Abstract
Riociguat (Adempas®), a soluble guanylate cyclase stimulator, is a new, first-in-class drug approved for the treatment of patients with chronic thromboembolic pulmonary hypertension (CTEPH) [inoperable or persistent/recurrent following surgery] or pulmonary arterial hypertension (PAH). It has been designated an orphan medicine by the European Medicines Agency and the US FDA. This article reviews the available pharmacological properties of oral riociguat and its clinical efficacy and tolerability in adults with CTEPH or PAH. Riociguat is effective and well tolerated in patients with inoperable CTEPH or persistent/recurrent CTEPH following pulmonary endarterectomy, and in patients with PAH. It has a positive result on exercise capacity and pulmonary haemodynamics, and improves WHO functional class. Most adverse events can be attributed to the vasodilatory mechanism of riociguat; however, there is a potential for serious bleeding and fetal harm, and riociguat use is contraindicated in pregnant patients. Pulmonary endarterectomy remains the first treatment of choice for CTEPH, as it is potentially curative. Head-to-head trials comparing riociguat with the approved phosphodiesterase type 5 inhibitors in patients with PAH would be of value for the placement of riociguat in the management of this disease. Riociguat is a promising addition to the treatment options for patients with CTEPH or PAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
First in class (soluble guanylate cyclase stimulators), dual mechanism of action |
Significantly improves exercise capacity, WHO functional class and pulmonary haemodynamics in patients with chronic thromboembolic pulmonary hypertension that is inoperable or persistent/recurrent following pulmonary endarterectomy, and in patients with pulmonary arterial hypertension |
Well tolerated; most common adverse events include headache, dyspepsia and gastritis, dizziness, nausea and diarrhoea |
Potential for fetal harm; riociguat use is contraindicated in pregnant patients |
1 Introduction
Pulmonary hypertension, defined as a mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest [1–3], has been further classified by the World Health Organisation (WHO) into five different groups: pulmonary arterial hypertension (PAH), pulmonary hypertension with left heart disease, pulmonary hypertension associated with lung diseases and/or hypoxaemia, chronic thromboembolic pulmonary hypertension (CTEPH), and pulmonary hypertension with unclear and/or multifactorial mechanisms [1, 2].
The cumulative incidence of CTEPH was estimated to be 1.0 % 6 months after an acute pulmonary embolism, 3.1 % after 1 year and 3.8 % after 2 years [1]; however, most experts have settled on an incidence of 0.5–2 % after acute pulmonary embolism [2]. The lowest estimated prevalence of PAH is 5.9–15 cases per million adult population [1, 2] (PAH prevalence in Europe has been estimated at 15–50 cases per million population [2]), and the lowest estimated incidence is 2.4 cases per million adult population per year [2]. PAH has a poor prognosis; patients on modern therapy have an ≈15 % risk of mortality within one year, and an estimated median survival of 2–3 years has been reported in patients with idiopathic PAH [1].
Pulmonary endarterectomy is the first choice of treatment for patients with CTEPH, as it can potentially cure the disease; however, not all patients are candidates for this surgery, and some may experience residual or recurrent pulmonary hypertension following surgery [1, 2]. When treatment guidelines for patients with CTEPH were published, data on efficacy of medication in these patients were limited [1, 2], but suggested that haemodynamic and clinical benefits were possible with prostanoid, endothelin receptor antagonist and phosphodiesterase type 5 (PDE5) inhibitor treatment [2].
While CTEPH can be cured in some instances by surgery [1, 2], PAH is a chronic disease with no known cure, and ongoing therapy is necessary [2]. Aside from supportive therapy, the currently recommended treatments for PAH include calcium channel blockers, PDE5 inhibitors, prostanoids, and endothelin receptor antagonists [1, 2].
Riociguat (Adempas®), a soluble guanylate cyclase (sGC) stimulator, is a new, first-in-class drug approved for the treatment of patients with CTEPH (inoperable or persistent/recurrent following surgery) or PAH. It has been designated an orphan medicine by the European Medicines Agency [4] and the US FDA [5]. This article reviews the available pharmacological properties of oral riociguat and its clinical efficacy and tolerability in adults with CTEPH or PAH.
2 Pharmacodynamic Properties
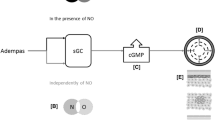
Riociguat (see Fig. 1 for its chemical structure) stimulates sGC [the receptor for nitric oxide (NO)] via a dual mechanism of action; it both sensitizes sGC to endogenous NO (via the stabilization of NO–sGC binding) and directly stimulates sGC independently of endogenous NO [6, 7]. Once active, either by binding to NO or by direct stimulation, sGC catalyses the synthesis of cyclic guanosine monophosphate (cGMP; a signalling molecule); intra-cellular cGMP is involved with regulating processes that influence vascular tone, proliferation, fibrosis and inflammation [6, 7]. Figure 2 shows a schematic representation of the effects of riociguat on the NO–sGC–cGMP pathway, as well as those of PDE-5 inhibitors, which target a different part of the same pathway.
Pulmonary hypertension is associated with impaired synthesis of NO and insufficient stimulation of the NO–sGC–cGMP pathway, as well as endothelial dysfunction [6, 7]. Riociguat, by stimulating the NO–sGC–cGMP pathway, is associated with improvement of pulmonary vascular haemodynamics [6, 7] and increased exercise ability [7]. Riociguat plasma concentration is directly related to the extent of changes in haemodynamic parameters [6, 7].
In vitro, riociguat at concentrations of 0.1–100 µmol/L stimulated recombinant rat sGC, with a 2- to 73-fold increase in activity from basal levels, and there was strong synergistic enzyme activation when riociguat was combined with NO-releasing agents [8]. In several animal models of pulmonary hypertension, riociguat was associated with improved haemodynamic parameters, improved cardio-renal function, improved survival, and/or protection against cardiac and renal end-organ damage [8–17]. Several animal trials also demonstrated that riociguat appeared more effective than sildenafil (a PDE5 inhibitor) in these endpoints [9, 11, 13, 14], likely due to its ability to stimulate sGC independently of NO levels, whereas PDE5 inhibitors require a sufficient supply of NO to exert their action [11].
A single dose of riociguat in healthy volunteers was associated with a significant increase versus placebo in heart rate over 1 min (a measure of the vasodilating effect on the cardiovascular system) at 2.5 and 5 mg (both p < 0.001) and significant decreases in diastolic and mean arterial blood pressure at 1 mg (both p < 0.05) and 5 mg (both p < 0.01); no significant difference in systolic blood pressure (SBP) was evident at any dosage [18]. Riociguat 1–5 mg was also associated with significant (p < 0.01) increases in plasma renin activity, and riociguat 2.5 and 5 mg were associated with significant (p < 0.01) increases in plasma cGMP levels, compared with placebo.
