Abstract
Extensive small-cell lung cancer (SCLC) is commonly treated with multiple cycles of chemotherapy. Reducing the time interval between cycles of chemotherapy (dose-dense chemotherapy) may improve outcomes in the treatment of extensive SCLC, as it has in other chemosensitive malignancies. To evaluate the feasibility of dose-dense chemotherapy in patients with extensive SCLC, this study evaluates a dose-dense doxorubicin/cyclophosphamide/etoposide (ACE) regimen, supported by the once-per-cycle administration of the hematopoietic growth factor pegfilgrastim. Patients received up to six 14-day cycles of ACE chemotherapy (doxorubicin 40 mg/m,2 cyclophosphamide 1000 mg/m2, etoposide 120 mg/m2 on day 1 IV, plus oral etoposide 240 mg/m2 daily on days 2–3). On day 4 of each cycle, patients received pegfilgrastim 6 mg by subcutaneous injection. Of 30 patients enrolled, 27 started chemotherapy and received pegfilgrastim. Full-dose, on-schedule chemotherapy was given to all 22 patients starting cycle 2, and in 107 (88%) of 121 cycles. Eighteen of the 27 patients (67%) received full-dose, on-schedule chemotherapy for all 6 cycles. The overall response rate was 17/27 (63%). Nine patients (33%) experienced hematologic toxicities that investigators considered severe or life-threatening. Four patients (15%) had febrile neutropenia. Full-dose, on-schedule dose-dense ACE chemotherapy is feasible with once-per-cycle pegfilgrastim support in extensive SCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small-cell lung cancer (SCLC) is characterized by a high proliferation rate, early hematogenous metastases, and initial chemosensitivity. Extensive SCLC is commonly treated by multi-cycle chemotherapy. Although SCLC is a chemosensitive disease, the emergence of drug resistance results in a poor prognosis, with a mean survival after treatment for extensive disease of 6–12 months [22] and a 5-year survival rate of 2%–12% [12]. Several combination chemotherapy regimens have been used for extensive SCLC, with varying response rates.
In an effort to improve outcomes in SCLC, clinical studies have evaluated regimens in which the dose of chemotherapy given in each cycle was increased (dose intensification). These regimens showed little therapeutic benefit [13, 14]. Similarly, a dose-intensified regimen in which the dose was increased by 25% and the interval between cycles was reduced by 33% did not result in a significant improvement in either response rate or survival, but led to an increase in hematologic and nonhematologic toxicity [1].
An alternative approach to dose intensification is reducing the time interval between cycles of standard-dose chemotherapy (dose-dense chemotherapy), which may improve outcomes in the treatment of SCLC, as it has in chemosensitive malignancies including breast cancer and lymphoma [5, 7, 24]. In two studies of SCLC in patients with a good or moderate prognosis, dose-dense regimens of vincristine with ifosfamide, carboplatin, and etoposide (V-ICE) resulted in median survival rates of 443 days and 483 days (69 weeks); 2-year survival rates were 32% [32] and 33% [25]. A dose-dense regimen of doxorubicin/cyclophosphamide/etoposide (ACE) chemotherapy every 14 days with granulocyte colony-stimulating factor (G-CSF) support improved the complete response rate (40% vs. 28%) and the 2-year survival rate (13% vs. 8%) compared with similar doses at 21-day cycle intervals [28].
However, many chemotherapy regimens, including ACE, are associated with febrile neutropenia, for which the risk grows with increasing dose density. Neutropenia often occurs in the first cycle of chemotherapy and may result in dose reductions or treatment delays [18]. Dose reductions and treatment delays have a negative impact on survival. Conversely, maintenance of the scheduled chemotherapy regimen was shown to improve survival for patients with chemosensitive tumors in small-cell lung cancer [6], breast cancer [3–5], and lymphoma [8, 16, 17, 19].
By reducing the incidence and duration of febrile neutropenia, hematopoietic growth factor support enables administration of full-dose chemotherapy on schedule in conventional and dose-dense regimens, potentially increasing the chance of improved survival. An 11–14-day regimen of daily injections of the hematopoietic growth factor filgrastim in each cycle reduced the incidence of febrile neutropenia after ACE chemotherapy for SCLC in 21-day cycles (77% vs. 40% [13] and 53% vs. 26% [30]). Similar results were seen in dose-dense 14-day cycles [27].
