochondrial calcium uniporter induces metabolic reprogramming to fatty acid β-oxidation and promotes the binding of carnitine palmitoyltransferase 1a (CPT1A) in mitochondria to the BH3 domain of mitochondrial B-cell lymphoma-2 (Bcl-2), decreases the proapoptotic proteins (Puma and Noxa), and inhibits the apoptosis of pulmonary macrophages. The interaction between CPT1A and Bcl-2 increases TGF-β1 in pulmonary macrophages, reduces antifibrosis protein (TNF-α), and promotes pulmonary fibrosis [30].
Potential Therapy Targeting AT2 Cells
Senescence and Regeneration
Dasatinib plus quercetin (DQ) can reduce aging markers in lung tissue of Sin3a-deficient mice and alleviate pulmonary fibrosis [9]. The clinical trial of DQ in the treatment of IPF shows that this drug can improve the physical function of patients [61]. Nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme in the production of NAD+, and abnormal metabolism of NAMPT occurs in some aging-related diseases [62]. Research has found that MSCs inhibit AT2 cell aging by inhibiting lysosome-mediated degradation of NAMPT [63]. Human clinical trials have shown that a high-dose of allogeneic MSCs can delay the progress of IPF patients [64]. Recent studies propose that serum-free cultured MSCs (SF-MSCs) are easier to implant into mouse lungs after intravenous administration than serum cultured MSCs (S-MSCs). The antifibrosis effect of SF-MSCs is more pronounced compared with S-MSCs [65]. Lung niche mesenchymal cells promote the repair and regeneration of AT2 cells by secreting growth hormones, Wnt5A and chemokines. Ghr-enriched EVs promote the expression of Ghr in AT2 cells and the regeneration of AT2 cells [66].
ER Stress
Tauroursodeoxycholic acid (TUDCA) reduces ER stress markers (GRP94 and CHOP) as well as mesenchymal markers [12]. TUDCA inhibits BLM-induced CHOP mRNA expression in a dose-dependent manner and presents protective effects against BLM-induced pulmonary fibrosis in mice [67]. Calcium and calmodulin-dependent kinase II (CaMKII) inhibition blocks ER stress and apoptosis induced by bleomycin in MLE12 cells. A transgenic mouse model of CaMKII inhibition in type II pneumocytes exhibited a lower degree of pulmonary fibrosis than WT mice [68].
Mitochondrial Damage
MitoROS is involved in CS-induced senescence of AT2 cells. Mitochondria-targeted antioxidant mitoquinone (MitoQ) protects mice from CS-induced pulmonary fibrosis. SIRT1 activator and supplementation of NAD with its precursors can restore SIRT1 activity, prevent AT2 cell senescence, and inhibit CS-induced pulmonary fibrosis [16]. Thyroid hormone restores mitochondrial respiration in bleomycin-induced AT2 cells and limits the severity of pulmonary fibrosis in mice [69].
Conclusion and Future Insights
The pathogenesis of IPF mainly involves cell aging and apoptosis, mitochondria and endoplasmic reticulum dysfunction, abnormality of AT2 cell transitional state, paracrine signals in AT2 cells and inflammatory response. Mitochondria and endoplasmic reticulum oxidative stress, which can damage mitochondrial DNA, mitochondrial autophagy and the UPR, promote AT2 cell aging and apoptosis. The transformation of fibroblasts into myofibroblasts through the paracrine pathway of AT2 cells and AT2 cell transitional state also participate in IPF. The study on the pathogenesis of IPF should not be limited to AT2 cells themselves, and the paracrine effect on surrounding cells should also be studied. More potential therapies targeting AT2 cells including DQ, TUDCA, MitoQ, and Ghr-enriched EVs need to be further explored.
