Abstract
Introduction
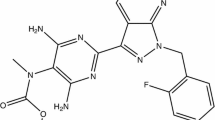
Epoprostenol, a synthetic prostaglandin I2 (PGI2) analog, has been the mainstay of treatment for severe pulmonary arterial hypertension (PAH) for the last two decades. Treprostinil, another synthetic prostaglandin analog, and selexipag, an oral selective Inositol Phosphate (IP) prostacyclin receptor agonist, have also been approved for treatment of PAH. Prostacyclin and its analogs cause a variety of side effects in patients with PAH; however, thyroid dysfunction is rarely reported.
Methods
After treating an index case of thyroid dysfunction occurring after initiation of epoprostenol, we reviewed our databases of PAH patients treated with epoprostenol, treprostinil or selexipag to identify the occurrence of this association.
Results
We identified six cases of thyroid dysfunction in our cohort: five after initiation of an intravenous prostacyclin (epoprostenol) and one after initiation of an oral prostacyclin receptor agonist (selexipag). Four of the patients presented with hyperthyroidism and two with a large autoimmune goiter. Graves’ disease was seen in three patients, Hashimoto’s disease in two patients and thyrotoxicosis in one patient.
Conclusion
Therapy with medications targeting the prostacyclin pathway is a potential risk factor for the development of symptomatic thyroid disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary Arterial Hypertension (PAH) has been defined as a mean pulmonary arterial pressure (mPAP) of ≥ 25 mm of Hg at rest, a mean pulmonary-capillary wedge pressure or left ventricular end-diastolic pressure of ≤ 15 mm Hg, and a pulmonary vascular resistance (PVR) > 3 Wood Units; however, the recent World Pulmonary Hypertension Symposium (WPHS) has suggested changing the diagnostic mPAP to > 20 mm Hg [1]. Although there is no cure for PAH, treatment regimens during recent decades have improved symptoms, exercise tolerance, and survival. Many co-morbid entities have been associated with an increased risk of development of PAH. In this regard, observational studies and case series have reported an increased incidence of thyroid disease in patients at diagnosis of PAH [2,3,4,5,6,7]; as such, screening for thyroid abnormalities is recommended in these patients. Of equal importance, because of the physiological effects, thyroid dysfunction has been associated with poorer outcome in patients with pulmonary hypertension (PH) [2, 8,9,10,11].
Therapies for PAH have fallen into four major classes: endothelin receptor antagonists (ERA), phosphodiesterase type 5 inhibitors (PDE5i), soluble guanylate cyclase stimulators, and prostacyclin analogs [12, 13]. In regard to therapies that affect the prostacyclin pathway, epoprostenol, a synthetic form of prostaglandin I2 or prostacyclin, and treprostinil, a prostaglandin analog similar to epoprostenol, have been shown to improve symptoms and functional class, increase exercise tolerance, and improve pulmonary hemodynamics; however, only epoprostenol has been shown to improve survival [14,15,16,17,18,19,20]. Recently, following a positive pivotal clinical trial, selexipag, an oral non-prostanoid IP receptor agonist, has also been approved for treatment of PAH [21].
Interestingly, one of the less common side effects associated with treatment of PAH with epoprostenol is thyroid dysfunction, especially hyperthyroidism [22, 23]. Reports of thyroid dysfunction with treprostinil or selexipag are even less common [21, 24]. For example, in the GRIPHON trial of selexipag, hyperthyroidism occurred in 8 patients in the treated cohort (~ 1%) and in no patient in the placebo group [21]. However, any occurrence of this side effect can have significant clinical import in these patients and can often be overlooked because of the complex underlying pathophysiology of PAH. In this report, we describe our experience with thyroid abnormalities in six patients who were treated with epoprostenol, treprostinil, or selexipag for PAH.
Materials and Methods
After an index case was observed, we reviewed databases at Boston Medical Center and Houston Methodist Hospital for PAH patients who were treated with epoprostenol (Flolan, GSK or Veletri, Actelion Pharmaceuticals), treprostinil (Remodulin, United Therapeutics), or selexipag (Uptravi, Actelion Pharmaceuticals). For this analysis, PAH was diagnosed in all cases by the previous hemodynamic criteria, not the more recently suggested WPHS criteria. We identified a total of six cases of thyroid dysfunction occurring after initiation of these therapies. Institutional Review Board (IRB) approval was obtained prior to comprehensive chart review. Then, a review of the literature and corporate data were undertaken to define the epidemiology and to attempt to understand the mechanism of this association.