A single dose of riociguat 1 mg (n = 5) or 2.5 mg (n = 10) in patients with moderate-to-severe pulmonary hypertension (including pulmonary arterial hypertension, distal chronic thromboembolic pulmonary hypertension or pulmonary hypertension with mild to moderate interstitial lung disease) was associated with clinically relevant and statistically significant (p < 0.05 to p < 0.0001) reductions from baseline in mPAP, SBP, pulmonary vascular resistance (PVR) and systemic vascular resistance (SVR), and increases in cardiac index [19]. The single dose of riociguat 2.5 mg was, in addition, associated with a significant (p < 0.0001) increase in heart rate. Moreover, both doses were associated with significantly (p < 0.05 to p < 0.0001) greater changes than those observed with inhaled NO in SBP, PVR, SVR and cardiac index, and the 2.5 mg dose was also associated with significantly (p < 0.05 to p < 0.01) greater changes than those observed with inhaled NO in mPAP and heart rate.
See Sect. 4 for haemodynamic data from the phase II trial in patients with CTEPH or PAH [20] and from the phase III trials: Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator Trial 1 (CHEST-1) [in patients with CTEPH] [21] and Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator Trial 1 (PATENT-1) [in patients with PAH] [22].
Data from CHEST-1 and PATENT-1 (see Sect. 4.2 for trial details) have demonstrated that riociguat has no clinically relevant effects on QT prolongation [23].
Co-administration of riociguat with warfarin [6, 7, 24] or aspirin [6, 7, 25] had no clinically relevant pharmacodynamic effects [prothrombin time (warfarin) and bleeding time and platelet aggregation (aspirin)]. However, coadministration of riociguat with nitrates or PDE5 inhibitors (such as sildenafil [26]) led to increased lowering of blood pressure and a higher risk of hypotension; thus, coadministration of these drugs is contraindicated [6, 7].
3 Pharmacokinetic Properties
3.1 General Properties
The pharmacokinetics of riociguat are linear and dose proportional [6, 7, 19, 27]. Areas under the plasma riociguat concentration-time curve (AUCs), across all doses, vary between individuals by ≈60 % [6, 7] and within individuals by ≈30 % [6]. Riociguat undergoes rapid absorption, with the maximum plasma concentration (Cmax) generally reached within 1–1.5 h [6, 7]. If riociguat is taken concomitantly with food, Cmax values decrease minimally (by 35 %) [7, 27]; these changes are not clinically relevant [6, 27]. Riociguat has a high absolute bioavailability of ≈94 % [6, 7, 27]. Table 1 presents pharmacokinetic data for patients with moderate-to-severe pulmonary hypertension receiving a single dose of riociguat 1 or 2.5 mg [19].
Riociguat binds extensively to plasma proteins (≈95 %); the main binding components are serum albumin and α1-acidic glycoprotein [6, 7]. The volume of distribution at steady state is ≈30 L [6, 7].
The main metabolic pathway for riociguat involves N-demethylation [catalysed by cytochrome P450 (CYP)1A1, CYP3A4, CYP2C8 and CYP2J2], leading to its major circulating active metabolite, M1 (catalysed by CYP1A1 in liver and lungs) [6, 7], which has a pharmacological activity one tenth to one third that of riociguat [7]. CYP1A1 is inducible by polycyclic aromatic hydrocarbons, which are present in cigarette smoke [6, 7]. M1 is further metabolized to N-glucuronide, which is pharmacologically inactive [6, 7]. In patients with PAH, M1 plasma concentrations are approximately half those of riociguat [6].
Following the administration of oral, radiolabelled riociguat in healthy volunteers, 33–45 % of the total radioactivity was recovered in the urine and 48–59 % in the faeces [6, 7]. Respective proportions of the administered dose for unchanged riociguat were ≈4–19 and ≈9–44 % [7]; metabolites were the major components excreted in most subjects [6]. Riociguat has a low systemic clearance of ≈3–6 L/h according to the EU summary of product characteristics (SPC) [7]; the US prescribing information states that average systemic clearance was ≈1.8 L/h in patients with PAH and ≈3.4 L/h in healthy volunteers [6]. The terminal elimination half-life is ≈12 h in patients and ≈7 h in healthy volunteers [6, 7].
3.2 Potential Drug Interactions
Riociguat and M1 are both substrates of the transporter proteins P-glycoprotein and breast cancer resistance protein (BCRP) [6, 7]. Coadministration of riociguat and ketoconazole, a strong multi-pathway CYP and P-glycoprotein/BCRP inhibitor, resulted in an increase in riociguat AUC and Cmax values, as well as an increase in riociguat terminal half-life and a decrease in total body clearance [7, 28]. Thus, the EU SPC recommends that riociguat should not be coadministered with drugs that are strong multi-pathway CYP and P-glycoprotein/BCRP inhibitors, such as ketoconazole, itraconazole and ritonavir, and that caution should be used when riociguat is coadministered with drugs that are strong inhibitors of P-glycoprotein/BCRP, such as cyclosporine A [7]. The US prescribing information recommends the consideration of a lower starting dosage of riociguat (0.5 mg three times daily) in patients also receiving strong CYP and P-glycoprotein/BCRP inhibitors [6].
Riociguat and M1 are not strong inhibitors or inducers of major CYP isoforms (e.g. CYP3A4) or transporters (e.g. P-glycoprotein or BCRP), according to in vitro trials [7, 29]. However, they are strong inhibitors of CYP1A1 in vitro, implying that there is a potential for clinically relevant drug interactions between riociguat and drugs which are significantly metabolized by CYP1A1 (e.g. erlotinib, gefitinib or granisetron), particularly in smokers [7].
In patients with PAH receiving concomitant riociguat and bosentan, a moderate inducer of CYP3A4, steady-state plasma riociguat concentrations were decreased by a small amount [7]; however, the US prescribing information states that no dosage adjustment is necessary [6]. Concomitant administration of riociguat and strong CYP3A4 inducers such as rifampin, phenytoin, carbamazepine, phenobarbitone or St. John’s Wort may lead to decreased riociguat plasma concentrations [6, 7]; however, no data confirming this are available [6].
M1 exposure may be increased by coadministration of riociguat with UDP-glycosyltransferase (UGT) 1A1 and 1A9 inhibitors [7].
No clinically relevant pharmacokinetic interactions were discovered with the coadministration of riociguat and warfarin [6, 7, 24], aspirin [6, 25], midazolam [6, 28] or sildenafil [6]. While riociguat bioavailability was decreased slightly when administered after or concurrently with omeprazole or concurrently with an antacid [6, 7, 30], this was considered to be of minor clinical relevance [30]; however, antacids should be taken ≥1 h after [6, 7, 30] or ≥1 [6] or ≥2 [7] h before riociguat administration. The coadministration of riociguat and clarithromycin led to an increase in riociguat AUC values but not Cmax values [28].
3.3 Special Patient Populations
Mean riociguat AUC values showed no clinically relevant change in patients with mild (Child-Pugh class A) hepatic impairment, but increased in those with moderate (Child-Pugh class B) hepatic impairment, compared with healthy volunteers [7, 31]. No data are available for patients with severe (Child-Pugh class C) hepatic impairment or patients with alanine aminotransferase levels greater than three times the upper limit of normal (ULN) or bilirubin levels >2× ULN [7].
