Abstract
Parkinson’s disease (PD) is a chronic neurodegenerative disorder characterized by motor symptoms such as bradykinesia, rest tremor, postural disturbances, and rigidity. PD is also characterized by non-motor symptoms such as sleep disturbances, cognitive deficits, and psychiatric disorders such as psychosis, depression, and anxiety. The pharmacological treatment for these symptoms is limited in efficacy and induce significant adverse reactions, highlighting the need for better treatment options. Cannabidiol (CBD) is a phytocannabinoid devoid of the euphoriant and cognitive effects of tetrahydrocannabinol, and preclinical and preliminary clinical studies suggest that this compound has therapeutic effect in non-motor symptoms of PD. In the present text, we review the clinical studies of cannabinoids in PD and the preclinical and clinical studies specifically on CBD. We found four randomized controlled trials (RCTs) involving the administration of agonists/antagonists of the cannabinoid 1 receptor, showing that these compounds were well tolerated, but only one study found positive results (reductions on levodopa-induced dyskinesia). We found seven preclinical models of PD using CBD, with six studies showing a neuroprotective effect of CBD. We found three trials involving CBD and PD: an open-label study, a case series, and an RCT. CBD was well tolerated, and all three studies reported significant therapeutic effects in non-motor symptoms (psychosis, rapid eye movement sleep behaviour disorder, daily activities, and stigma). However, sample sizes were small and CBD treatment was short (up to 6 weeks). Large-scale RCTs are needed to try to replicate these results and to assess the long-term safety of CBD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative disorder with an incidence in the world population over 65 years of age of 1–2%. It is mainly characterized by classical motor symptoms such as bradykinesia, rest tremor, postural disturbances, and rigidity. PD patients often also suffer with non-motor symptoms, including constipation, urinary incontinence, sexual dysfunction, orthostatic hypotension, sleep disturbances, cognitive deficits, and psychiatric disorders such as psychosis, depression, and anxiety [1]. The pathophysiology of PD involves a progressive loss of dopamine-containing neurons of the basal ganglia, specifically in the pars compacta region of the substantia nigra. This neuron degeneration seems to be related to mitochondrial dysfunction, oxidative stress, and reduced protein degradation, resulting in degeneration of the nigrostriatal tract (possible responsible for mot motor symptoms) and accumulation of Lewy bodies in surviving neurons (the hallmark of PD) [1].
Pharmacological treatments for PD basically involve the administration of dopamine precursors such as levodopa (L-DOPA) and dopamine degradation inhibitors [dopa-decarboxylase inhibitors, monoamine oxidase (MAO) inhibitors, and catechol-O-methyl transferase (COMT) inhibitors]. However, some patients do not respond to L-DOPA, the main medication used to treat motor symptoms in PD. Moreover, L-DOPA produces important motor effects after long-term use (dyskinesia), thus decreasing its therapeutic effect over time. Moreover, PD patients also use other medications to treat their non-motor symptoms, including antidepressants, anxiolytics, sedatives, and antipsychotics, which also have limited efficacy and induce significant adverse reactions. These non-motor symptoms do not respond to dopaminergic drugs, and the handling of these non-motor symptoms is one of the most difficult current challenges in the pharmacological treatment of PD [1].
Cannabidiol (CBD) is one of the over 100 cannabinoids identified in Cannabis sativa plant, being the second most abundant constituent after ∆9-tetrahydrocannabinol (THC). Unlike THC, CBD does not induce psychological effects; however, several studies demonstrated that this cannabinoid modulates the effects of different compounds of the plant.
Basic, pre-clinical and clinical studies have suggested continuously to encouraging positive effects of CBD on the treatment of movement disorders, such as dystonia, Huntington’s (HD), and Parkinson’s (PD) diseases. Moreover, CBD presents multiple actions in the central nervous system that may have an essential role in the pharmacotherapy of the non-motor effects of PD, including anxiolytic, antipsychotic, antidepressant, and sleep effects. Therefore, we review here the clinical and pre-clinical data to the potential of the cannabinoids in the treatment of the non-motor effects of PD with a greater emphasis on CBD.
PD and the endocannabinoid system (ECS)
Cannabinoid receptors (cannabinoid receptors 1 and 2 or CB1 and CB2), their ligands or endocannabinoids (N-arachidonoyl ethanolamine or anandamide, and 2-aracdonoyl glycerol or 2-AG), and the enzymes that synthetize and metabolize them (fatty acid amide hydrolase or FAAH and monoacylglycerol lipase or MAGL) form the endocannabinoid system (ECS) [2, 3]. Endocannabinoids are found in large concentrations in brain areas involved in the processing and execution of body movements, such as the basal ganglia. Animal and human studies demonstrate that the endocannabinoid system undergoes neurochemical changes during the course of PD, including downregulation of CB1 receptors in the early stages of the disease and upregulation of these receptors (as well as CB2 receptors) and increased endocannabinoid tone in the intermediate and more advanced phases of the disease [2,3,4,5,6,7,8,9].