References
Lederer DJ, Martinez FJ (2018) Idiopathic pulmonary fibrosis. N Engl J Med 379(8):797–798. https://doi.org/10.1056/NEJMc1807508
Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J et al (2015) An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 192(2):3–19
Olson AL, Gifford AH, Inase N, Fernandez PER, Suda T (2018) The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur Respir Rev 27(150):180077
Parimon T, Yao C, Stripp BR, Noble PW, Chen P (2020) Alveolar epithelial type II cells as drivers of lung fibrosis in idiopathic pulmonary fibrosis. Int J Mol Sci 21(7):2269
Alysandratos K-D, Russo SJ, Petcherski A, Taddeo EP, Acin-Perez R, Villacorta-Martin C et al (2021) Patient-specific iPSCs carrying an SFTPC mutation reveal the intrinsic alveolar epithelial dysfunction at the inception of interstitial lung disease. Cell Rep. https://doi.org/10.1016/j.celrep.2021.109636
Tan W, Wang Y, Chen Y, Chen C (2021) Cell tracing reveals the transdifferentiation fate of mouse lung epithelial cells during pulmonary fibrosis in vivo. Exp Ther Med. https://doi.org/10.3892/etm.2021.10622
Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM et al (2015) Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA 112(16):5099–5104. https://doi.org/10.1073/pnas.1504780112
Chen H, Chen H, Liang J, Gu X, Zhou J, Xie C et al (2020) TGF-beta1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp Mol Med 52(1):130–151
Yao C, Guan X, Carraro G, Parimon T, Liu X, Huang G et al (2021) Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med 203(6):707–717. https://doi.org/10.1164/rccm.202004-1274OC
Yang L, Wang Y, Pan Z, Gao S, Zou B, Lin Z et al (2018) Tetraspanin 1 inhibits TNFα-induced apoptosis via NF-κB signaling pathway in alveolar epithelial cells. Inflamm Res 67(11–12):951–964. https://doi.org/10.1007/s00011-018-1189-9
Jiang C, Liu G, Cai L, Deshane J, Antony V, Thannickal VJ et al (2021) Divergent regulation of alveolar type 2 cell and fibroblast apoptosis by plasminogen activator inhibitor 1 in lung fibrosis. Am J Pathol 191(7):1227–1239. https://doi.org/10.1016/j.ajpath.2021.04.003
Borok Z, Horie M, Flodby P, Wang H, Liu Y, Ganesh S et al (2020) Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. Am J Respir Crit Care Med 201(2):198–211
Tat V, Ayaub EA, Ayoub A, Vierhout M, Naiel S, Padwal MK et al (2021) FK506-binding protein 13 expression is upregulated in interstitial lung disease and correlated with clinical severity. A potentially protective role. Am J Respir Cell Mol Biol 64(2):235–246. https://doi.org/10.1165/rcmb.2020-0121OC
Bueno M, Brands J, Voltz L, Fiedler K, Mays B et al (2018) ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell 17(2):e12720
Knoell J, Chillappagari S, Knudsen L, Korfei M, Dartsch R, Jonigk D et al (2022) PACS2-TRPV1 axis is required for ER-mitochondrial tethering during ER stress and lung fibrosis. Cell Mol Life Sci 79(3):151. https://doi.org/10.1007/s00018-022-04189-2
Zhang Y, Huang W, Zheng Z, Wang W, Yuan Y, Hong Q et al (2021) Cigarette smoke-inactivated SIRT1 promotes autophagy-dependent senescence of alveolar epithelial type 2 cells to induce pulmonary fibrosis. Free Radic Biol Med 166:116–127
Bindu S, Pillai VB, Kanwal A, Samant S, Mutlu GM, Verdin E et al (2017) SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage. Am J Physiol Lung Cell Mol Physiol 312(1):L68–L78. https://doi.org/10.1152/ajplung.00188.2016
Kim SJ, Cheresh P, Eren M, Jablonski RP, Yeldandi A, Ridge KM et al (2017) Klotho, an antiaging molecule, attenuates oxidant-induced alveolar epithelial cell mtDNA damage and apoptosis. Am J Physiol Lung Cell Mol Physiol 313(1):L16–L26
Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z et al (2020) Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell 180(1):107. https://doi.org/10.1016/j.cell.2019.11.027
Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J et al (2020) Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol 22(8):934–946. https://doi.org/10.1038/s41556-020-0542-8
Duerr J, Leitz DHW, Szczygiel M, Dvornikov D, Fraumann SG, Kreutz C et al (2020) Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat Commun 11(1):2012. https://doi.org/10.1038/s41467-020-15743-6
Guan S, Liu H, Zhou J, Zhang Q, Bi H (2022) The MIR100HG/miR-29a-3p/Tab1 axis modulates TGF-beta1-induced fibrotic changes in type II alveolar epithelial cells BLM-caused lung fibrogenesis in mice. Toxicol Lett. https://doi.org/10.1016/j.toxlet.2022.04.003
Ciechomska M, O’Reilly S, Suwara M, Bogunia-Kubik K, van Laar JM (2014) MiR-29a reduces TIMP-1 production by dermal fibroblasts via targeting TGF-beta activated kinase 1 binding protein 1, implications for systemic sclerosis. PLoS ONE 9(12):e115596. https://doi.org/10.1371/journal.pone.0115596
Yao L, Conforti F, Hill C, Bell J, Drawater L, Li J et al (2019) Paracrine signalling during ZEB1-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in lung fibrosis. Cell Death Differ 26(5):943–957. https://doi.org/10.1038/s41418-018-0175-7
Gokey JJ, Snowball J, Green J, Waltamath M, Spinney JJ, Black KE et al (2021) Pretreatment of aged mice with retinoic acid supports alveolar regeneration via upregulation of reciprocal PDGFA signalling. Thorax 76(5):456–467
Wilson C, Mertens TC, Shivshankar P, Bi W, Collum SD, Wareing N et al (2022) Sine oculis homeobox homolog 1 plays a critical role in pulmonary fibrosis. JCI Insight. https://doi.org/10.1172/jci.insight.142984
Tian Y, Lv J, Su Z, Wu T, Li X, Hu X et al (2021) LRRK2 plays essential roles in maintaining lung homeostasis and preventing the development of pulmonary fibrosis. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2106685118
Young LR, Gulleman PM, Short CW, Tanjore H, Sherrill T, Qi A et al (2016) Epithelial–macrophage interactions determine pulmonary fibrosis susceptibility in Hermansky–Pudlak syndrome. JCI Insight 1(17):e88947. https://doi.org/10.1172/jci.insight.88947
Hou J, Ji J, Chen X, Cao H, Tan Y, Cui Y et al (2021) Alveolar epithelial cell-derived Sonic hedgehog promotes pulmonary fibrosis through OPN-dependent alternative macrophage activation. FEBS J 288(11):3530–3546. https://doi.org/10.1111/febs.15669
Gu L, Surolia R, Larson-Casey JL, He C, Davis D, Kang J et al (2022) Targeting Cpt1a-Bcl-2 interaction modulates apoptosis resistance and fibrotic remodeling. Cell Death Differ 29(1):118–132. https://doi.org/10.1038/s41418-021-00840-w
Armanios M (2013) Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest 123(3):996–1002. https://doi.org/10.1172/JCI66370
van Batenburg AA, Kazemier KM, van Oosterhout MFM, van der Vis JJ, Grutters JC, Goldschmeding R et al (2021) Telomere shortening and DNA damage in culprit cells of different types of progressive fibrosing interstitial lung disease. ERJ Open Res. https://doi.org/10.1183/23120541.00691-2020
Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR et al (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123(7):3025–3036. https://doi.org/10.1172/JCI68782
Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A et al (2010) Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181(3):254–263. https://doi.org/10.1164/rccm.200810-1615OC
Garcia O, Hiatt MJ, Lundin A, Lee J, Reddy R, Navarro S et al (2016) Targeted type 2 alveolar cell depletion. A dynamic functional model for lung injury repair. Am J Respir Cell Mol Biol 54(3):319–330. https://doi.org/10.1165/rcmb.2014-0246OC
Pfaffenbach KT, Lee AS (2011) The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol 23(2):150–156
Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC et al (2011) Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 108(26):10562–10567. https://doi.org/10.1073/pnas.1107559108