Mean AUC values were increased by 43–104 % in patients with mild [creatinine clearance (CLCR) 80–50 mL/min], moderate (CLCR 49–30 mL/min) and severe (CLCR <30 mL/min) renal impairment, compared with healthy volunteers; however, exposure was highly variable in patients with renal impairment, and the ranges overlapped in all groups [32]. Values for M1 were also higher in patients with renal impairment than in healthy volunteers [7, 32]. Data are limited for patients with severe renal impairment, and no data are available for patients undergoing dialysis [7]. Riociguat is not expected to be dialyzable, as a result of its high plasma protein binding [7].
The plasma concentration of riociguat in smokers is 50–60 % lower than that in non-smokers; dosage adjustment may be required in these patients [6, 7]. Patient sex [6, 7, 33], bodyweight [6, 7] and race [6, 7] have no clinically relevant effect on the pharmacokinetics of riociguat or M1. The US prescribing information states that age has no clinically relevant effect [6]; the EU SPC clarifies that elderly patients aged ≥65 years show higher plasma concentrations than those in younger patients (mean AUC values increased by ≈40 %), largely due to reduced apparent total and renal clearance, and that no studies in paediatric patients have been undertaken [7].
4 Therapeutic Efficacy
4.1 Phase II Trial
Following an initial, proof-of-concept trial in patients with several subtypes of pulmonary hypertension (see Sect. 2 for further trial details and some results) [19], the efficacy of riociguat 0.5–2.5 mg three times daily in patients with CTEPH (inoperable disease or who had refused an operation; n = 42) or PAH (n = 33) and WHO functional class II or III was investigated in a 12-week, noncomparative, phase II study [20]. The primary endpoints in this study were safety and tolerability; however, efficacy was also investigated.
After 12 weeks, the median 6-min walking distance had increased from baseline by 55 m in patients with CTEPH and 57 m in patients with PAH (both p < 0.0001; baseline values 390 and 337 m, respectively) [20]. Median PVR was significantly decreased from baseline in both patient populations (−200 and −245 dyn·s/cm5, respectively; both p < 0.0001; baseline values 691 and 748 dyn·s/cm5). WHO functional class improved in 31 % of patients (both groups combined) and deteriorated in 1 %.
Patients in this study were eligible to enter a long-term extension trial [34, 35]. A total of 68 patients entered the extension trial; the most recent analyses available are after a mean treatment duration of 36.5 months (treatment duration of ≤4.5 years as of November 2011) [34]. Over 42 months of the extension, the mean 6-min walking distance showed a generally sustained improvement similar to that observed in the original trial (e.g. a 78 m improvement from baseline of the original trial to 36 months of treatment [35]), and 54 % of patients had a WHO functional class of I or II at 42 months (45 months overall) [from a baseline of 21 % [35]] [34]. Between months 6 and 36 of the extension (months 9–39 overall), the percentage of patients with a WHO functional class of I or II ranged between 63 and 70 % [34].
4.2 Phase III Trials
The efficacy of riociguat has been investigated in two phase III trials: one in patients with CTEPH (CHEST-1) [21], the other in patients with PAH (PATENT-1) [22].
4.2.1 In Patients with Chronic Thromboembolic Pulmonary Hypertension
In CHEST-1, eligible patients with technically inoperable CTEPH or persistent or recurrent pulmonary hypertension following pulmonary endarterectomy were randomized to 16 weeks’ treatment with riociguat 0.5–2.5 mg three times daily or placebo [21]. Riociguat recipients initially received a starting dosage of 1 mg three times daily; this dosage was adjusted based on efficacy and tolerability over the first 8 weeks to the final dosage, which was stable for the remaining 8 weeks [21]. The final dosages of riociguat at week 16 were 0.5, 1, 1.5, 2 and 2.5 mg three times daily in 1, 4, 6, 12 and 77 % of patients, respectively. Dosage was decreased in 10 % of riociguat versus 3 % of placebo recipients during the study. All patients received anticoagulation therapy for at least 3 months before enrollment and throughout CHEST-1 [36]. Further study details and baseline patient characteristics are presented in Table 2. Upon completion of CHEST-1, patients were eligible for participation in a long-term extension study (CHEST-2) [Sect. 4.2.1.1].
Baseline characteristics did not significantly differ between treatment groups [21]. Most patients had a WHO functional class of II or III. A total of 72 % of patients had inoperable CTEPH; the remaining 28 % had postoperative persistent or recurrent pulmonary hypertension.
Riociguat was significantly more effective than placebo with regard to increasing 6-min walking distance from baseline (primary endpoint) in patients with CTEPH (Table 3), with a mean treatment difference in change from baseline of 46 m [95 % confidence interval (CI) 25–67; p < 0.001] at 16 weeks [21]. Patient subgroups (disease classification, baseline WHO functional class, baseline 6-min walking distance, sex, age) had no significant effect on the treatment effect.
At 16 weeks, riociguat was also significantly more effective than placebo (Table 3) at decreasing PVR [mean difference in change from baseline −246 dyn·s/cm5 (95 % CI −303 to −190; p < 0.001)], N-terminal pro-brain natriuretic peptide levels [mean difference −444 pg/mL (95 % CI −843 to −45; p < 0.001)], and Borg dyspnoea score (p = 0.004; considered to be exploratory on the basis of the prespecified hierarchical testing procedure), and was associated with significant improvements in WHO functional class (Fig. 3a; p = 0.003) [21].
In general, haemodynamic exploratory parameters were also improved with riociguat versus placebo [21]. There was a significant relative decrease in mPAP [Table 3; mean difference in change from baseline −5 mmHg (95 % CI −7 to −3; p < 0.001)] and mean arterial pressure [− 9 vs. −0.3 mmHg (baseline values both 95 mmHg); mean difference −9 mmHg (95 % CI −12 to −6); p < 0.001] and a significant relative increase in cardiac output [Table 3; mean difference 0.9 L/min (95 % CI 0.6–1.1); p < 0.001] in riociguat recipients.
No significant difference between riociguat and placebo was found in change from baseline in right atrial pressure [− 1 vs. −0.6 mmHg (baseline values both 9 mmHg); mean difference −0.6 mmHg (95 % CI −1.7 to 0.6)] or pulmonary-capillary wedge pressure [0.6 vs 0.2 mmHg (baseline values both 9 mmHg); mean difference 0.6 mmHg (95 % CI −0.4 to 1.5)] [21]. Changes in arterial oxygen saturation, heart rate and partial pressure of oxygen in the blood were similar between treatment groups.
Health-related quality of life (HR-QOL) was significantly improved with riociguat versus placebo treatment with regard to score on the EuroQol Group 5-Dimension Self-Report Questionnaire [EQ-5D; scores range from −0.6 to 1.0 (higher score indicates better quality of life)] and the EuroQol Visual Analogue Scale [EQ-VAS; scores range from 0 to 100 (higher score indicates better quality of life)], but not on the Living with Pulmonary Hypertension questionnaire [LPH; scores range from 0 to 105 (higher score indicates worse quality of life)] [21, 37]. Scores on the EQ-5D increased to a significantly greater extent in riociguat recipients than in placebo recipients [0.06 vs. −0.08 (baseline values 0.64 and 0.66); mean difference 0.13 (95 % CI 0.06–0.21); p < 0.0001] [21, 37], as did scores on the EQ-VAS [treatment difference in change from baseline 10.0 (95 % CI 5.4–14.7); p < 0.0001] [37]. LPH scores changed by −6.7 in riociguat recipients and −2.1 in placebo recipients [baseline values 41 and 46; mean difference −5.8 (95 % CI −10.5 to −1.1)] [21, 37].
