Abstract
Purpose
Obstructive sleep apnea (OSA) and insomnia are very prevalent disorders, especially in sleep-lab setting, and insomnia may be the presenting complaint of OSA. Here, we aimed to validate No-Apnea as screening tool for OSA in patients with self-reported insomnia complaints and to compare its performance with other models.
Methods
This cross-sectional study involved evaluation of No-Apnea as well as STOP-Bang, NoSAS and Epworth Sleepiness Scale (ESS) in subjects with insomnia being evaluated with full in-lab polysomnography. Discrimination was assessed by area under the curve (AUC), while predictive parameters were calculated by contingency tables. OSA severity was classified based on the apnea/hypopnea index: ≥ 5.0/h as any OSA (OSA≥5), ≥ 15.0/h as moderate/severe OSA (OSA≥15), and ≥ 30.0/h as severe OSA (OSA≥30).
Results
Overall, 2591 patients with a clinical diagnosis of insomnia were included. Diagnosis of OSA≥5, OSA≥15, and OSA≥30 was of 76.3%, 53.1%, and 32.6%, respectively. At all levels of OSA severity, No-Apnea had sensitivity ranging from 84.5 to 94.1% and specificity ranging from 58.2 to 35.1%. For screening of OSA≥5, OSA≥15, and OSA≥30, discriminatory ability (AUC) of No-Apnea was: 0.790 [95% confidence interval (CI) 0.770–0.810], 0.758 (95% CI 0.740–0.777), and 0.753 (95% CI 0.734–0.772), respectively. Based on AUCs, No-Apnea, STOP-Bang, and NoSAS performed similar at all levels of OSA severity. The ESS did not present satisfactory discrimination as OSA screening model.
Conclusions
In a large sample of patients with insomnia, No-Apnea, STOP-Bang, and NoSAS, but not ESS, enable satisfactory and similar discrimination at all levels of OSA severity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a very prevalent disease [1, 2], especially in subjects referred to a sleep laboratory, or in specific populations such as individuals undergoing preoperative assessments for bariatric surgery [3] and those with resistant hypertension [4] or stroke [5]. Currently, the prevalence of OSA has been revisited and appears to be in the rise, possibly due to the obesity epidemic and the aging of the population, with estimated prevalence of moderate to severe OSA of 13% among men and of 6% among women [1], and of 49.7% in men and 23.4% in women [2]. OSA is characterized by recurrent upper airway obstructive episodes, resulting in intermittent hypoxemia, sleep fragmentation, cardiovascular and metabolic consequences, and increased overall mortality [6].
Another highly prevalent sleep disorder is insomnia, and according to the definition used insomnia rates will vary ranging from 6 to 48% [7]. In general, the clinical diagnosis of insomnia is reached by evaluating specific sets of symptoms and their duration, namely difficulty in starting sleep, difficulty in maintaining sleep, and early morning awakenings [8, 9]. Consistent risk factors for insomnia include aging, female gender, underlying psychiatric disorder, shift work, unemployment, and lower socioeconomic status [8,9,10]. Therefore, appropriate screening for potential co-morbid OSA in insomnia patients may be imperative, particularly since the presence of co-morbid insomnia may also affect OSA treatment outcomes [11].
Specifically in limited-resource areas with a high prevalence of OSA, the use of screening instruments to identify high-risk patients for this disorder can be extremely helpful. The No-Apnea model [12] is a recently developed and validated practical instrument that includes only two objective parameters: neck circumference (NC) and age, with a total score ranging from 0 to 9 points. The cutoff point chosen for this tool was ≥ 3 to classify patients at high-risk of having OSA at all levels of severity. In the derivation cohort, the discrimination, assessed by area under the curve (AUC), for screening of any OSA (OSA≥5), moderate/severe (OSA≥15), and severe OSA (OSA≥30) was as follows: 0.784, 0.758, and 0.754, respectively. Subsequently, the model was validated confirming its reproducibility. Importantly, No-Apnea discriminatory ability, in a high pre-test probability of OSA population, was similar to those of STOP-Bang questionnaire or NoSAS score [12].