Results
We observed thyroid dysfunction in five patients after initiation of epoprostenol and in one patient after initiation of selexipag. We did not identify thyroid abnormalities in any patient after the initiation of systemic treprostinil in this cohort. In each of the cases, the patient had normal thyroid function prior to the initiation of the drug. The characteristics of these patients are summarized in Table 1.
Epoprostenol Cases
Case 1: A 37-year-old Caucasian female with PAH associated with limited systemic sclerosis treated with epoprostenol was diagnosed with hyperthyroidism during an inpatient admission for suspected tunnel line infection; the entry site cellulitis was treated with IV antibiotics and resolved; all blood cultures were negative. She reported a recent weight loss of 20 lb. and worsening diarrhea for several weeks. Physical exam revealed tachycardia at 120 beats per minute, positive lid lag, and a firm micronodular and mobile thyroid without cervical adenopathy. Laboratory testing confirmed hyperthyroidism: TSH < 0.01 µIU/mL (ref 0.4–4.0), a total T3 > 400 ng/dL (ref 83–160), and free T4 of 2.09 ng/dL (ref 0.6–1.8). Anti-Thyroid Peroxidase Antibody (Anti-TPO Ab) was 1.5 IU/mL (ref < 5.0), but Thyroid-Stimulating Immunoglobulin activity (TSI) was 208% (ref < 140%). Ultrasound (US) of the thyroid demonstrated a diffusely enlarged gland with minimal hypo-echogenicity and overall hypervascularity consistent with Graves’ disease. She had been treated with epoprostenol for approximately 48 months prior to the onset of symptoms. She was treated with methimazole and had resolution of symptoms and normalization of thyroid function tests within 8 weeks. Epoprostenol was continued.
Case 2: A 33-year-old Caucasian male with Idiopathic PAH (IPAH) treated with epoprostenol presented to emergency department with a chief complaint of difficulty breathing and rapid enlargement of his neck. On examination, he was in mild respiratory distress and was tachycardic to 120 beats per minute. He had a plethoric facies but no peripheral signs of hyperthyroidism, such as lid lag or exophthalmos. He had a markedly enlarged lobular thyroid and bilateral cervical lymphadenopathy. A computed tomography (CT) scan of the neck showed a diffusely enlarged thyroid gland with cervical adenopathy. Thyroid function tests confirmed hyperthyroidism: TSH < 0.02µIU/mL (ref 0.4–4.0), Free T4: 4.48 ng/dL (ref 0.6–1.8), total T3: 301 ng/dL (ref. 83–160) thyroid-stimulating immunoglobulin activity: 334% (ref < 140%), and anti-TPO Abs: < 1.0 IU/mL (ref < 5.0). The patient was treated with methimazole and a beta-blocker and thyroid function tests normalized. However, the patient eventually required a total thyroidectomy because of continued compressive symptoms, particularly dysphagia and orthopnea, from the enlarged gland. Histopathological analysis of the thyroid confirmed hyperplastic thyroid follicles with fibrosis consistent with Graves’ disease. He had been treated with epoprostenol for approximately 18 months prior to the onset of symptoms and was continued on this therapy.
Case 3: A 66-year-old Caucasian female with IPAH treated with epoprostenol was diagnosed with hyperthyroidism during admission for worsening dyspnea; she also complained of a recent 20 lb. weight loss, heartburn, and tremors. Physical examination did not reveal a goiter, tremors, proptosis, or lid lag. However, thyroid function tests confirmed hyperthyroidism: TSH < 0.01 µIU/mL (ref. 0.4–4.0), Free T4: 2.83 ng/dL. (ref. 0.6–1.8), total T3: 282 ng/dL (ref. 83–160), thyroid-stimulating immunoglobulin activity 496% (ref. < 140%), and positive anti-TPO Abs of 774 IU/mL (ref. < 5.0). Thyroid US demonstrated a diffusely heterogeneous gland with a single sub-centimeter nodule, but normal size and vascularity. A radio-iodine uptake scan showed an elevated thyroid uptake of 32.9% at 4 h (ref. 5–15%) with no hyperactive nodules to suggest a hyper-functioning adenoma. She had been treated with epoprostenol for approximately 10 months prior to onset of symptoms. She was treated with methimazole and a beta-blocker and thyroid function tests normalized. The patient eventually underwent bilateral lung transplantation because of the severity of the IPAH; epoprostenol was discontinued at that time. Methimazole and the beta-blocker were continued for 3 years post-transplant, but eventually discontinued. Since then, she has had normal thyroid function testing for greater than one year.