Riociguat and placebo recipients did not significantly differ with regard to the incidence of clinical worsening (2 vs. 6 %; p = 0.17) or the time to clinical worsening (p = 0.1724) [21]. A total of 1 % of patients in both treatment groups withdrew from the study as a result of lack of efficacy.
4.2.1.1 Long-Term Extension
A total of 237 patients from CHEST-1 entered CHEST-2; 2-year data (cut-off March 2014; final analysis before most patients switched to the commercial drug) from this extension trial are available as an abstract [38]. Patients received their optimal dosage (≤2.5 mg three times daily). At the time of analysis, 172 patients were still receiving treatment and 171 patients had received ≥2 year of treatment. A total of 8 % had switched to treatment with the commercial drug.
After 2 years in CHEST-2, riociguat treatment was associated with an increase of 50 m in 6-min walking distance from CHEST-1 baseline [38]. Proportions of patients with improved, stable or worsened WHO functional class were 39, 58 and 3 %, respectively, versus CHEST-1 baseline. A total of 10 % of patients received additional medication for pulmonary hypertension, as a result of worsened disease. In this analysis, 2-year survival was 93 %.
Subgroup analyses at 1 year demonstrated that there was a similar change from baseline in 6-min walking distance, regardless of baseline functional class (+51 vs. +52 m in patients with baseline WHO functional class I/II vs. III/IV) [39]. At 1 year, proportions of patients with improved, stable or worsened WHO functional class were 25, 67 and 8 % in patients with baseline WHO functional classes of I or II and 58, 40 and 1 % in patients with baseline WHO functional classes of III or IV [39].
According to the EU SPC, at 1, 2 and 3 years, the probability of survival was 97, 94 and 88 %, respectively [7]. In patients with WHO functional class II and III at baseline, the respective probabilities of survival were 97 and 97 % at 1 year, 94 and 94 % at 2 years, and 88 and 87 % at 3 years [7].
4.2.2 In Patients with Pulmonary Arterial Hypertension
In PATENT-1, eligible patients with PAH were randomized to 12 weeks’ treatment with riociguat 0.5–1.5 or 0.5–2.5 mg three times daily or placebo [22]. The riociguat 0.5–1.5 mg group was included for exploratory purposes only; data from that group did not undergo statistical analyses, and are not discussed in detail. Riociguat 0.5–2.5 mg recipients initially received a starting dosage of 1 mg three times daily, which was adjusted based on efficacy and tolerability over the first 8 weeks to the final dosage, which was stable for the final 4 weeks. Riociguat 0.5–1.5 mg recipients followed the same dosage adjustment regimen as riociguat 0.5–2.5 mg recipients, but received sham adjustment once a dosage of 1.5 mg was reached. A total of 96 % of patients in the riociguat 0.5–1.5 mg group were receiving 1.5 mg three times daily as their final dosage at week 12; the final dosages in the riociguat 0.5–2.5 mg group were 0.5, 1, 1.5, 2 and 2.5 mg three times daily in 2, 3, 6, 15 and 75 % of patients, respectively. Dosage was decreased in 12 % of riociguat 0.5–2.5 mg versus 9 % of placebo recipients during the study. Further study details and baseline patient characteristics are presented in Table 2. Upon completion of PATENT-1, patients were eligible for participation in a long-term extension study (PATENT-2) [Sect. 4.2.2.1].
Baseline characteristics did not significantly differ between treatment groups [22]. Most patients had a WHO functional class of II or III. PAH classifications included idiopathic (61 % of patients), associated with connective-tissue disease (25 %), associated with congenital heart disease (8 %), associated with portopulmonary hypertension (3 %), familial (2 %) and associated with anorexigen or amphetamine use (1 %). A total of 50 % of patients received additional treatment for PAH [endothelin-receptor antagonists (mainly bosentan) 44 % or prostanoids (mainly inhaled iloprost) 6 %].
Riociguat 0.5–2.5 mg three times daily was significantly more effective than placebo with regard to increasing 6-min walking distance from baseline (primary endpoint) in patients with PAH (Table 3), with a mean treatment difference in change from baseline of 36 m (95 % CI 20–52; p < 0.001) at 12 weeks [22]. Most patient subgroups (baseline therapy, disease classification, baseline 6-min walking distance, sex, age) had no significant effect on the treatment effect; however, riociguat had significantly greater efficacy versus placebo in patients with a WHO functional class of III or IV than in those with a WHO functional class of I or II (mean treatment difference 59 vs. 14 m; p = 0.005).
After 12 weeks’ treatment, riociguat 0.5–2.5 mg three times daily was also significantly more effective than placebo (Table 3) at decreasing PVR [mean difference in change from baseline −226 dyn·s/cm5 (95 % CI −281 to −170; p < 0.001)], N-terminal pro-brain natriuretic peptide levels [mean difference −432 pg/mL (95 % CI −782 to −82; p < 0.001)], and Borg dyspnoea score (p = 0.002), and was associated with significant improvements in WHO functional class (Fig. 3b; p = 0.003) [22].
As in patients with CTEPH, haemodynamic exploratory parameters were also improved with riociguat 0.5–2.5 mg three times daily versus placebo in patients with PAH [22]. There was a significant relative decrease in mPAP [Table 3; mean difference in change from baseline −4 mmHg (95 % CI −6 to −2; p < 0.001)] and mean arterial pressure [− 9 vs. −1 mmHg (baseline values 90 and 91 mmHg); mean difference −7 mmHg (95 % CI −10 to −5); p < 0.001] and a significant relative increase in cardiac output [Table 3; mean difference 0.9 L/min (95 % CI 0.7–1.2); p < 0.001] in riociguat recipients.
No significant difference between riociguat and placebo was found in change from baseline in right atrial pressure [− 0.2 vs. 1 mmHg (baseline values 8 and 7 mmHg); mean difference −1.0 mmHg (95 % CI −2.2 to 0.1)] or pulmonary-capillary wedge pressure [1 vs 0.5 mmHg (baseline values both 9 mmHg); mean difference 0.4 mmHg (−0.4 to 1.2)] [22]. Mixed venous oxygen saturation increased to a significantly greater extent in riociguat than in placebo recipients [3 vs. −2 % (baseline values 65 and 66 %); mean difference 5 % (95 % CI 3–7); p < 0.001]. Changes in heart rate were similar between treatment groups.
Correlation analyses found that an improvement in 6-min walking distance was significantly (but weakly) correlated with improvements in PVR (r = −0.21; p < 0.001) and cardiac index (r = 0.16; p = 0.002) [40].
HR-QOL was significantly improved with riociguat 0.5–2.5 mg three times daily versus placebo with regard to score on the LPH, but not on the EQ-5D [22]. Scores on the LPH decreased to a significantly greater extent in riociguat recipients than in placebo recipients [− 6 vs. 0.4 (baseline values both 42); mean difference −6 (95 % CI −10 to −3); p = 0.002]. EQ-5D scores changed by 0.03 in riociguat recipients and −0.03 in placebo recipients [baseline values both 0.7; mean difference 0.06 (95 % CI 0.01 to 0.11)].