Despite the high frequency of insomnia symptoms in patients referred for polysomnography (PSG), the use of instruments for OSA screening in this population is scarce. In addition, insomnia cohorts exhibit higher prevalence of women in relation to the general population, and the symptoms of OSA among women differ from those in men [13]. Based on aforementioned considerations, it remains unclear whether questionnaires frequently applied to patients with suspected OSA could also be successfully implemented in insomniac patients. Accordingly, the aims of the present study were: (i) to validate the No-Apnea tool in a sample of consecutive adult patients with self-reported insomnia complaints and (ii) to compare its performance with those obtained from three other frequently used screening instruments, namely STOP-Bang questionnaire, NoSAS score, and Epworth Sleepiness Scale (ESS).
Methods
Study Design and Patient Selection
This cross-sectional study prospectively enrolled consecutive subjects with self-reported insomnia, from January 2017 to December 2018. All patients were referred for sleep test evaluation by their respective attending physicians. Inclusion criteria were subjects of both genders, aged ≥ 18 years, with least one symptom compatible with the clinical diagnosis of insomnia. Patients were excluded for any of the following reasons: previously diagnosed OSA, use of portable studies for OSA diagnosis, incomplete clinical data, and technically inadequate PSG. Patient characteristics included gender, age, body-mass index (BMI), NC, and self-reported comorbidities (hypertension, diabetes mellitus, and smoking). The BMI was calculated by dividing the weight in kilograms by the square of the height in meters (kg/m2), while NC (in cm) was systematically measured using a tape measure. On the evening of the PSG, all demographic, anthropometric, and clinical data were collected by qualified sleep technicians, in addition to completing the instruments: No-Apnea, STOP-Bang, NoSAS, and ESS.
Ethical Considerations
The study protocol complied with the Declaration of Helsinki and was approved by Ethics Committee of the Federal University of Rio de Janeiro (#1.764.165). All participants provided written informed consent before study enrollment.
Insomnia Definition
Insomnia was defined as present if a patient indicated one or more of the following complaints, which were investigated through a semi-structured interview: (i) difficulty initiating sleep, (ii) difficulty maintaining sleep, and/or (iii) waking up earlier than desired, representing initial insomnia, middle insomnia, and late insomnia, respectively. Furthermore, this sleep disorder has to occur at least three nights a week for a period of ≥ 3 months and to be related to the presence of daytime impairments [14].
Screening Instruments
No-Apnea [12] is a 2-item instrument (NC and age): NC is scored as follows: 37.0–39.9 cm (1 point), 40.0–42.9 cm (3 points), and ≥ 43.0 cm (6 points), while age is scored as follows: 35–44 years (1 point), 45–54 years (2 points), ≥ 55 years (3 points), totaling a score of 0–9 points. A score ≥ 3 points was considered as high risk of presence of OSA.
STOP-Bang [15] is a tool containing eight yes-or-no questions (1 point for each positive answer): loud snoring, tiredness, observed apnea, hypertension, BMI > 35 kg/m2, age > 50 years, NC > 40 cm, and male gender, totaling a score of 0–8 points. A score ≥ 3 points was considered as high risk of presence of OSA.
NoSAS [16] is an instrument containing five parameters: NC > 40 cm (4 points), BMI 25.0–29.9 kg/m2 (3 points), BMI ≥ 30.0 kg/m2 (5 points), snoring (2 points), age > 55 years (4 points), male gender (2 points); totaling a score of 0–17 points. A score ≥ 8 points was considered as high risk of presence of OSA.
ESS [17] is a widely and extensively used 8-item questionnaire that assesses the subjective likelihood of falling asleep in various settings. Each item is scored from zero (would never doze) to three (high chance of dozing), totaling a score of 0–24 points. A score ≥ 11 points was considered indicative of excessive daytime sleepiness.
Sleep Test
All PSG were conducted at a single Brazilian sleep center: Sleep Laboratory - Centro Medico BarraShopping, Rio de Janeiro. All patients underwent an attended, full PSG (EMBLA® S7000, Embla Systems, Inc., Broomfield, CO, USA), consisting of continuous monitoring of electroencephalography, electrooculography, electromyography (chin and legs), electrocardiography, airflow, thoracic and abdominal impedance belts for respiratory effort, oxygen saturation (SpO2), snoring microphone, and body position sensors. Data from PSG were manually scored by two board-certified sleep physicians in accordance with previous guidelines [18], and both physicians were blinded for No-Apnea, STOP-Bang, NoSAS, and ESS results. Apneas were classified with a drop ≥ 90% of baseline in airflow lasting at least 10 s, while hypopneas were defined as a drop ≥ 30% of pre-event during ≥ 10 s and were associated with more than 3% oxygen desaturation or an arousal [18]. Diagnosis of OSA was based on an apnea/hypopnea index (AHI) ≥ 5.0/h, being its severity classified according to AHI thresholds: ≥ 5.0/h (OSA≥5), ≥ 15.0/h (OSA≥15), and ≥ 30.0/h (OSA≥30).