Case 4: A 31-year-old African-American female with IPAH treated with epoprostenol noticed gradual onset of neck swelling; she had no signs or symptoms of thyroid dysfunction except for the progressively enlarging goiter. Thyroid function tests, were within normal limits: TSH of 3.22 µIU/mL (ref. 0.4–4.0) and a free T4 of 0.9 ng/dL (ref. 0.6–1.8); serum thyroglobulin antibody was elevated at 1000 IU/ml. Thyroid uptake and scan showed a 6-h radio-iodine uptake of 32% (ref. 8–20%) and 24-h uptake of 60% (ref. 10–35%). Thyroid US was notable for a goiter affecting both lobes and isthmus and a heterogeneous echotexture with no discrete nodules. A fine needle aspirate of her thyroid revealed chronic lymphocytic thyroiditis consistent with Hashimoto’s thyroiditis. She had been treated with epoprostenol for approximately 12 months prior to onset of symptoms. The patient was initially treated with small doses of levothyroxine to suppress thyromegaly for cosmetic reasons but it was discontinued due to development of subclinical hyperthyroidism.
Case 5: A 35-year-old African-American female with IPAH treated with epoprostenol for approximately 96 months presented with throat pain, difficulty swallowing, and worsening dyspnea. She was found to have an enlarged thyroid gland, but no other clinical symptoms or signs of thyroid dysfunction. Thyroid US demonstrated an enlarged gland with diffuse heterogeneity of the echotexture. Two large nodules were identified: one in the isthmus (1.6 cm) and one in the left lobe (2.0 cm). Biopsy of the larger nodule showed colloid and benign follicular cells consistent with an adenomatous nodule. TSH level was 0.20 µIU/ml (ref. 0.27–4.20). Serum thyroglobulin was 147.6 ng/ml (ref. 1.3–31.8). FT4 was 1.6 ng/dL (ref. 0.9–1.7). Thyroid peroxidase antibody was 70.4 IU/ml (ref. 0–9). These findings and biopsy results, in the absence of a preceding neck pain or upper respiratory infection were consistent with autoimmune Hashimoto’s thyroiditis. Due to the compressive symptoms, the patient has been referred for elective thyroidectomy.
Selexipag Case
Case 6: A 36-year-old Caucasian female with PAH associated with congenital heart disease was diagnosed with hyperthyroidism during an admission for congestive heart failure. She had undergone ostium primum repair and stenting of the left pulmonary artery at age 8 and was diagnosed with PAH after a second pregnancy. At that time, she was treated with a PDE5i and an ERA. Because of continuing symptoms, as well as limited clinical and hemodynamic change, selexipag was added to the treatment regimen. 8 weeks after initiation, she was admitted for severe decompensated congestive heart failure symptoms. Her physical examination at that time was notable for elevated jugular venous distension (JVD), a new IV/VI systolic murmur at the left lower sternal border, and tachycardia at 129 beats per minute. Further evaluation was consistent with hyperthyroidism: TSH < 0.01 (ref. 0.27–4.2) and an elevated free T4 (FT4) of 4.3 (ref. 0.9–1.7). Her Anti-TPO Ab level was negative at 0.4 (ref. 0–9) as was her thyroid-stimulating immunoglobulin activity. Thyroid US revealed diffuse enlargement of the gland without cysts or masses. She was treated with beta-blockers, methimazole, and steroids. Selexipag was eventually discontinued due to insurance issues and intolerable side effects. 2 months following discontinuation of selexipag, the patient discontinued methimazole against medical advice; however, repeat thyroid hormone testing has demonstrated normal thyroid function.