Pre-clinical studies performed in the last 20 years show that, depending on the PD stage and the different sub-areas of the basal ganglia involved, modulation of the ECS by cannabinoids (natural and synthetic) can regulate the neurochemical changes in the glutamate and GABA (gamma-aminobutyric acid) neural systems caused by reduced dopamine levels through activation and/or inhibition of CB1/2 receptors [2, 3, 7, 9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Moreover, these studies also show the anatomical and functional complexity of the distribution of CB1 receptors and endocannabinoids in different subareas of the basal ganglia. Both the expression of these receptors and the endocannabinoid tone are modified in the different phases of PD. In accordance, preclinical studies indicate that CB1 receptor agonists and antagonist and drugs modulating endocannabinoid metabolism have a potential medicinal use in this disorder [2, 3, 7, 9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Several mechanisms have been proposed to be responsible for the possible therapeutic effect of cannabinoids in PD, including antioxidant, anti-excitotoxic, and anti-inflammatory effects (mediated not only by the activation of the CB1 receptor, but also the CB2 receptor), inhibition of anandamide hydrolysis, and other mechanisms of action that are independent of cannabinoid receptors, such as modulation of the receptor channel TRPV1 (transient receptor potential vanilloid 1) and of the G protein-coupled receptor 55 (GPR55), among others [2, 3, 7, 9,10,11,12,13,14,15,16,17,18,19,20,21,22].
Clinical studies on the effects of cannabinoids in Parkinson’s disease
Since 2014, several reviews on the possible therapeutic effects of cannabinoids in neurological diseases in general and in motor disorders specifically have been published [2, 3, 23,24,25,26,27]. However, most of these reviews focused on behavioral and neurochemical effects in preclinical models. Indeed, a systematic review on the efficacy and safety of medical cannabis in neurologic disorders from the Guideline Development Subcommittee of the American Academy of Neurology concluded, based on two randomized controlled trials (RCTs) [28, 29] that “oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson disease” [24].
Since the publication of that 2014 review, other two RCTs have evaluated the effects of cannabinoids in PD patients [30, 31]. Thus, four RCTs with a total sample of 49 patients assessed the effects of CB1 receptor agonists/antagonists in PD patients. These studies included 2 trials with the synthetic THC analogue and CB1 receptor agonist nabilone [28, 30], 1 trial with a cannabis standardized extract (2.5 mg THC/1.25 mg CBD) [29], and 1 trial with the synthetic CB1 receptor antagonist rimonabant [31].
The main information from each study is presented in Table 1.
In a randomized, double-blind, placebo-controlled, crossover trial (n = 7), oral administration of nabilone (single oral dose, 0.03 mg/kg) significantly reduced levodopa-induced dyskinesia in PD according to the Rush Dyskinesia Disability Scale [28]. Nabilone administration was safe. Both nabilone and placebo induced a postural fall in systolic blood (no significant difference between nabilone and placebo), but two patients were withdrawn after nabilone treatment due to vertigo (n = 1) and symptomatic postural hypotension (n = 1). Nabilone also induced other transient adverse effects (n = 5), including mild sedation, “floating sensation”, dizziness, hyperacusis, partial disorientation, and visual hallucinations.
In another double-blind, randomised, placebo-controlled, crossover study (n = 15), administration of nabilone (single oral dose, 0.03 mg/kg) to patients with generalized and segmental primary dystonia was not associated with significant reductions in dystonia according to the Burke, Fahn, Marsden Dystonia Scale [29]. However, four patients described subjective improvements in dystonia severity 2–3 days after nabilone. Nabilone was well tolerated, but two patients were withdrawn from the study due to significant postural hypotension (n = 1) and marked sedation, (n = 1). No significant difference was observed between placebo and nabilone in postural fall in blood pressure, and no other adverse effects were observed. It is important to consider that the patients in this study had dystonia, which seems to have a different physiology than L-DOPA-induce dyskinesia.