Statistical Analysis
Data analysis was performed using SPSS (version 21.0; Chicago, IL, USA). Results were reported as mean ± standard deviation for quantitative variables and as number and percentage for qualitative variables. Comparisons between groups were performed using the Chi-square test for dichotomous variables, Student’s t test and univariate analysis of variance (ANOVA) for continuous variables. Discrimination, the ability of a model to distinguish between patients with and without different outcomes, was estimated from the AUC obtained by receiver operator characteristic (ROC) curves, which may range from 0.5 (no discrimination) to 1.0 (perfect discrimination) [19]. An AUC > 0.7 was considered as clinically significant, being that the AUCs obtained were compared using prior algorithm [20]. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy (rate of correctly classified patients) were calculated using contingency tables, being all estimates reported with their respective 95% confidence interval (CI). All two-tailed tests were performed at a 5% significance level.
Results
Study Population
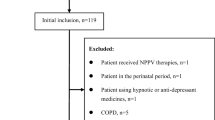
A flowchart illustrating the study approach is summarized in Fig. 1, being the study population composed by 2591 subjects, with subtypes of insomnia divided as follows: 1160 subjects (44.8%) had sleep-onset insomnia, 1164 subjects (44.9%) had middle of the night insomnia, and 1682 subjects (64.9%) had early morning insomnia. Characteristics of the patients with insomnia are listed in Table 1, being that 56.3% were females. Overall, 74.4%, 79.1%, 64.3%, and 42.8% of the patients were classified as high risk of OSA patients according to No-Apnea, STOP-Bang, NoSAS, and ESS, respectively. As would be anticipated from the study design and the high pre-test risk inherent to a sleep-lab referral cohort, we found a high prevalence of OSA≥5 (76.3%), OSA≥15 (53.1%), and OSA≥30 (32.6%). The prevalence of OSA≥5, OSA≥15, and OSA≥30 was statistically higher in males than in females: 88.2% versus 67.1% (p < 0.001), 69.1% versus 40.6% (p < 0.001), and 48.8% versus 20.0% (p < 0.001), respectively. The probability of having OSA≥5, OSA≥15, and OSA≥30 was higher in men than in women: odds ratio (OR) 3.656 (95% CI 2.961–4.513), OR 3.276 (95% CI 2.781–3.859), and OR 3.813 (95% CI 3.207–4.535), respectively.
Predicting OSA
Table 2 shows the predictive performance of the four screening tools evaluated. For screening of different levels of OSA severity, No-Apnea tool had sensitivity ranging from 84.5 to 94.1% and specificity ranging from 58.2 to 35.1%. Among all instruments, STOP-Bang showed a highest sensitivity for screening of OSA≥5 (88.2%), OSA≥15 (92.5%), and OSA≥30 (96.7%). For screening of OSA≥5, NoSAS showed a higher specificity (69.3%), while for screening of OSA≥15 and OSA≥30, the highest specificity was obtained by ESS: 63.8% and 62.2%, respectively. As can be seen in Fig. 2, ESS did not show adequate discrimination for screening of OSA≥5, OSA≥15, and OSA≥30. Conversely, No-Apnea, STOP-Bang, and NoSAS emerged as adequate screening tools for OSA in patients with insomnia (all AUCs > 0.7 at all levels of OSA severity). In addition, at all levels of OSA severity, there were no statistically significant differences when comparing the discriminatory power obtained by No-Apnea, STOP-Bang, and NoSAS (all comparisons with p value > 0.05).