Discussion
We report six patients with PAH who developed thyroid dysfunction after initiation of treatment with epoprostenol or selexipag. Our findings suggest that there may be an increased occurrence of thyroid dysfunction in patients who are treated with these agents. Four patients presented with hyperthyroidism, while two presented with goitrous enlargement of thyroid gland. In our patients, mean time to develop thyroid abnormalities was 31 months (2–96 months).
Thyroid Dysfunction at the Time of PAH Diagnosis
Several reports demonstrate an increased incidence of baseline thyroid disease in patients at diagnosis of PAH [2,3,4,5,6,7]. A prospective observational study, showed a prevalence of thyroid dysfunction of 49% in patients with PAH [7]. Most of these patients had autoimmune disease (Graves’ disease, Hashimoto’s thyroiditis and thyroid autoantibodies). The authors hypothesized that the association between the incidence of PAH and autoimmune thyroid dysfunction (AITD) was consistent with a common immunogenetic susceptibility. Another study suggested 20% prevalence of thyroid disease in PAH at the time of diagnosis [3]. Additional studies have established a strong correlation between thyroid dysfunction and PAH/PH [4,5,6]. Hyperthyroidism as well as hypothyroidism have been associated with development of Group 5 PH. In such cases, treatment of the underlying thyroid disease improves pulmonary artery pressures [6, 11, 25]. In addition, thyroid abnormalities, both hypothyroidism and hyperthyroidism, can exacerbate pre-existent PAH by affecting the metabolic requirements and stress on the body [26].
Thyroid Dysfunction After Initiation of Therapy that Effects the Prostacyclin Pathway
Previous investigators have suggested a potential relationship between thyroid abnormalities and epoprostenol treatment of PAH [22, 23]. For example, in an adult cohort of 134 patients, 26 developed thyroid dysfunction (hyper- or hypothyroidism); 20 of these patients were treated with epoprostenol and 11 developed thyroid dysfunction after being initiated on epoprostenol [22]. In a separate cohort of 78 children with primary pulmonary hypertension (now IPAH), the same authors reported that eight of these children had thyroid dysfunction, six of whom developed disease after starting epoprostenol [22]. Another study of 58 patients with PAH found an odds ratio of 5.25 (95% CI 1.46–18.85, p = 0.008) for development of thyroid dysfunction in patients treated with epoprostenol [27]. There are several other reports of hyperthyroidism in patients with PAH after initiation of epoprostenol [28, 29]. And, lastly, in a cohort of PAH patients receiving epoprostenol long term, the cumulative rate of hyperthyroidism was 45.2% over 10 years [30].
In regard to the prostacyclin analog treprostinil, we found no cases of thyroid dysfunction associated with its use in our databases. However, pursuant to our request to United Therapeutics (manufacturer of treprostinil), review of their database identified one case of Graves’ disease after initiation of treprostinil [24]. Additionally, prominent clinical trials that studied ERA’s and PDE5i’s for PAH do not describe thyroid dysfunction as a major adverse effect of therapy either [31,32,33]. Interestingly, thyroid dysfunction was also noticed among the adverse events in the pivotal selexipag trial (GRIPHON); eight patients in the treatment arm developed thyroid abnormalities (p = 0.004 compared to placebo) [21]. (ClinicalTrials.gov number, NCT01106014.)
Possible Mechanism(s) of Thyroid Dysfunction
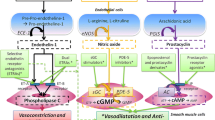
The mechanism(s) by which these molecules lead to thyroid dysfunction is not understood. However, activation of prostaglandin receptors in the thyroid gland leads to production of cAMP (cyclic Adenosine Mono-Phosphate) which, in turn, stimulates thyroid hormone production [34,35,36]. The association of hyperthyroidism with positive antibodies suggest an immune-genetic mechanism; however, seronegative hyperthyroidism can also occur via direct stimulation of cAMP in thyroid follicular cells by prostacyclin. Chadha et al. noted a high prevalence (6.7%) of thyroid-stimulating immunoglobulin-negative hyperthyroidism (seronegative hyperthyroidism) in patients treated with epoprostenol, in the absence of other mechanisms or drugs [37]. In this report, they described three patients with hyperthyroidism while on prostacyclin therapy; autoimmune markers were negative in all three patients, but each had increased uptake on thyroid scintigraphy. In either case, autoimmune or immune negative, it is not clear why only a small subset of patients treated with these agents develop hyperactive thyroid disease. To complicate this issue further, a recent study suggested a protective effect of ERAs [38]. In theory, the opposing effects of prostacyclin and ERAs on the Th17/IL17 axis might explain the difference in occurrence of autoimmune thyroid disease in these patients (Fig. 1).