Riociguat 0.5–2.5 mg three times daily recipients had a significantly lower incidence of clinical worsening than placebo recipients (1 vs. 6 %; p = 0.0285), and there was a significantly longer time to clinical worsening with riociguat recipients (p = 0.0046) [22]. A total of 1 % of placebo recipients withdrew from the study as a result of lack of efficacy; no patients in the riociguat 0.5–2.5 mg group withdrew for this reason.
4.2.2.1 Long-Term Extension
A total of 396 patients from PATENT-1 entered PATENT-2; 2-year data (cut-off March 2014; final analysis before most patients switched to the commercial drug) from this extension trial are available as an abstract [41]. Patients received riociguat 0.5–2.5 mg three times daily. At the time of analysis, 275 patients were still receiving treatment and 307 patients had received ≥2 year of treatment. A total of 3 % had switched to commercial drug.
After 2 years in PATENT-2, riociguat treatment was associated with an increase of 47 m in 6-min walking distance from PATENT-1 baseline [41]. Proportions of patients with improved, stable or worsened WHO functional class were 33, 58 and 9 %, respectively, versus PATENT-1 baseline. Among those patients who were formerly not receiving additional medication for pulmonary hypertension, 17 % had changed to receive additional medication at 2 years, as a result of worsened disease. In this analysis, 2-year survival was 93 %.
Subgroup analyses showed a change from baseline in 6-min walking distance at 1 year of +59 vs. +45 m in patients with baseline WHO functional class I/II vs. III/IV [42]. At 1 year, proportions of patients with improved, stable or worsened WHO functional class were 10, 80 and 10 % in patients with baseline WHO functional classes of I or II and 51, 45 and 4 % in patients with baseline WHO functional classes of III or IV [42].
According to the EU SPC, at 1, 2 and 3 years, the probability of survival was 97, 93 and 91 %, respectively [7]. In patients with WHO functional class II and III at baseline, the probabilities of survival were 98 and 96 % at 1 year, 96 and 91 % at 2 years, and 96 and 87 % at 3 years [7].
5 Tolerability
Riociguat is generally well tolerated in patients with CTEPH [6, 7, 21] or PAH [6, 7, 22]. In the phase III trials, adverse events occurred in 92 and 86 % of riociguat 0.5–2.5 mg and placebo recipients with CTEPH [21] and 89, 92 and 86 % of riociguat 0.5–2.5 mg, riociguat 0.5–1.5 mg and placebo recipients with PAH [22], respectively. The most common adverse events in these trials are presented (as pooled data; the safety profile of riociguat appeared to be similar in patients with CTEPH to those with PAH [6, 7]) in Fig. 4; most can be attributed to the vasodilatory mechanism of riociguat [6, 7].
Tolerability of riociguat 0.5–2.5 mg three times daily versus placebo in patients with chronic thromboembolic pulmonary hypertension or pulmonary arterial hypertension (pooled analysis of the 16-week CHEST-1 and 12-week PATENT-1 trials) [6]. Adverse events that occurred in ≥5 % of riociguat recipients and in ≥3 % more riociguat than placebo recipients
In patients with CTEPH, serious adverse events considered by the investigators to be drug related included syncope in 2 % of patients and gastritis, acute renal failure and hypotension each in 1 % of patients in the riociguat treatment group and syncope and trauma each in 1 % of patients in the placebo group [21].
Of the 3 and 2 % of riociguat and placebo recipients, respectively, with CTEPH who discontinued treatment as a result of adverse events, one patient in the riociguat group discontinued as a result of drug-related adverse events (diarrhoea, heartburn, nausea, vomiting and headache), and four and two patients in the riociguat and placebo groups discontinued as a result of serious adverse events [right heart decompensation, vaginal bleeding, study drug overdose as attempted suicide and worsening of general condition (riociguat recipients), and right heart failure and cardiocirculatory arrest (placebo recipients); these were not considered to be drug-related] [21].
A total of 1 and 3 % of patients with CTEPH in the riociguat and placebo groups died as a result of adverse events; one of these deaths (acute renal failure in a riociguat recipient) was considered to be treatment related [21].
In patients with PAH, drug-related serious adverse events included three cases of syncope (in 1 % of patients) and one case each of increased hepatic enzyme levels, dizziness, presyncope, acute renal failure and hypotension [in a total of 0.4 % of patients (some patients experienced more than one event)] in the riociguat 0.5–2.5 mg treatment group and one case each of diarrhoea, presyncope, syncope, dyspnoea and worsening pulmonary hypertension [in a total of 1 % of patients (some patients experienced more than one event)] in the placebo group [22].
A total of 3 and 7 % of riociguat 0.5–2.5 mg and placebo recipients, respectively, with PAH discontinued treatment as a result of adverse events [22]. Of the adverse events leading to discontinuation in the riociguat group, increased hepatic enzyme levels, acute renal failure and syncope were serious, and oesophageal pain/swelling, supraventricular tachycardia, hypotension, generalized oedema and neck pain were considered to be drug related; of those in the placebo group, syncope, hypoxaemia and worsening of pulmonary hypertension were serious, and diarrhoea and dyspnoea were considered to be drug related.
Adverse event-related death occurred in 1 and 2 % of riociguat 0.5–2.5 mg and placebo recipients, respectively, with PAH; none were considered to be related to treatment [22].
Syncope, as an adverse event of special interest (as riociguat is a vasodilator), occurred in 2 and 3 % of patients with CTEPH [21] and 1 and 4 % of patients with PAH [22] receiving riociguat 0.5–2.5 mg and placebo, respectively.
In placebo-controlled trials, serious bleeding occurred in 2.4 and 0 % of riociguat and placebo recipients and serious haemoptysis occurred in 1 and 0 % (one event was fatal) [6, 7]. Serious haemorrhagic events included vaginal haemorrhage (2 patients), catheter-site haemorrhage (2 patients) and subdural haematoma, haematemesis, and intra-abdominal haemorrhage (1 patient each) [6, 7]. There is an increased risk of respiratory tract bleeding in patients with PAH (particularly those receiving anticoagulation treatment); this risk may be increased with riociguat use, particularly in patients with risk factors such as recent serious haemoptysis, and the EU SPC states that riociguat treatment should be avoided in these patients [7].
Long term, the safety profile of riociguat is similar to that in shorter-term trials [6]. A total of 5 % of patients with CTEPH [38] and 10 % of patients with PAH [41] withdrew from treatment as a result of adverse events in analyses where patients had received at least 2 years of treatment. The most common drug-related adverse events were dizziness (11 %), dyspepsia (8 %) and hypotension (5 %) in patients with CTEPH [38] and dizziness (10 %), dyspepsia (9 %) and headache (8 %) in patients with PAH [41]. In patients with CTEPH, 7 drug-related serious syncope events occurred [38]; in patients with PAH, 13 drug-related serious syncope events and 4 drug-related serious pulmonary bleeding events occurred [41].