Discriminatory ability–reported as area under the curve (95% confidence interval)–obtained by No-Apnea, STOP-Bang, NoSAS, and Epworth Sleepiness Scale (ESS) for screening of obstructive sleep apnea (OSA). OSA severity was classified based on the apnea/hypopnea index: ≥ 5.0/h as any OSA (OSA≥5), ≥ 15.0/h as moderate/severe OSA (OSA≥15), and ≥ 30.0/h as severe OSA (OSA≥30). In predicting OSA≥5, OSA≥15, and OSA≥30, No-Apnea performed similar when compared with STOP-Bang or NoSAS: p = 0.532 and p = 0.592; p = 0.444 and p = 0.705; p = 0.182 and p = 0.743, respectively. In predicting OSA≥5, OSA≥15, and OSA≥30, STOP-Bang performed similar when compared with NoSAS: p = 0.246; p = 0.253; and p = 0.096, respectively. No-Apnea, STOP-Bang, and NoSAS presented higher discrimination than that presented by ESS at all levels of OSA severity (all p-values < 0.001)
Discussion
The present study, with a large sample of prospectively recruited patients with insomnia and who were referred to a sleep laboratory, showed that the screening instruments No-Apnea, NoSAS, and STOP-Bang, but not the ESS, were useful to detect patients at-risk of OSA. Furthermore, despite its obvious simplicity and objectivity, the discrimination obtained by No-Apnea was similar to those exhibited by the STOP-Bang and NoSAS models. The discriminatory ability of a model to distinguish between patients with and without a specific condition was estimated from the AUC, which plots the true positive rate against false positive rate for consecutive cut-points for the probability of a defined condition being present or absent [19]. In addition, it is possible to compare the AUCs obtained by different screening approaches using previously described algorithms [20]. Similar to the No-Apnea derivation and validation study [12], the cutoff point ≥ 3 was associated with high sensitivity and moderate specificity. Of particular importance is the fact that No-Apnea is a 2-item tool, while the STOP-Bang is an 8-item instrument and the NoSAS is a 5-item instrument: this simplified approach clearly enables much greater facility and ease of screening of OSA, particularly among high pre-test probability populations [12, 21]. Identical findings evidencing similar discriminatory capacity between No-Apnea, STOP-Bang, and NoSAS were also observed in the No-Apnea derivation and validation study [12] as well as in the study involving morbidly obese patients [22].
Accordingly, high-risk patients can be properly designated for portable diagnostic methods and thus reduce long waiting lists in sleep centers across many countries. Furthermore, since the No-Apnea does not contain subjective variables, it can be used in patients in whom sleep-related information from the bed partner is not always available. These findings are particularly relevant, since some studies have shown a high prevalence of insomnia symptoms among patients referred for PSG [23,24,25].
Both OSA and insomnia are considered as risk factors for cardiovascular disease [26, 27], end organ damage [28, 29], and are associated with an increase in direct and indirect healthcare and overall economic costs [30, 31]. Although OSA and insomnia are very prevalent disorders, especially in sleep-lab individuals, some differences should be emphasized: (i) the diagnosis of OSA is based on objective sleep study, while the diagnosis of insomnia relies on clinical history [32] and (ii) OSA is a disease that most commonly affects men, while insomnia is typically more common among women [33, 34]. Our findings also showed a preponderance of women over men in individuals with insomnia; however, the male gender was associated with a higher prevalence of OSA than female gender at all levels of OSA severity.
Sleep laboratories around the world have large numbers of patients with suspected OSA waiting to be tested. The gold standard for diagnosis of OSA is overnight in-lab PSG; however, the prevalence of OSA is far higher than the volume of patients that can be handled by the available sleep laboratories around the world. To better address the utilization of scarce resources and detect those patients more likely to benefit from such onerous diagnostic test, several instruments have been developed and published in the literature. Moreover, we should also emphasize that the performance of an OSA screening tool may exhibit considerable variability, which is usually related to the patient population and AHI thresholds employed [35].
The STOP-Bang is a nowadays widely used mnemonic screening approach that was initially developed for screening surgical patients showing the following reported characteristics: sensitivity: 83.6%, specificity: 56.4%, PPV: 81.0%, and NPV: 60.8% [15]. The yield of the STOP-Bang in screening sleep clinic patients for OSA was previously evaluated [36]: to detect OSA≥5, OSA≥15, and OSA≥30, sensitivity ranged from 90 to 96% and specificity ranged from 49 to 25%, respectively. In addition, the AUC was consistently > 0.72 for all OSA severities [36].