In our cohort, three patients had antibodies normally associated with Graves’ disease; two had a high titer of anti-thyroglobulin antibodies and pathologically proven Hashimoto’s thyroiditis while one did not demonstrate any sign of autoimmune thyroid disease.
In addition, there is a significant decrease in the receptor density for prostacyclin binding sites in Graves’ disease, suggesting the possibility of a feedback mechanism and association between the two [39]. Increases in the plasma levels of the prostacyclin metabolite 6-keto prostaglandin F1-alpha in Graves’ disease has also been observed [40]. It is also possible that autoimmune thyroid disease in patients treated with drugs increasing prostacyclin may be a manifestation of their latent genotype. Seronegative hyperthyroidism with uniform radio-iodine uptake [37] may represent another phenotype that is susceptible to exposure to selexipag. As noted among the adverse events in the GRIPHON trial, and with our sixth case, hyperthyroidism after initiation of selexipag may not be an isolated event and may be observed more frequently as the number of patients treated with this medication increases.
In sum, the etiology of thyroid dysfunction in these patients is likely complex and multifactorial with the clinical phenotype representing the net effect of many contributing genetic and environmental factors [41]. Thus, the mechanism(s) by which epoprostenol, selexipag, or treprostinil might act as an environmental trigger for inducing Graves’ disease require further investigation. Of further interest and as additional validation that this truly is a drug effect, thyroid function returned to normal in two of our patients after discontinuation of the offending agent. In the selexipag case, there was rapid remission of hyperthyroidism after its discontinuation. And, in one epoprostenol case, thyroid function normalized after lung transplantation and discontinuation of epoprostenol. These two cases suggest that should they occur, this untoward side effect may, in fact, be reversible.
Effect of Thyroid Dysfunction in PAH Patients
Thyroid dysfunction can be an important cause of clinical deterioration in patients with PAH. For example, decreased T3 levels often observed with long-standing Graves’ disease have been associated with poor prognosis in patients with PAH and with chronic heart failure [42]. Development of Graves’ disease and the subsequent hyperthyroid/hyperdynamic state can lead to elevated pulmonary artery pressures as well as increases the metabolism of PAH medication leading to treatment failure. Moreover, many of the signs of excess thyroid stimulation are similar to those of worsening right ventricular function (tachycardia, edema, etc.) and likely overlooked. Thus, early recognition and treatment of the hyperthyroidism is exceptionally important and can lead to marked clinical improvement.
Limitations
This is a small, two-site study; thus, generalizability to other cohorts is unknown. However, data from other groups and from the multi-center GRIPHON trial suggest that this is an important clinical untoward effect of these drugs. In addition, the retrospective nature of the study limits identifying etiopathogenesis; further studies are warranted to elucidate the mechanism of development and to identify susceptible subgroups. A major limitation of the current study is the inability to determine accurately the prevalence of thyroid dysfunction associated with these agents. Because of the nature of clinical practice, patients were lost to follow-up, overlapped with other providers, and/or transitioned to other providers within the same area or even a geographically different area. Thus, it is difficult, if not impossible, to determine the prevalence of the described adverse effect. Although we estimate it to be ~ 1% (similar to what was observed in the GRIPHON trial), a longitudinal study or a Registry will be required to determine the prevalence accurately. In addition, we also were struck by the temporal heterogeneity in the occurrence of this adverse event, but do not have a cogent explanation for this phenomenon.
Conclusion
This case series and review of available literature and drug databases adds to the literature of thyroid disease after treatment with epoprostenol, but also suggests similar occurrences with selexipag and treprostinil or potentially all agents affecting the prostacyclin pathway. Large-scale observational studies and comprehensive post-marketing surveillances are warranted to define further a causal effect between drugs that affect the prostanoid pathway and the development of thyroid disease. Lastly, practitioners should be aware of this possibility since development of thyroid disease in PAH patients can be devastating and can easily be overlooked.