Katzen J, Wagner BD, Venosa A, Kopp M, Tomer Y, Russo SJ et al (2019) An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis. JCI Insight. https://doi.org/10.1172/jci.insight.126125
Kim SJ, Cheresh P, Jablonski RP, Rachek L, Yeldandi A, Piseaux-Aillon R et al (2020) Mitochondrial 8-oxoguanine DNA glycosylase mitigates alveolar epithelial cell PINK1 deficiency, mitochondrial DNA damage, apoptosis, and lung fibrosis. Am J Physiol Lung Cell Mol Physiol 318(5):L1084–L1096
Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y et al (2005) PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J 24(4):717–729. https://doi.org/10.1038/sj.emboj.7600559
Weng H, Ma Y, Chen L, Cai G, Chen Z, Zhang S et al (2020) A new vision of mitochondrial unfolded protein response to the sirtuin family. Curr Neuropharmacol 18(7):613–623. https://doi.org/10.2174/1570159X18666200123165002
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51
Mencke R, Hillebrands JL (2017) The role of the anti-ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res Rev 35:124–146. https://doi.org/10.1016/j.arr.2016.09.001
Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC et al (2018) alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553(7689):461–466
Erben RG (2018) alpha-Klotho’s effects on mineral homeostasis are fibroblast growth factor-23 dependent. Curr Opin Nephrol Hypertens 27(4):229–235
Shaghaghi H, Para R, Tran C, Roman J, Ojeda-Lassalle Y, Sun J et al (2021) Glutamine restores mitochondrial respiration in bleomycin-injured epithelial cells. Free Radic Biol Med 176:335–344. https://doi.org/10.1016/j.freeradbiomed.2021.10.006
Wang S, Li X, Ma Q, Wang Q, Wu J, Yu H et al (2022) Glutamine metabolism is required for alveolar regeneration during lung injury. Biomolecules. https://doi.org/10.3390/biom12050728
Yoo HC, Yu YC, Sung Y, Han JM (2020) Glutamine reliance in cell metabolism. Exp Mol Med 52(9):1496–1516. https://doi.org/10.1038/s12276-020-00504-8
Tran TQ, Lowman XH, Kong M (2017) Molecular pathways: metabolic control of histone methylation and gene expression in cancer. Clin Cancer Res 23(15):4004–4009. https://doi.org/10.1158/1078-0432.CCR-16-2506
Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F et al (2020) Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv 6(28):eaba1983. https://doi.org/10.1126/sciadv.aba1983
Kathiriya JJ, Wang C, Zhou M, Brumwell A, Cassandras M, Le Saux CJ et al (2022) Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5(+) basal cells. Nat Cell Biol 24(1):10–23. https://doi.org/10.1038/s41556-021-00809-4
Strunz M, Simon LM, Ansari M, Kathiriya JJ, Angelidis I, Mayr CH et al (2020) Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun 11(1):3559. https://doi.org/10.1038/s41467-020-17358-3
Wang Y, Liu J, Chen J, Feng T, Guo Q (2015) MiR-29 mediates TGFbeta 1-induced extracellular matrix synthesis through activation of Wnt/beta-catenin pathway in human pulmonary fibroblasts. Technol Health Care 23(Suppl 1):S119–S125. https://doi.org/10.3233/thc-150943
Yao L, Zhou Y, Li J, Wickens L, Conforti F, Rattu A et al (2021) Bidirectional epithelial–mesenchymal crosstalk provides self-sustaining profibrotic signals in pulmonary fibrosis. J Biol Chem 297(3):101096. https://doi.org/10.1016/j.jbc.2021.101096
El-Hashash AH, Al Alam D, Turcatel G, Rogers O, Li X, Bellusci S et al (2011) Six1 transcription factor is critical for coordination of epithelial, mesenchymal and vascular morphogenesis in the mammalian lung. Dev Biol 353(2):242–258. https://doi.org/10.1016/j.ydbio.2011.02.031
Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL et al (2016) Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1(14):e86704. https://doi.org/10.1172/jci.insight.86704