Inadvertent riociguat overdosages of 9–25 mg/day have been reported, and resulted in adverse events similar to those at therapeutic dosages [7].
6 Dosage and Administration
Riociguat is indicated in adult patients with CTEPH (inoperable disease or persistent/recurring disease following surgery) for the improvement of exercise capacity [6, 7] and WHO functional class [6], and (as monotherapy or in combination with endothelin receptor antagonists) in adult patients with PAH for the improvement of exercise capacity [6, 7] and WHO functional class and to delay clinical worsening [6].
As the forms of PAH in which riociguat has been investigated mainly include idiopathic or heritable PAH and PAH associated with connective tissue disease, the EU SPC states that the use of riociguat in other forms of PAH is not recommended [7]. In addition, the EU SPC states that expert assessment of operability should be undertaken before treatment with riociguat in patients with CTEPH, as pulmonary endarterectomy remains the treatment of choice [7].
The recommended dosage of riociguat is 0.5–2.5 mg three times daily (with or without food [7]), with a recommended starting dosage of 1 mg three times daily (or 0.5 mg three times daily in patients who may not tolerate the hypotensive effect [6]) and subsequent up-titration (in 0.5 mg increments every two weeks if the SBP remains >95 mmHg and the patient has no signs or symptoms of hypotension) to an established individual dosage to be maintained [6, 7]. Dosage can be decreased if hypotension occurs at any time [6, 7].
Patients who smoke may require dosages of riociguat that exceed 2.5 mg three times daily [6, 7], although smokers should be advised to stop smoking during treatment [7]. Patients receiving concurrent strong CYP or P-glycoprotein/BCRP inhibitors may require a starting riociguat dosage of 0.5 mg three times daily [6].
No data are available regarding the use of riociguat in patients with severe hepatic impairment [6, 7], and riociguat use is contraindicated in these patients in the EU [7]. The EU SPC states that care should be taken when administering riociguat to patients with moderate hepatic impairment [7]. Data are limited for the use of riociguat in patients with severe renal impairment, and there are no data in patients on dialysis [6, 7]; use of riociguat is not recommended by the EU SPC in these patients [7]. There is a higher risk of hypotension in patients with renal impairment; the EU SPC recommends care during individual dosage titration in patients with moderate and mild renal impairment [7].
The US prescribing information contains a boxed warning regarding the potential for fetal harm if riociguat is administered to pregnant women [6], and treatment with riociguat is contraindicated in these patients in both the USA and the EU [6, 7]. Riociguat was associated with teratogenic and embryotoxic effects and placental transfer in animal studies [6, 7]. Further contraindications include concomitant treatment with nitrates or nitric oxide donors and concomitant treatment with specific and nonspecific phosphodiesterase inhibitors (in both the USA and the EU) [6, 7]. Moreover, riociguat should not be administered in paediatric patients, due to a lack of data in these patients [7].
As riociguat reduces blood pressure, there is a potential for symptomatic hypotension or ischaemia in patients with certain underlying conditions, such as hypovolaemia, severe left ventricular outflow obstruction, resting hypotension, autonomic dysfunction, or concomitant treatment with antihypertensives or strong CYP or P-glycoprotein/BCRP inhibitors [6, 7]. The EU SPC states that riociguat must not be used in patients with an SBP less than 95 mmHg, and that patients older than 65 years are at an increased risk of hypotension (and thus caution should be exercised if riociguat is administered in these patients) [7]. In addition, the cardiovascular status of patients with pulmonary veno-occlusive disease may worsen with pulmonary vasodilator treatment; thus, riociguat use in these patients is not recommended [6, 7].
Local prescribing information should be consulted for further, detailed information, including contraindications, precautions, drug interactions, and use in special patient populations.
7 Place of Riociguat in the Management of Chronic Thromboembolic Pulmonary Hypertension or Pulmonary Arterial Hypertension
While the introduction of treatments such as PDE5 inhibitors, prostanoids, and endothelin receptor antagonists has advanced the treatment of pulmonary hypertension, additional therapeutic interventions remain necessary to fill the gaps in current treatment options. For example, treatments that directly elevate NO levels (either inhaled NO or NO-donor drugs) have short-lived effects, and there is a risk that patients will develop tolerance to them; moreover, NO is associated with nonspecific interactions with various other biomolecules [43]. PDE5 inhibitors, such as sildenafil, are one solution to this issue, as they augment the effects of endogenous NO (rather than relying directly on NO levels), thus increasing cGMP levels by preventing its degradation. However, a significant number of patients with pulmonary hypertension do not respond to sildenafil; this may be a result of the endogenous NO levels having decreased to the extent that sildenafil has no significant effect on cGMP levels [43].
The development of the first-in-class sGC stimulator riociguat, a drug which can directly stimulate sGC (independent of NO activity) as well as augment the effects of NO (Sect. 2), is a promising theoretical solution to these problems.
Riociguat 0.5–2.5 mg three times daily is effective in improving 6-min walking distance (a measure of exercise capacity) in patients with inoperable CTEPH, CTEPH that persisted or recurred following pulmonary endarterectomy (Sect. 4.2.1), or PAH (Sect. 4.2.2). Riociguat recipients also exhibited improvements versus placebo recipients in most secondary endpoints, including haemodynamic outcomes (such as PVR) and WHO functional class. Improvement in WHO functional class has historically been correlated with survival; long-term survival data in patients receiving riociguat are not yet available outside of clinical trials and their extensions, but would be of interest. Data from the extension studies showed a 3-year survival of >87 % with riociguat treatment in both CTEPH and PAH patients (Sects. 4.2.1.1, 4.2.2.1). Overall, the positive effects on 6-min walking distance and WHO functional class continued with longer-term treatment in patients with CTEPH and PAH (Sects. 4.2.1.1, 4.2.2.1).
Interestingly, WHO functional class at baseline had an effect on the efficacy (6-min walking distance) of riociguat in patients with PAH (Sect. 4.2.2) but not CTEPH (Sect. 4.2.1), implying that patients with PAH and a higher WHO functional class may be more likely to experience a clinically relevant effect.
Riociguat is generally well tolerated, in both the shorter and the longer term, in patients with inoperable CTEPH, CTEPH that persisted or recurred following pulmonary endarterectomy, or PAH (Sect. 5). Most adverse events can be attributed to the vasodilatory mechanism of riociguat; the most common adverse events include headache, dyspepsia and gastritis, dizziness, nausea and diarrhoea. Riociguat has been associated with teratogenic and embryotoxic effects in animals, and is contraindicated in pregnant women as a result (Sect. 6). Riociguat has been associated with serious bleeding events; however, these may also be related to the underlying disease or anticoagulant use. There is also a risk of hypotension or ischaemia in patients with certain underlying disorders or receiving certain medication already associated with these symptoms (Sect. 5).
As riociguat is an orphan drug and first-in-class, tolerability data are limited, and only the most common adverse events are well characterized. More information on the tolerability of the drug will appear over time, including the overall risk of more serious adverse events, such as haemoptysis and bleeding; a pharmacovigilance plan is being implemented in the EU [44].