References
Galiè N, Humbert M, Vachiery JL et al (2016) 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37(1):67–119
Badesch DB, Raskob GE, Elliott CG et al (2010) Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137(2):376–387
Curnock AL, Dweik RD, Higgins BH, Saadi HF, Arroliga AC (1999) High prevalence of hypothyroidism in patients with primary pulmonary hypertension. Am J Med Sci 318(5):289–292
Arroliga AC, Dweik RA, Rafanan AL (2000) Primary pulmonary hypertension and thyroid disease. Chest 118(4):1224–1225
Kashyap AS, Kashyap S (2001) Thyroid disease and primary pulmonary hypertension. JAMA 285(22):2853–2854
Marvisi M, Brianti M, Marani G, Del Borello P, Bortesi ML, Guariglia A (2002) Hyperthyroidism and pulmonary hypertension. Respir Med 96(4):215–220
Chu JW, Kao PN, Faul JL, Doyle RL (2002) High prevalence of autoimmune thyroid disease in pulmonary arterial hypertension. Chest 122(5):1668–1673
Iervasi G, Molinaro S, Landi P et al (2007) Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med 167(14):1526–1532
Iervasi G, Pingitore A, Landi P et al (2003) Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation 107(5):708–713
Miura Y, Fukumoto Y, Sugimura K et al (2010) Identification of new prognostic factors of pulmonary hypertension. Circ J 74(9):1965–1971
Vallabhajosula S, Radhi S, Alalawi R, Raj R, Nugent K, Cevik C (2011) Hyperthyroidism and pulmonary hypertension: an important association. Am J Med Sci 342(6):507–512
Macchia A, Marchioli R, Tognoni G, Scarano M, Marfisi R, Tavazzi L, Rich S (2010) Systematic review of trials using vasodilators in pulmonary arterial hypertension: why a new approach is needed. Am Heart J 159(2):245–257
Humbert M, Ghofrani HA (2016) The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 71(1):73–83
Rubin LJ, Mendoza J, Hood M et al (1990) Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol): results of a randomized trial. Ann Intern Med 112(7):485–491
Coeytaux RR, Schmit KM, Kraft BD et al (2014) Comparative effectiveness and safety of drug therapy for pulmonary arterial hypertension: a systematic review and meta-analysis. Chest 145(5):1055–1063
Barst RJ, Rubin LJ, McGoon MD, Caldwell EJ, Long WA, Levy PS (1994) Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Intern Med 121(6):409–415
McLaughlin VV, Shillington A, Rich S (2002) Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 106(12):1477–1482
Barst RJ, Rubin LJ, Long WA et al (1996) A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 334(5):296–301
Badesch DB, Tapson VF, McGoon MD et al (2000) Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease: a randomized, controlled trial. Ann Intern Med 132(6):425–434
Kuhn KP, Byrne DW, Arbogast PG, Doyle TP, Loyd JE, Robbins IM (2003) Outcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenol. Am J Respir Crit Care Med 167(4):580–586
Sitbon O, Channick R, Chin KM et al (2015) Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 373(26):2522–2533
Ferris A, Jacobs T, Widlitz AB, Robyn J, Morse JH (2001) Pulmonary arterial hypertension and thyroid disease. Chest 119(6):1980
Davies A, Frost A (1997) Epoprostenol-associated thyropathy in PPH patients [abstract]. Am J Respir Crit Care Med 155(suppl):A630
Gu Y, Schwarcz MD, Weiss IA (2016) Pulmonary hypertension aggravated by hyperthyroidism: a case report [abstract]. Endocr Rev 37(2):Supplement 1
Nakchbandi IA, Wirth JA, Inzucchi SE (1999) Pulmonary hypertension caused by Graves’ thyrotoxicosis: normal pulmonary hemodynamics restored by 131I treatment. Chest 116(5):1483–1485
Marvisi M, Balzarini L, Mancini C, Mouzakiti P (2013) Thyroid gland and pulmonary hypertension. What’s the link? Panminerva Med 55(1):93–97
Castillo MP, García FH, Montero PB, González RL, Ocaña CM, Sánchez JR (2009) Thyroid dysfunction in patients with pulmonary arterial hypertension. A cohort study of 58 patients. Med Clin (Barc) 132(18):695–700