Pegfilgrastim is a sustained-duration growth factor that preferentially stimulates the growth and differentiation of neutrophil precursors and the function of mature neutrophils. A single injection of pegfilgrastim per cycle of chemotherapy has been shown to be as effective and safe as multiple daily injections of filgrastim in studies of patients with breast cancer [9, 11], Hodgkin’s and non-Hodgkin’s lymphomas [10, 31], and non-small-cell lung cancer [15]. Preliminary studies in patients with hematologic malignancies suggest that pegfilgrastim may be useful in dose-dense chemotherapy [2, 21, 31]. Studies are needed to evaluate pegfilgrastim in dose-dense chemotherapy for patients with solid tumors.
This open-label, single-arm phase 2 multicenter study evaluates the use of pegfilgrastim to support a dose-dense ACE regimen in patients with extensive SCLC. It was hypothesized that patients receiving a fixed 6-mg dose of pegfilgrastim once per cycle beginning in cycle 1 would experience adequate absolute neutrophil count (ANC) and white blood cell (WBC) recovery, making it feasible to administer a full dose of cycle-2 chemotherapy on schedule. It was expected that pegfilgrastim would enable full-dose chemotherapy on schedule for subsequent cycles without increasing the risk of adverse events. The study also evaluates response rates and the safety of a dose-dense ACE regimen with pegfilgrastim support.
Patients and Methods
This study was conducted in accordance with the ethical principles defined in the International Conference on Harmonisation Tripartite Guideline on Good Clinical Practice effective in Europe from 17 January 1997. The protocol was approved by each institution’s ethics committee, and each enrolled patient provided written informed consent.
Patients were eligible if they were 19 years of age or older and had documented extensive SCLC not previously treated with chemotherapy or radiotherapy. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 [20], ANC ≥ 2.0 × 109/L, platelet count ≥ 100 × 109/L, adequate organ function, and a life expectancy ≥ 3 months. At least two weeks were required to have passed since patients underwent major thoracic or abdominal surgery.
Patients with symptomatic or previously untreated brain metastases, active infections or known HIV infection, clinically significant cardiac disease, or prior exposure to pegfilgrastim were excluded. Patients were also excluded if they had any premalignant or malignant myeloid condition or cancer other than SCLC within five years of enrollment, except for certain carcinomas.
This multicenter, open-label single-arm study evaluated a single, fixed 6-mg dose of pegfilgrastim (Neulasta® Amgen, Inc., Thousand Oaks, CA) in patients with SCLC who were receiving their first cycle of ACE chemotherapy on a dose-dense treatment schedule of 6 cycles with 14-day cycle intervals.
The primary endpoint was the proportion of patients receiving cycle-2 chemotherapy at full dose (> 75% and ≤ 125% of the protocol-specified dose) on schedule (14 ± 3 days after the start of the previous chemotherapy cycle). This endpoint reflects the extent of WBC and ANC recovery after cycle-1 chemotherapy.
Other efficacy endpoints included the proportion of all cycles given with full dose on schedule, the proportion of patients receiving full-dose, on-schedule chemotherapy over all cycles, and overall response to treatment [29]. The serum concentration of pegfilgrastim on the day before the next cycle of chemotherapy was also measured. A safety profile was developed for all cycles, including the incidence of febrile neutropenia (ANC < 0.5 × 109/L and oral temperature ≥ 38.2°C on the same day) and adverse events.
Patients underwent physical examinations, including measurement of baseline characteristics, disease staging, and ECOG performance status at baseline, at the start of each cycle and at the end of the study (within 7 days after cycle 6 or on the day of withdrawal from the study).
A complete blood count (CBC) was performed at screening, on day 1 of each cycle, weekly thereafter, and at the end of the study. Clinical chemistry tests were performed at screening, in each cycle within 7 days before the start of the next cycle, and at the end of the study. Serum was collected for measurement of pegfilgrastim on day 1 of each cycle and at the end of the study.
All patients received dose-dense ACE chemotherapy consisting of doxorubicin 40 mg/m2 and cyclophosphamide 1000 mg/m2 plus etoposide 120 mg/m2 by intravenous (IV) infusion on day 1, followed by oral etoposide 240 mg/m2 daily on days 2 and 3. This regimen was repeated every 14 days for up to 6 cycles. On day 4 of each cycle, patients received 6 mg of pegfilgrastim by subcutaneous injection. Disease assessment was performed after cycles 2, 4, and 6 (or on the day of withdrawal), and in case of clinical progression. Patients with progressive disease were withdrawn from the study. Cycles 2 through 6 were scheduled to start on day 1 if the patient had WBC ≥ 3.0 × 109/L, ANC ≥ 1.5 × 109/L, or platelets ≥ 100 × 109/L following the nadir. If WBC, ANC, or platelet counts were below these limits, chemotherapy was delayed until these counts were reached. If the next chemotherapy cycle was delayed by more than 14 days, the patient was withdrawn from the study. The protocol recommended that chemotherapy doses should not be reduced.