These trials used 6-min walking distance as the primary endpoint; this has historically been the gold standard efficacy endpoint for assessing potential drugs for pulmonary hypertension, and on this basis riociguat achieved approval for the improvement of exercise capacity in patients with CTEPH or PAH. According to the most recent trial-design guidelines [45], the 6-min walking distance remains an acceptable primary endpoint for assessing new drugs for PAH, especially if the indication is restricted to improving exercise capacity.
These guidelines also indicate that a composite primary endpoint that reflects time to clinical worsening and mortality may now be the most clinically relevant endpoint to use [45]. Of interest, in CHEST-1 and PATENT-1, time to clinical worsening was significantly longer and the incidence of clinical worsening was significantly lower with riociguat than with placebo in patients with PAH (Sect. 4.2.2) but not in those with CTEPH (Sect. 4.2.1) (both secondary endpoints). Further studies with riociguat evaluating this composite endpoint, both in clinical trials and in the real-world setting, would be of interest.
Previous trials of bosentan and sildenafil have not shown significant improvement in the primary endpoint in patients with CTEPH [44]; riociguat is the only drug so far to show an improvement in patients with CTEPH in 6-min walking distance as well as in pulmonary haemodynamics, N-terminal pro-brain natriuretic peptide levels and WHO functional class. However, any comparisons between data from trials involving other drugs and those seen in the trial of riociguat should be interpreted with caution.
As pulmonary endarterectomy is potentially curative, it is important that this remains the first treatment of choice for patients with CTEPH; current guidelines strongly recommend that patients with CTEPH be assessed by a multidisciplinary team, including experienced surgeons, for the best course of treatment, to ensure that all who are able to undergo surgery do [2]. Thus, riociguat should only be used in patients with CTEPH that is inoperable (approximately one-third of patients [21]) or persistent/recurrent following surgery (5–35 % of post-surgical patients [21]). Most centres do not perform pulmonary endarterectomy [46]; there is, therefore, a risk that patients may undergo alternative treatment instead of the potentially curative surgery, as the easier option. Riociguat should, ideally, not be used in patients who are eligible for the operation, as this may unnecessarily delay surgery and ultimately make removal of the thrombus more difficult. However, it would be of definite interest to determine the efficacy of riociguat in affecting the post-operative course following pulmonary endarterectomy, by improving preoperative haemodynamics and the physical condition of patients eligible for surgery.
A head-to-head trial with an approved PDE5 inhibitor, such as sildenafil, would be of great interest in patients with PAH, to determine whether the dual mechanism of action of riociguat conveys an advantage over the single action of the PDE5 inhibitors; they both use endogenous NO to increase cGMP levels, but riociguat alone can also stimulate sGC without NO. An ongoing, noncomparative trial is currently investigating the effects of riociguat in patients with PAH and an insufficient response to PDE-5 inhibitors [47]. In addition, further studies involving patients with subtypes of PAH other than idiopathic or heritable PAH or PAH associated with connective tissue disease are required to determine the efficacy of riociguat in these patients; currently, the use of riociguat in other forms of PAH is not recommended in the EU [4]. Well designed cost effectiveness trials would also be of great use in the placement of this drug in the management of PAH.
Further information on riociguat in patients with CTEPH will soon be available; a noncomparative study of riociguat 0.5–2.5 mg three times daily in patients with CTEPH that is inoperable or persistent/recurrent following surgery, who are not satisfactorily treated and are unable to participate in other CTEPH trials, is currently ongoing [48].
Data from preliminary trials of riociguat in patients with pulmonary hypertension associated with systolic [49] or diastolic [50] left ventricular dysfunction, chronic obstructive pulmonary disease [51], and interstitial lung disease [52] have recently become available, showing improvements in several haemodynamic parameters. Further investigation into the efficacy of riociguat in these and other forms of pulmonary hypertension would be of great interest, to discover whether riociguat is a viable and safe option for patients with these diseases.
In conclusion, riociguat is effective and well tolerated in patients with CTEPH that is inoperable or persistent/recurrent following pulmonary endarterectomy, and in patients with PAH. It has a positive result on exercise capacity and pulmonary haemodynamics, and improves WHO functional capacity. Most adverse events can be attributed to the vasodilatory mechanism of riociguat; however, there is a potential for serious bleeding and fetal harm, and riociguat use is contraindicated in pregnant patients. Pulmonary endarterectomy remains the first treatment of choice for CTEPH, as it is potentially curative. Head-to-head trials comparing riociguat with the approved PDE5 inhibitors in patients with PAH would be of value for the placement of riociguat in the management of this disease. Riociguat is a promising addition to the treatment options for patients with CTEPH or PAH.
Data selection sources:
Relevant medical literature (including published and unpublished data) on riociguat was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) [searches last updated 10 October 2014], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Riociguat, Adempas, pulmonary arterial hypertension, pulmonary hypertension, chronic thromboembolic pulmonary hypertension, PAH.
Study selection: Studies in patients with pulmonary arterial hypertension who received riociguat. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–94.
Galié N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493–537.
Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–50.
European Medicines Agency. Adempas (riociguat) EPAR summary for the public. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002737/WC500165037.pdf. Accessed 8 Sep 2014.
US FDA. Adempas (riociguat) FDA approval letter. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204819Orig1s000Approv.pdf. Accessed 8 Sep 2014.
Bayer Healthcare. Adempas® (riociguat tablets): US prescribing information. 2014. http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf. Accessed 9 July 2014.
European Medicines Agency. Adempas® (riociguat tablets): EU summary of product characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf. Accessed 9 July 2014.
Schermuly RT, Stasch JP, Pullamsetti SS, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J. 2008;32(4):881–91.
Becker EM, Stasch J-P, Bechem M, et al. Effects of different pulmonary vasodilators on arterial saturation in a model of pulmonary hypertension. PLoS One. 2013;8(8):e73502.
Dumitrascu R, Weissmann N, Ghofrani HA, et al. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113(2):286–95.
Evgenov OV, Zou L, Zhang M, et al. Nitric oxide-independent stimulation of soluble guanylate cyclase attenuates pulmonary fibrosis [abstract no. O9]. BMC Pharmacol. 2011;11(Suppl. 1).
Geschka S, Kretschmer A, Sharkovska Y, et al. Soluble guanylate cyclase stimulation prevents fibrotic tissue remodeling and improves survival in salt-sensitive Dahl rats. PLoS One. 2011;6(7):e21853.
Kojonazarov B, Lang M, Weissmann N, et al. Effects of riociguat on pulmonary vascular remodeling in severe experimental pulmonary hypertension [abstract no. A2516]. Am J Respir Crit Care Med. 2011;183(1 MeetingAbstracts).
Lang M, Kojonazarov B, Tian X, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS One. 2012;7(8):e43433.
Pichl A, Parajuli N, Seimetz M, et al. Stimulation of soluble guanylate cyclase by riociguat prevents tobacco smoke-induced pulmonary hypertension in mice [abstract no. A310]. Pneumologie. 2012;66(6).
Sharkovska Y, Kalk P, Lawrenz B, et al. Nitric oxide-independent stimulation of soluble guanylate cyclase reduces organ damage in experimental low-renin and high-renin models. J Hypertens. 2010;28(8):1666–75.