In another double-blind, randomised, placebo-controlled, crossover study (n = 19) with PD patients, placebo capsules were compared to capsules of a standardized ethanolic cannabis extract containing 2.5 mg THC and 1.25 mg CBD per capsule (maximum THC dose: 0.25 mg/kg per day) [30]. No significant differences were observed between cannabis extract capsules and placebo neither on the primary outcome measure (Unified Parkinson’s Disease Rating Scale) nor on secondary outcome measures (Bain Dyskinesia Scale, Rush Dyskinesia Scale, Quality of life Parkinson’s Disease Questionnaire, McGill Pain Scale, sleep visual analogue scale, activities of daily living, and pathophysiologic indicators of dyskinesia). The cannabis extract was well tolerated, and no serious adverse events were reported. Both the cannabis extract and placebo produced transient and mild adverse effects, such as feeling “drowsy/lethargic” and “dizzy/light-headed”, musculoskeletal pain, and dry mouth, but the following were associated only with the cannabis extract: nausea, constipation, feeling “detached”, paranoia, vivid dreams/nightmares, confusion, panic attacks, and poor concentration. The incidence of adverse events increased with higher cannabis extract doses, and all adverse effects were ameliorated by dose reduction.
In a double-blind, randomised, placebo-controlled, parallel-arm study (n = 8), eight PD patients with motor fluctuations and levodopa-induced dyskinesias for at least 6 months received placebo or the CB1 receptor antagonist rimonabant (single oral dose, 20 mg), but no significant difference between treatments were observed on motor symptoms and dyskinesia assessed with the Unified Parkinson’s Disease Rating Scale [31]. Rimonabant was well tolerated, without marked adverse events.
Regarding medicinal cannabis, since 2004 three observational studies involving surveys/interviews with patients using cannabis to treat their PD symptoms reported that a significant proportion of these patients described improvements in general symptoms, bradykinesia, muscle stiffness, tremors and dyskinesia, as well as improvements in mood and sleep [32,33,34]. More recently, an open-label study with 22 PD patients using medical cannabis described significant reductions in motor symptoms (bradykinesia, muscle stiffness, and tremors), as well as decreased pain and improved sleep [35]. In all four studies cannabis was well tolerated. To the best of our knowledge, there are no RCT with medical cannabis in PD patients.
Taken together, the available clinical data from RCT involving the direct modulation of the CB1 receptor either with agonists or antagonists do not provide a clear evidence of efficacy or lack of it, since there are few studies, with small samples, and with limited variety of doses (few studies with more than on dose of a cannabinoid). The data on medical cannabis is mostly from observational and non-controlled studies, thus causality assessment is difficult. Moreover, use of non-standardized cannabis products and unknown doses of the different phytocannabinoids also make causality assessment complicated. It is not clear if it is the combination of different phytocannabinoids that are producing both the desired and undesired effects, or if one phytocannabinoid is more effective and safer than the other.
CBD and PD: preclinical studies
To the best of our knowledge, the first preclinical study investigating the antiparkinsonian properties of CBD was published in 2005 [13]. Since then, at least eight new studies were published [20, 21, 36,37,38,39,40]. The main characteristics of these studies are shown in Table 2.
Four of the eight studies used a classic animal model of motor symptoms of PD that consists of dopamine (DA) depletion using the neurotoxin 6-hydroxydopamine (6-OHDA) [13, 21, 36, 39]. This toxin causes depletion of DA content and reduction of tyrosine hydroxylase (TH) activity in the striatum and of TH-mRNA levels in the substantia nigra, among other biochemical changes. The first study showed that CBD (3 mg/kg) produced significant reductions in 6-OHDA-induced neurotoxic effects in rats, a neuroprotective effect probably mediated by cannabinoid receptor-independent antioxidant and anti-inflammatory potentials [13]. These findings were replicated by the same group in a second study using the same model and the same CBD dose [36]. This study also showed that the neuroprotective effects of CBD were associated with an upregulation of mRNA levels of Cu, Zn-superoxide dismutase, an enzyme that regulated oxidative stress, thus suggesting that the effects of CBD were produced by cannabinoid receptor-independent antioxidant properties.