Concomitant medications or treatments deemed necessary for adequate supportive care were permitted, except for other investigational agents, other hematopoietic growth factors (except for erythropoietin for treatment of anemia), prophylactic corticosteroids (except as premedication for chemotherapy treatment), radiotherapy treatment, and WBC transfusions.
Results
Of 30 patients enrolled at four Austrian centers between November 2001 and May 2003, 27 (90%) started chemotherapy and received at least one pegfilgrastim injection; two patients died and one patient withdrew because of an allergic reaction before receiving pegfilgrastim. All patients who received pegfilgrastim were white, aged 46–76 yr (median, 60.0); 93% were male (Table 1).
Of the 27 patients included in the analysis, 17 (63%) completed all 6 cycles of chemotherapy and 15 (56%) completed the study. The most common reasons for discontinuation included death (3 patients, 11%), adverse events (2 patients, 7%), and disease progression (3 patients, 11%). A total of 121 cycles of chemotherapy were administered to 27 patients (range of cycles received, 1–6; mean, 4.5; median, 6).
All 22 patients who started cycle-2 chemotherapy received the full dose on schedule. Of the 5 patients who did not begin cycle-2 chemotherapy, 3 died and 2 withdrew (1 because of ineligibility [low platelet count] and 1 because of an adverse event [moniliasis]).
Of the 121 cycles of chemotherapy administered, 107 (88%) were at the full dose on schedule (Table 2). Of 27 patients, 18 (67%) received a full dose of chemotherapy on schedule for all cycles, while 8 patients (30%) experienced a delay in any cycle. One patient (4%) experienced a delay more than 3 days and a reduction in doses of doxorubicin and cyclophosphamide; no other patients had a greater than 25% dose reduction of any chemotherapy agent.
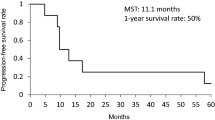
A treatment response at the end of the study was observed in 17 of 27 patients (63%) (77% of the 22 patients for whom an on-study disease assessment was made). Based on the analysis of all 27 patients, a complete response was reported for 2 patients (7%) and a partial response for 15 patients (56%) (Table 3).
Three patients (11%) died during the study, including one death from disease progression, one from sepsis, and one from pneumonia. One additional patient died of disease progression after withdrawing from the study. None of the deaths was considered pegfilgrastim-related.
As would be expected in this patient population, nearly all patients (26, 96%) reported 1 or more, adverse events (Table 4). Only one patient experienced a single adverse event that was considered study drug–related: moderate bone pain following pegfilgrastim administration in cycle 1. This event was not considered serious, no action was taken, and the patient continued in the study with no recurrence of bone pain in later cycles.
Nine patients (33%) experienced a hematologic toxicity considered by the investigator to be severe or life-threatening, and six patients (22%) experienced nonhematologic events considered severe or life-threatening (Table 5).
The mean pegfilgrastim serum concentration, assessed on day 1 of all cycles, was less than 1 ng/ml at the start of each cycle and correlated with ANC (Fig. 1). The maximum concentration was 2.8 ng/ml (a nonactive level), which occurred at the start of cycle 3 in one patient. The pegfilgrastim concentration at the start of cycle 2 was either below the active level or nondetectable in all patients for whom pharmacokinetic data were available
.
Discussion
Previous studies in patients with breast cancer [5, 24] and lymphoma [7], showed dose-dense chemotherapy with 14-day cycle intervals, supported by hematopoietic growth factors, may improve outcomes; similar results were seen in patients with SCLC who had a good or moderate prognosis [25, 32]. The present study builds on these findings, demonstrating that pegfilgrastim administered once per cycle enables the administration of dose-dense ACE chemotherapy to patients with extensive SCLC. All 22 patients who started cycle-2 chemotherapy received full-dose cycle-2 chemotherapy on schedule, and 88% of cycles overall (in 67% of patients) were administered at full dose on schedule.