In the HypnoLaus cohort, NoSAS identified individuals at high-risk of having clinically OSA (defined as an AHI ≥ 20.0 events/h) with an AUC of 0.74, while in the EPISONO cohort, it performed with an AUC of 0.81 [16]. Afterwards, this instrument was validated in different settings, always reporting adequate performance as screening model for OSA: in a multiethnic Asian cohort [37], in a hospital-based sample [38, 39], and in subjects suffering from depressive disorder [40].
Similar to our findings, ESS was deemed insufficiently accurate as a screening tool for OSA, possibly because it is based on the level of excessive daytime sleepiness, which is not always present in OSA [41,42,43,44]. Although ESS was not specifically developed for OSA screening but rather for excessive daytime sleepiness, this tool has been widely used in several OSA-related studies.
Limitations and Strengths
Our study has some obvious limitations based on its design, since it examined the screening instruments in referred patients with a high pre-test probability, which may limit its external validity. In addition, it was performed at a single institution, and its implications for the general population or other sleep centers may also vary. Another possible limitation is that the diagnosis of insomnia was merely subjective through self-reported data, being that patients suffering from insomnia may underestimate their sleep times and overestimate their waking times. Conversely, the present study has several important strengths: a large sample of patients consecutively and prospectively recruited, all of them evaluated with full PSG and scored according to the current guidelines proposed in 2012 by the American Academy of Sleep Medicine [18]. Furthermore, this is the first study that was effectively designed to assess differences in No-Apnea, STOP-Bang, NoSAS, and ESS performance among subjects with insomnia and referred for PSG.
Conclusions
The No-Apnea, a 2-item instrument, showed adequate discrimination and predictive performance for diagnosis of OSA≥5, OSA≥15, and OSA≥30 with a cutoff point ≥ 3. Its performance was comparable to those of STOP-Bang and NoSAS, besides being superior to ESS. Further studies evaluating the applicability of No-Apnea as a referral tool for OSA diagnosis in the context of primary care setting among patients with a primary complaint of insomnia should be forthcoming.
References
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177:1006–1014
Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N et al (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3:310–318
Duarte RL, Magalhães-da-Silveira FJ (2015) Factors predictive of obstructive sleep apnea in patients undergoing pre-operative evaluation for bariatric surgery and referred to a sleep laboratory for polysomnography. J Bras Pneumol 41:440–448
Muxfeldt ES, Margallo VS, Guimarães GM, Salles GF (2014) Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens 27:1069–1078
Johnson KG, Johnson DC (2010) Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med 6:131–137
Muraja-Murro A, Kulkas A, Hiltunen M, Kupari S, Hukkanen T, Tiihonen P et al (2013) The severity of individual obstruction events is related to increased mortality rate in severe obstructive sleep apnea. J Sleep Res 22:663–669
Ohayon MM (2002) Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 6:97–111
Chung KF (2005) Insomnia subtypes and their relationships to daytime sleepiness in patients with obstructive sleep apnea. Respiration 72:460–465
Krell SB, Kapur VK (2005) Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath 9:104–110
Taylor D, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ (2007) Comorbidity of chronic insomnia with medical problems. Sleep 30:213–218
Wallace DM, Sawyer AM, Shafazand S (2018) Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath 22:5–15
Duarte RLM, Rabahi MF, Magalhães-da-Silveira FJ, de Oliveira-e-Sá TS, Mello FCQ, Gozal D (2018) Simplifying the screening of obstructive sleep apnea with a 2-item model, No-Apnea: a cross-sectional study. J Clin Sleep Med 14:1097–1107
Nigro CA, Dibur E, Borsini E, Malnis S, Ernst G, Bledel I et al (2018) The influence of gender on symptoms associated with obstructive sleep apnea. Sleep Breath 22:683–693
American Association of Sleep Medicine (2014) ICSD-3 International Classification of Sleep ICSD-3 Disorders. American Association of Sleep Medicine, Dartmouth
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S et al (2008) STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 108:812–821
Marti-Soler H, Hirotsu C, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M et al (2016) The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 4:742–748
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK et al (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med 8:597–619
Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N et al (2010) Assessing the performance of prediction models: a framework for some traditional and novel measures. Epidemiology 21:128–138
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Duarte RLM, Fonseca LBM, Magalhães-da-Silveira FJ, Silveira EAD, Rabahi MF (2017) Validation of the STOP-Bang questionnaire as a means of screening for obstructive sleep apnea in adults in Brazil. J Bras Pneumol 43:456–463
Duarte RLM, Mello FCQ, Magalhães-da-Silveira FJ, Oliveira-e-Sá TS, Rabahi MF, Gozal D (2019) Comparative performance of screening instruments for obstructive sleep apnea in morbidly obese patients referred to a sleep laboratory: a prospective cross-sectional study. Sleep Breath. https://doi.org/10.1007/s11325-019-01791-w
Hagen C, Patel A, McCall WV (2009) Prevalence of insomnia symptoms in sleep laboratory patients with and without sleep apnea. Psychiatry Res 170:276–277
Mysliwiec V, Gill J, Lee H, Baxter T, Pierce R, Barr TL et al (2013) Sleep disorders in US military personnel. A high rate of comorbid insomnia and obstructive sleep apnea. Chest 144:549–557
Smith S, Sullivan K, Hopkins W, Douglas J (2004) Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS). Sleep Med 5:449–456
Li M, Zhang XW, Hou WS, Tang ZY (2014) Insomnia and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol 176:1044–1047
Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF (2014) Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 21:57–64
Lu JL, Freire AX, Molnar MZ, Kalantar-Zadeh K, Kovesdy CP (2018) Association of chronic insomnia with mortality and adverse renal outcomes. Mayo Clin Proc 93:1563–1570
Molnar MZ, Mucsi I, Novak M, Szabo Z, Freire AX, Huch KM et al (2015) Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax 70:888–895
Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J (2009) The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 32:55–64
Léger D, Bayon V (2010) Societal costs of insomnia. Sleep Med Rev 14:379–389
Subramanian S, Guntupalli B, Murugan T, Bopparaju S, Chanamolu S, Casturi L et al (2011) Gender and ethnic differences in prevalence of self-reported insomnia among patients with obstructive sleep apnea. Sleep Breath 15:711–715
Lichstein KL, Thomas SJ, Woosley JA, Geyer JD (2013) Co-occurring insomnia and obstructive sleep apnea. Sleep Med 14:824–829
Krakow B, Melendrez D, Ferreira E, Clark J, Warner TD, Sisley B et al (2001) Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest 120:1923–1929
Abrishami A, Khajehdehi A, Chung F (2010) A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anesth 57:423–438
Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S et al (2015) Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS ONE 10:e0143697. https://doi.org/10.1371/journal.pone.0143697.eCollection2015
Tan A, Hong Y, Tan LWL, van Dam RM, Cheung YY, Lee CH (2017) Validation of NoSAS score for screening of sleep-disordered breathing in a multiethnic Asian population. Sleep Breath 21:1033–1038
Hong C, Chen R, Qing S, Kuang A, Yang H, Su X et al (2018) Validation of the NoSAS score for the screening of sleep-disordered breathing: a hospital-based retrospective study in China. J Clin Sleep Med 14:191–197
Peng M, Chen R, Cheng J, Li J, Liu W, Hong C (2018) Application value of the NoSAS score for screening sleep-disordered breathing. J Thorac Dis 10:4774–4781
Guichard K, Marti-Soler H, Micoulaud-Franchi JA, Philip P, Marques-Vidal P, Vollenweider P et al (2018) The NoSAS score: a new and simple screening tool for obstructive sleep apnea syndrome in depressive disorder. J Affect Disord 227:136–140
Vana KD, Silva GE, Goldberg R (2013) Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health 36:84–94
Panchasara B, Poots AJ, Davies G (2017) Are the Epworth Sleepiness Scale and Stop-Bang model effective at predicting the severity of obstructive sleep apnoea (OSA); in particular OSA requiring treatment? Eur Arch Otorhinolaryngol 274:4233–4239
Duarte RLM, Rabahi MF, Oliveira-e-Sá TS, Magalhães-da-Silveira FJ, Mello FCQ, Gozal D (2019) Fractional exhaled nitric oxide measurements and screening of obstructive sleep apnea in a sleep-laboratory setting: a cross-sectional study. Lung 197:131–137
Mediano O, Barceló A, de la Peña M, Gozal D, Agustí A, Barbé F (2007) Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J 30:110–113
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duarte, R.L.M., Magalhães-da-Silveira, F.J., Oliveira-e-Sá, T.S. et al. Predicting Obstructive Sleep Apnea in Patients with Insomnia: A Comparative Study with Four Screening Instruments. Lung 197, 451–458 (2019). https://doi.org/10.1007/s00408-019-00232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-019-00232-5