Furthermore, a third study by the same group [21] replicated these results with chronic (14 days) CBD administration using the same rat model but involving the administration of a CBD-enriched cannabis extract (4.63 mg/kg). According to the authors, this extract contains 64.8% CBD, 2.3% THC, 1.1% cannabigerol, 3.0% cannabichromene, and 1.5% other phytocannabinoids. The administered CBD dose was equivalent to the 3 mg/kg dose of pure CBD used in the previous studies of the group. A subsequent study by this group used the same dose of this CBD-enriched cannabis extract during a month in a rodent model of multisystemic neurological disease (frontotemporal dementia, Parkinsonism, and amyotrophy/lower motor neuron disease) [37]. Animals receiving the extract showed significant reductions in stress-related behaviors, auto- and hetero-aggression, stereotypy, levels of free radicals in the limbic system, gliosis in cortex and hippocampus, and in inducible nitric oxide synthase (iNOS) levels in the cortex. CBD-treated mice also showed significant increases in the ratio of reduced/oxidized glutathione in the limbic system and in the cortical levels of complex IV. Finally, this group of mice also showed a significant reduction of tau and amyloid protein deposition in the cortex and hippocampus and a significant increase in autophagy. The improvements in oxidative stress are especially relevant for the pathophysiology of PD. Importantly, these studies assessed the neuroprotector effect of CBD, not its symptomatic/antiparkinsonian effects.
A recent study assessed the effects of CBD (15, 30 and 60 mg/kg, for 3 days) in L-DOPA-induced dyskinesia in mice [39]. After 6-OHDA treatment, animals received L-DOPA for 21 days. Although no significant effects were observed with the administration of CBD alone, its combination with capsazepine (antagonist of the Transient Receptor Potential Vanilloid-1 or TRPV-1) at the 30 mg/kg dose induced a significant reduction in L-DOPA-induced involuntary movements. Moreover, this combination also significantly reduced the expression of the pro-inflammatory markers cyclooxygenase-2 (COX-2) and nuclear factor-kappa B (NF-κB). All these effects were blocked by antagonists of CB1 and PPARγ (Peroxisome Proliferator-Activated type gamma) receptors. Thus, this study suggests that the neuroprotective effects of CBD (in association with a TRPV-1 antagonist) are produced by anti-inflammatory properties mediated by CB1 and PPARγ receptors.
In a cell culture model of PD, human neuroblastoma cells were exposed to three toxins that model biochemical abnormalities related to PD: reduced mitochondrial activity (1-methyl-4-phenylpyridinium or MPP+), production of free radical (paraquat), and ubiquitin proteasome system (UPS) inhibition (lactacystin) [20]. Administration of MPP+ and lactacystin produced a significant up-regulation of CB1 receptors. No protective effects of CBD (0.01–1.0 µM) were observed in this model.
Two studies have used the drug-induced catalepsy test, commonly used to investigate motor impairments related to changes in striatal function [38, 40]. The first one evaluated the acute pretreatment with the CBD (5, 15, 30, or 60 mg/kg) in the catalepsy induced in male Swiss mice by haloperidol (D2 receptor antagonist), L-nitro-N-arginine (selective nitric oxide synthase [NOS] inhibitor), and WIN55,212 (CB1 receptor agonist). The increased catalepsy time produced by all three drugs was attenuated dose-dependently by CBD. The second study involved the repeated administration of reserpine (an irreversible inhibitor of vesicular monoamine transporters 1 and 2 or VMAT1/2) to rats to induce motor impairments and cognitive deficits to model both tardive dyskinesia and PD [40]. Both CBD doses of 0.5 or 5 mg/kg significantly reduced reserpine-related catalepsy and chewing movements (but not locomotor activity), and the lowest dose also significantly improved reserpine-induced memory/learning deficits in the discriminative avoidance task.
Taken together, the preclinical data reviewed above suggests that CBD improved PD-related symptoms in animal models of motor, biochemical, and cognitive symptomatology. However, most studies used a single model of motor symptoms using 6-OHDA, with a limited number of studies assessing other aspects of PD. Moreover, the total number of studies is still limited, and their methodology is heterogeneous regarding both CBD doses and the presence of other phytocannabinoids (when using the CBD-enriched cannabis extract).
CBD and PD: clinical evidence
To the best of our knowledge, only three clinical trials involving the administration of CBD specifically to PD patients were published [41,42,43]. One study was published in 2009 [41] and the other two studies were published in 2014 [42, 43]. All studies were performed by our research group in the Ribeirão Preto Medical School of the University of São Paulo, Brazil. The main information of each study is presented in Table 3:
The first of the trials was an open-label pilot study with six PD patients (four men and two women) with psychotic symptoms [41]. Inclusion criteria included patients with psychotic symptoms for at least 3 months before study entry that could not be controlled with reduction of antiparkinsonian medications and that were in stable doses of antiparkinsonian drugs for at least 7 days. CBD was orally administered at doses ranging from 150 mg in the first week to 400 mg in the fourth and last week of treatment, according to the patients’ clinical response. Patients were evaluated before CBD intake and then weekly with the Brief Psychiatric Rating Scale, the Parkinson Psychosis Questionnaire, the Unified Parkinson’s Disease Rating Scale, the Clinical Global Impression–Improvement scale, the Mini-Mental Status Examination, and the Frontal Assessment Battery. CBD administration was associated with significant improvements in psychotic symptoms (assessed by the Brief Psychiatric Rating Scale and the Parkinson Psychosis Questionnaire) and in global functioning (assessed by the Unified Parkinson’s Disease Rating Scale and the Clinical Global Impression–Improvement scale). No other significant effects were observed, and no adverse effects were reported during the study.