Efficacy results in this study of patients with extensive disease are similar to those reported in the 1995 Thatcher study of filgrastim support for ACE chemotherapy in both limited and extensive SCLC. In that study, cycle-2 chemotherapy was administered on schedule to 72% of patients, and 58% of all cycles were administered on schedule [27].
In this study, 17 of 27 patients (63%) experienced an overall treatment response. This response rate is similar to those obtained in other studies of patients with SCLC. In a 1995 Thatcher study in which 35% of patients had extensive disease, the overall response rate was 85% [26]. In the 2000 Thatcher study of a dose-dense regimen, 22% of patients had extensive disease; the overall response rate was 78% [28]. In a 2002 study of irinotecan plus cisplatin versus etoposide plus cisplatin in which all patients had extensive SCLC, overall treatment response rates of 84.4% and 67.5%, respectively, were observed [23]. The 7% complete response rate in this study—only about one-third of the complete response rate observed in the Thatcher 2000 study—was disappointing. It may reflect the fact that all the patients in this study had extensive disease compared with only 22% of patients in the Thatcher study. The complete response rate in this study is similar to the 9.1% rate of complete response observed for patients receiving etoposide plus cisplatin in the Noda study [23], in which all patients had extensive disease.
Administration of dose-dense ACE 14 chemotherapy in the present study was possible without an increased safety risk, and adverse event profiles were similar to those seen in similar patient populations. Severe or life-threatening hematologic toxicities were mainly anemia (15%), febrile neutropenia (15%), thrombocytopenia (11%), and leukopenia (7%), but overall toxicity rates were low. In contrast to our study, in the Noda study in which growth factor support was used only after neutropenia or leukopenia occurred, a high proportion of patients experienced Grade 3 or 4 neutropenia (65% in the irinotecan–cisplatin group and 92.2% in the etoposide – cisplatin group; febrile neutropenia was not identified separately) [23]. Pegfilgrastim was self-regulating, as demonstrated by the pharmacokinetic observation that pegfilgrastim concentrations at the start of cycle 2 were below active or detectable levels.
The results of our study indicate that pegfilgrastim use allows for dose-dense ACE chemotherapy in patients with extensive SCLC with acceptable toxicity. The sustained-duration formulation of pegfilgrastim permits patients to receive hematopoietic support in a single injection per cycle. The results of the present study are important in advancing the development of chemotherapy treatment strategies for extensive SCLC, but further evaluation of this dose-dense regimen in comparison to a conventional-schedule chemotherapy regimen is warranted.
References
Ardizzoni A, Tjan-Heijnen VCG, Postmus PE, et al. (2002) Standard versus intensified chemotherapy with granylocyte colony-stimulating factor support in small-cell lung cancer: a prospective European Organization for Research and Treatment of Cancer—Lung Cancer Group Phase III Trial 08923. J Clin Oncol 20:3947–3955
Bentley MP, Norvath N, Lewis ID, et al. (2003) Single dose per cycle pegfilgrastim successfully supports full dose intensity CHOP-14 in patients over 60 years with non-Hodgldn’s lymphoma (NHL) and successfully mobilizes peripheral blood progenitor cells (PBPC). Blood 102:abstract 2348
Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332:901–906
Budman DR, Berry DA, Cirrincione CT, et al. (1998) Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 90:1205–1211
Citron ML, Berry DA, Cirrincione C, et al. (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21:1431–1439
Crawford J, Ozer H, Stoller R, et al. (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325:164–170
Dunlop DJ, Eatock MM, Paul J, et al. (1998) Randomized multicentre trial of filgrastim as an adjunct to combination chemotherapy for Hodgkin’s disease. West of Scotland Lymphoma Group. Clin Oncol (R Coll Radiol) 10:107–114
Epelbaum R, Faraggi D, Ben-Arie Y, et al. (1990) Survival of diffuse large cell lymphoma: a muitivariate analysis including dose intensity variables. Cancer 66:1124–1129
Green MD, Koelbl H, Baselga J, et al. (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35
Grigg A, Solal–Celigny P, Hoskin P, et al. (2003) Open-label, randomized study of pegfilgrastim vs. daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin’s lymphoma. Leuk Lymphoma 44:1503–1508
Holmes FA, Jones SE, O’Shaughnessy J, et al. (2002) Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol 13:903–909