Srimatkandada P, Farber HW, Coviello AD (2014) Rapid development of compressive goiter and severe hyperthyroidism in a patient with pulmonary arterial hypertension after treatment with epoprostenol [abstract]. Endocr Rev 35(3)
Srimatkandada P, Coviello AD (2015) Severe autoimmune hyperthyroidism in two patients with pulmonary arterial hypertension after treatment with epoprostenol. J Thyroid Disord Ther. 4(176):2
Funasako M, Miyaji K, Sakuma M, Takagi Y, Kyotani S, Nakanishi N (2010) Long-term epoprostenol infusion therapy could induce hyperthyroidism in patients with idiopathic and hereditary pulmonary arterial hypertension [abstract]. Circulation 122(21_MeetingAbstracts):Supplement 1
Galie N, Rubin LJ, Hoeper MM et al (2008) Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 371(9630):2093–2100
Galiè N, Olschewski H, Oudiz RJ et al (2008) Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 117(23):3010–3019
Rubin LJ, Badesch DB, Fleming TR et al (2011) Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: the SUPER-2 study. Chest 140(5):1274–1283
Zor U, Kaneko T, Lowe IP, Bloom G, Field JB (1969) Effect of thyroid-stimulating hormone and prostaglandins on thyroid adenyl cyclase activation and cyclic adenosine 3′, 5′-monophosphate. J Biol Chem 244(19):5189–5195
Virgolini I, Steurer G, Keminger K, Sinzinger H, Kraupp O (1987) Evaluation of prostaglandin receptors in the human thyroid gland. Prog Clin Biol Res 242:35–42
Kasai K, Hiraiwa M, Suzuki Y et al (1986) Prostacyclin stimulation of adenylate cyclase activity in human thyroid membranes. Horm Metab Res 18(09):625–629
Chadha C, Pritzker M, Mariash C (2009) Effect of epoprostenol on the thyroid gland: enlargement and secretion of thyroid hormone. Endocr Pract 15(2):116–121
Satoh M, Aso K, Nakayama T, Saji T (2017) Effect of treatment with epoprostenol and endothelin receptor antagonists on the development of thyrotoxicosis in patients with pulmonary arterial hypertension. Endocr J 64(12):1173–1180
Virgolini I, Hermann M, Sinzinger H (1989) Decrease of the prostaglandin I2 binding capacity in thyroids from patients with Graves’ disease. Prostaglandins Leukot Essent Fatty Acids 37(2):121–128
Yoh-ichiro K, Kanemaru Y, Noguchi T, Onaya T (1987) Circulating prostacyclin and thromboxane in patients with Graves’ disease. Prostaglandins Leukot Essent Fatty Acids 26(1):75–84
Brix TH, Hegedüs L (2011) The complexity of the etiology of autoimmune thyroid disease is gravely underestimated. Thyroid 21(12):1289–1292
Frey A, Kroiss M, Berliner D et al (2013) Prognostic impact of subclinical thyroid dysfunction in heart failure. Int J Cardiol 168:300–305
Author information
Authors and Affiliations
Contributions
AAM, SS, and HWF were involved in the study design, data collection (including figures), data interpretation, and literature review; all authors contributed to the writing and revision of the manuscript. AAM, SS, and HWF are the guarantors of and take responsibility for the content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AA.M and L.E.B have no conflicts of interest to disclose. S.S has received consultant fees from Actelion Pharmaceuticals and speaker fees from Actelion Pharmaceuticals, Bayer AG and United Therapeutics Corporation. H.W.F. has received research grants from Gilead; Actelion Pharmaceuticals US Inc; and United Therapeutics Corporation and consulting fees from Gilead; Actelion Pharmaceuticals US, Inc; United Therapeutics Corporation; Bayer AG; Arena, Boehringer-Ingelheim; and Bellerophon. He also has served on a speaker’s bureau or given presentations on behalf of Actelion Pharmaceuticals US, Inc; Gilead; and Bayer AG.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Menon, A.A., Sahay, S., Braverman, L.E. et al. Thyroid Dysfunction in Patients with Pulmonary Artery Hypertension (PAH): The Effect of Therapies Affecting the Prostanoid Pathway. Lung 197, 761–768 (2019). https://doi.org/10.1007/s00408-019-00283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-019-00283-8