Gunther S, Fagone P, Jalce G, Atanasov AG, Guignabert C, Nicoletti F (2019) Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: from pathogenic factors to therapeutic targets. Drug Discov Today 24(2):428–439. https://doi.org/10.1016/j.drudis.2018.11.003
Gunther S, Bordenave J, Hua-Huy T, Nicco C, Cumont A, Thuillet R et al (2018) Macrophage migration inhibitory factor (MIF) inhibition in a murine model of bleomycin-induced pulmonary fibrosis. Int J Mol Sci. https://doi.org/10.3390/ijms19124105
Aumiller V, Balsara N, Wilhelm J, Günther A, Königshoff M (2013) WNT/β-catenin signaling induces IL-1β expression by alveolar epithelial cells in pulmonary fibrosis. Am J Respir Cell Mol Biol 49(1):96–104. https://doi.org/10.1165/rcmb.2012-0524OC
Baran CP, Opalek JM, McMaken S, Newland CA, O’Brien JM Jr, Hunter MG et al (2007) Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 176(1):78–89. https://doi.org/10.1164/rccm.200609-1279OC
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK et al (2019) Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40:554–563. https://doi.org/10.1016/j.ebiom.2018.12.052
Dahl TB, Holm S, Aukrust P, Halvorsen B (2012) Visfatin/NAMPT: a multifaceted molecule with diverse roles in physiology and pathophysiology. Annu Rev Nutr 32:229–243. https://doi.org/10.1146/annurev-nutr-071811-150746
Lai X, Huang S, Lin S, Pu L, Wang Y, Lin Y et al (2022) Mesenchymal stromal cells attenuate alveolar type 2 cells senescence through regulating NAMPT-mediated NAD metabolism. Stem Cell Res Ther 13(1):12. https://doi.org/10.1186/s13287-021-02688-w
Averyanov A, Koroleva I, Konoplyannikov M, Revkova V, Lesnyak V, Kalsin V et al (2020) First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl Med 9(1):6–16. https://doi.org/10.1002/sctm.19-0037
Takao S, Nakashima T, Masuda T, Namba M, Sakamoto S, Yamaguchi K et al (2021) Human bone marrow-derived mesenchymal stromal cells cultured in serum-free media demonstrate enhanced antifibrotic abilities via prolonged survival and robust regulatory T cell induction in murine bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther 12(1):506. https://doi.org/10.1186/s13287-021-02574-5
Xie T, Kulur V, Liu N, Deng N, Wang Y, Rowan SC et al (2021) Mesenchymal growth hormone receptor deficiency leads to failure of alveolar progenitor cell function and severe pulmonary fibrosis. Sci Adv. https://doi.org/10.1126/sciadv.abg6005
Tanaka Y, Ishitsuka Y, Hayasaka M, Yamada Y, Miyata K, Endo M et al (2015) The exacerbating roles of CCAAT/enhancer-binding protein homologous protein (CHOP) in the development of bleomycin-induced pulmonary fibrosis and the preventive effects of tauroursodeoxycholic acid (TUDCA) against pulmonary fibrosis in mice. Pharmacol Res 99:52–62. https://doi.org/10.1016/j.phrs.2015.05.004
Winters CJ, Koval O, Murthy S, Allamargot C, Sebag SC, Paschke JD et al (2016) CaMKII inhibition in type II pneumocytes protects from bleomycin-induced pulmonary fibrosis by preventing Ca2+-dependent apoptosis. Am J Physiol Lung Cell Mol Physiol 310(1):L86-94. https://doi.org/10.1152/ajplung.00132.2015
Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A et al (2018) Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med 24(1):39. https://doi.org/10.1038/nm.4447
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82000065), Beijing Key Clinical Specialty Construction Project (2020–2022).
Author information
Authors and Affiliations
Contributions
WZ conceived, wrote, and edited the manuscript. CT and JZ conceived and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, W., Tan, C. & Zhang, J. Alveolar Epithelial Type 2 Cell Dysfunction in Idiopathic Pulmonary Fibrosis. Lung 200, 539–547 (2022). https://doi.org/10.1007/s00408-022-00571-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-022-00571-w