Weissmann N, Lobo B, Pichl A, et al. Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am J Respir Crit Care Med. 2014;189(11):1359–73.
Frey R, Muck W, Unger S, et al. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521: an ascending-dose study in healthy male volunteers. J Clin Pharmacol. 2008;48(8):926–34.
Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J. 2009;33(4):785–92.
Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J. 2010;36(4):792–9.
Ghofrani H-A, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29.
Ghofrani H-A, Galié N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–40.
Bayer HealthCare Pharmaceuticals Inc. Briefing document for the Cardiovascular and Renal Drugs Advisory Committee: riociguat (BAY 63-2521). 2013. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm363543.pdf. Accessed 5 June 2014.
Frey R, Muck W, Kirschbaum N, et al. Riociguat (BAY 63-2521) and warfarin: a pharmacodynamic and pharmacokinetic interaction study. J Clin Pharmacol. 2011;51(7):1051–60.
Frey R, Muck W, Unger S, et al. No pharmacodynamic (PD) and pharmacokinetic (PK) interaction of riociguat (BAY 63-2521) and aspirin [abstract no. P3977]. In: 21st Annual Congress of the European Respiratory Society; 24–28 Sep 2011; Amsterdam.
Galie N, Neuser D, Muller K, et al. A placebo-controlled, double-blind phase II interaction study to evaluate blood pressure following addition of riociguat to patients with symptomatic pulmonary arterial hypertension (pah) receiving sildenafil (patent Plus) [abstract no. A3530]. Am J Respir Crit Care Med. 2013;187(1_MeetingAbstracts).
Becker C, Frey R, Hesse C, et al. Absorption behavior of riociguat: bioavailability, food effects, and dose-proportionality [abstract no. P7]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):22.
Becker C, Frey R, Unger S, et al. Pharmacokinetic interaction of ketoconazole, clarithromycin, and midazolam with riociguat [abstract no. P5]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):19–22.
Rickert V, Haefeli WE, Weiss J. Pharmacokinetic interaction profile of riociguat, a new soluble guanylate cyclase stimulator, in vitro. Pulm Pharmacol Ther. 2014. doi:10.1016/j.pupt.2014.02.004.
Frey R, Becker C, Unger S, et al. Effect of omeprazole and AlOH/MgOH on riociguat absorption [abstract no. P4079]. In: 23rd Annual Congress of the European Respiratory Society; 7–11 Sep 2013; Barcelona.
Frey R, Becker C, Unger S, et al. Pharmacokinetics of the soluble guanylate cyclase stimulator riociguat in individuals with hepatic impairment [abstract no. P21]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):33–4.
Frey R, Becker C, Unger S, et al. Pharmacokinetics of the soluble guanylate cyclase stimulator riociguat in individuals with renal impairment [abstract no. P22]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):34–6.
Frey R, Lettieri J, Nadel A, et al. Effects of age and gender on the pharmacokinetics of the soluble guanylate cyclase stimulator riociguat [abstract no. P23]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):36–7.
US FDA. Center for Drug Evaluation and Research medical review document for riociguat. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204819Orig1s000MedR.pdf. Accessed 5 Sep 2014.
Ghofrani H, Hoeper M, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: long-term safety, tolerability, and efficacy [abstract no. A2370]. Am J Respir Crit Care Med. 2012;185(Online abstracts).
Ghofrani H-A, Simonneau G, Rubin LJ, et al. Riociguat for pulmonary hypertension. N Engl J Med. 2013;369(23):2266–8.
Ghofrani HA, Grimminger F, Hoeper MM, et al. Impact of riociguat on health-related quality of life (HRQoL) in patients with chronic thromboembolic pulmonary hypertension (CTEPH) [abstract no. P3418]. In: 23rd Annual Congress of the European Respiratory Society; 7–11 Sep 2013; Barcelona.
Simonneau G, D’Armini A, Ghofrani AH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension (CTEPH): 2-year results from the CHEST-2 long-term extension [abstract]. In: European Respiratory Society International Congress; 6–10 Sep 2014; Munich.
Wang C, D’Armini AM, Ghofrani H-A, et al. Long-term riociguat treatment in inoperable and persistent/recurrent CTEPH patients in WHO functional class (FC) I/II versus FC III/IV at baseline: results from the 16-week phase III CHEST-1 study and CHEST-2 open-label extension [abstract no. 535B]. Chest. 2014;145(3_MeetingAbstracts).
Galié N, Grimminger F, Grünig E, et al. Correlation of improvements in hemodynamics and exercise capacity in patients with PAH: Results from the phase III PATENT-1 study [abstract no. P1784]. In: 23rd Annual Congress of the European Respiratory Society; 7–11 Sep 2013; Barcelona.
Rubin L, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): 2-year results from the PATENT-2 long-term extension [abstract] In: European Respiratory Society International Congress; 6–10 Sep 2014; Munich.
Meyer G, Galié N, Grimminger F, et al. Long-term riociguat treatment in PAH patients in WHO functional class (FC) I/II versus FC III/IV at baseline: results from the 12-week phase III PATENT-1 study and PATENT-2 open-label extension [abstract no. 513A]. Chest. 2014;145(3_MeetingAbstracts).
Mittendorf J, Weigand S, Alonso-Alija C, et al. Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. Chem Med Chem. 2009;4(5):853–65.
European Medicines Agency. Adempas (riociguat): Committee for Medicinal Products for Human Use assessment report. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002737/WC500165036.pdf. Accessed 8 July 2014.
European Medicines Agency. Committee for Medicinal Products for Human Use guideline on the clinical investigations of medicinal products for the treatment of pulmonary arterial hypertension. 2009. http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500016686. Accessed 3 Oct 2014.
Archer SL. Riociguat for pulmonary hypertension–a glass half full. N Engl J Med. 2013;369(4):386–8.
Bayer. Riociguat clinical effects studied in patients with insufficient treatment response to phosphodiesterase-5 inhibitor (RESPITE) [ClinicalTrials.gov identifier NCT02007629]. US National Institutes of Health, ClinicalTrials.gov. 2014. http://www.clinicaltrials.gov. Accessed 8 Sep 2014.
Bayer. Riociguat in patients with chronic thromboembolic pulmonary hypertension (CTEPH) (EAS) [ClinicalTrials.gov identifier NCT01784562]. US National Institutes of Health, ClinicalTrials.gov. 2014. http://www.clinicaltrials.gov. Accessed 8 July 2014.
Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128(5):502–11.
Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;. doi:10.1378/chest.14-0106.
Ghofrani HA, Staehler G, Gruenig E, et al. The effect of the soluble guanylate cyclase stimulator riociguat on hemodynamics in patients with pulmonary hypertension due to chronic obstructive pulmonary disease [abstract no. A6127]. Am J Respir Crit Care Med. 2011;183(1_MeetingAbstracts).
Hoeper MM, Halank M, Wilkens H, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J. 2013;41(4):853–60.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit. K. P. Garnock-Jones is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garnock-Jones, K.P. Riociguat: A Review of Its Use in Patients with Chronic Thromboembolic Pulmonary Hypertension or Pulmonary Arterial Hypertension. Drugs 74, 2065–2078 (2014). https://doi.org/10.1007/s40265-014-0317-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0317-2