Ihde DC (1995) Small cell lung cancer. State-of-the-art therapy 1994. Chest 107:243S–248S
Ihde DC, Mulshine JL, Kramer BS, et al. (1994) Prospective randomized comparison of high-dose and standard-dose etoposide and cisplatin chemotherapy in patients with extensive-stage small-cell lung cancer. J Clin Oncol 12:2022–2034
Johnson DH, Einhorn LH, Birch R, et al. (1987) A randomized comparison of high-dose versus conventional-dose cyclophosphamide, doxorubicin, and vincristine for extensive-stage small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 5:1731–1738
Johnston E, Crawford J, Blackwell S, et al. (2000) Randomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. J Clin Oncol 18:2522–2528
Kwak LW, Halpern J, Olshen RA, Horning SJ (1990) Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol 8:963–977
Lepage E, Gisselbrecht C, Haioun C, et al. (1993) Prognostic significance of received relative dose intensity in non-Hodgkin’s lymphoma patients: Application to LNH-87 protocol. Ann Oncol 4:651–656
Lyman GH, Morrison VA, Dale DC, et al. (2003) Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 44:2069–2076
Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI (2004) Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol 22:4302–4311
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Moore TD, Patel T, Segal ML, et al. (2003) A single pegfilgrastim dose per cycle supports dose-dense (q14d) CHOP-R in patients with non-Hodgkin’s lymphoma. Blood 102:abstract 2365
National Cancer Institute (2003) Small Cell Lung Cancer Treatment, vol 2004. US National Institutes of Health, Washington, DC
Noda K, Nishiwaki Y, Kawahara M, et al. (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91
Pfreundschuh M, Truemper L, Kloess M, et al. (2004) 2-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 104:634–641
Steward WP, von Pawel J, Gatzemeier U, et al. (1998) Effects of granulocyte–macrophage colony-stimulating factor and dose intensification of V-ICE chemotherapy in small-cell lung cancer: a prospective randomized study of 300 patients. J Clin Oncol 16:642–650
Thatcher N, Anderson H, Bleehen NM, et al. (1995) The feasibility of using glycosylated recombinant human granulocyte colony-stimulating factor (G-CSF) to increase the planned dose intensity of doxorubicin, cyclophosphamide and etoposide (ACE) in the treatment of small cell lung cancer. Medical Research Council Lung Cancer Working Party. Eur J Cancer 31A:152–156
Thatcher N, Clark PI, Smith DB, et al. (1995) Increasing and planned dose intensity of doxorubicin, cyclophosphamide and etoposide (ACE) by adding recombinant human methionyl granulocyte colony-stimulating factor (G-CSF; filgrastim) in the treatment of small cell lung cancer (SCLC). Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 7:293–299
Thatcher N, Girling DJ, Hopwood P, et al. (2000) Improving survival without reducing quality of life in small-cell lung cancer patients by increasing the dose-intensity of chemotherapy with granulocyte colony-stimulating factor support: results of a British Medical Research Council Multicenter Randomized Trial. Medical Research Council Lung Cancer Working Party. J Clin Oncol 18:395–404
Therasse P, Arbuck SG, Eisenhauer EA, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Trillet-Lenoir V, Green J, Manegold C, et al. (1993) Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer 29A:319–324
Vose JM, Crump M, Lazarus H, et al. (2003) Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol 21:514–519
Woll PJ, Hodgetts J, Lomax L et al. (1995) Can cytotoxic dose-intensity be increased by using granulocyte colony-stimulating factor? A randomized controlled trial of lenograstim in small-cell lung cancer. J Clin Oncol 13:652–659
Younes A, Fayad L, Romaguera J, et al. (2003) Single administration of a fixed dose pegfilgrastim (Neulasta) in inducing neutrophil count recovery after 170 consecutive doses of ABVD chemotherapy in patients with Hodgkin lymphoma: safety of pegfilgrastim with q14-day chemotherapy regimens. Blood 102:637a
Acknowledgments
The authors thank their coinvestigators and study nurses, particularly, Wilma Minar, RN, Dr. Christine Gomar–Höss, and Dr. Michael Shuhmacher. They also acknowledge the site and study management assistance of Thomas Horak and Felicity Norman, and the medical writing assistance of Joan O’Byrne and Sue Hudson
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pirker, R., Ulsperger, E., Messner, J. et al. Achieving Full-Dose, On-Schedule Administration of ACE Chemotherapy Every 14 Days for the Treatment of Patients with Extensive Small-Cell Lung Cancer. Lung 184, 279–285 (2006). https://doi.org/10.1007/s00408-005-2594-8
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00408-005-2594-8