The second study was a randomized, double-blind, placebo-controlled, parallel-group trial involving the oral administration of placebo or CBD (75 or 300 mg) for 6 weeks to PD patients without dementia or comorbid psychiatric conditions (n = 21, seven volunteers per group) [42]. Motor and general PD symptoms and well-being and quality of life were assessed in the week before the trial and in the last week of the experiment with the Unified Parkinson’s Disease Rating Scale and the Parkinson’s Disease Questionnaire–39, respectively. Moreover, the possible neuroprotective effects of CBD were investigated by measuring brain-derived neurotrophic factor (BDNF) plasma levels and N-acetylaspartate to creatine ratios in bilateral basal ganglia (putamen) through magnetic resonance spectroscopy (H1-MRS). Compared to placebo, 300 mg/day CBD was associated with significant improvements in the Parkinson’s Disease Questionnaire-39 total score, and significant improvements were also observed between placebo and both CBD doses in the scale factors “activities of daily living” and “stigma”. These results suggest the PD patients had a significant improvement in their well-being and quality of life. No significant effects were observed for other measures (including in the motor score of the Parkinson’s Disease Questionnaire-39), and no adverse reactions were recorded.
The third and final study was a case series of four PD patients with a diagnosis of rapid eye movement sleep behaviour disorder that had participated in the previous RCT and received CBD treatment (75 or 300 mg for 6 weeks) [43]. The study was performed after breaking the blind, and there were no patients at the placebo group with this diagnosis. Inclusion criteria included a clinical assessment by a neurologist specialized in sleep disorders and at least two episodes of complex sleep-related behaviours per week (nightmares and active behaviour during dreaming). Two patients had symptoms and polysomnography results confirmig the diagnosis, while the other two patients had symptoms but no polysomnography results. Three patients received the 75 mg/day CBD dose and the other received the 300 mg/day dose for 6 weeks. None of them had been previously treated for this sleep disorder. CBD was well tolerated, and all patients had a rapid and persistent reduction in the frequency of symptoms, which included swearing, talking, yelling, pushing, kicking, punching, gesturing, etc. Three patients had no symptoms at all after the 4-week CBD treatment, and the other patient had only one symptom per week. After the CBD treatment was interrupted, symptoms returned to the same frequency and intensity of baseline.
CBD for non-motor symptoms of PD
Below, we will discuss the possible therapeutic uses of CBD on neurological and neuropsychiatric non-motor symptoms of PD including psychiatric disorders (psychosis, anxiety, and depression), sleep disorders, cognitive decline, and in quality of life.
Psychosis
Psychosis is common in PD, affecting nearly one-third of patients, particularly in later stages of the disease [1, 41, 44]. The pathophysiology of psychosis in PD is poorly understood, but seems to be multifactorial, involving antiparkinsonian medications, dopaminergic neurotoxicity, and Lewy Body pathology. Treatment of psychosis in PD is difficult and remains a clinical challenge, since it usually involves reduction of dosage or complete elimination of antiparkinsonian medications (which could increase symptoms) and/or add-on therapy with conventional antipsychotics (which can worse motor symptoms). Treatment with atypical antipsychotics such as clozapine is not associated with worsening of motors symptoms, but can produce significant hematological, cardiovascular, and neurological side-effects [1, 41, 44]. Thus, there is a need for safer and more effective pharmacological treatments for psychosis in PD.
Several reviews published in the last 6 years [45,46,47,48,49,50] showed that studies in animals and humans (including randomized, controlled trials with psychotic patients [51, 52]) consistently demonstrate that CBD has antipsychotic effects. Importantly, an open-label study with six PD with psychotic symptoms showed that CBD administration (150–400 mg for 4 weeks) was associated with significant improvements in psychotic symptoms and in global functioning and was well tolerated [41]. However, the number of clinical trials with CBD administration to psychotic patients published so far is small, and most studies had small sample sizes and a short duration, focused on symptomatic or first episode patients, and did not focus on negative/cognitive symptoms specifically. Therefore, large-scale clinical trials are needed to assess the long-term efficacy and safety of CBD.
The mechanisms of action behind the antipsychotic effects of CBD are not completely understood, but CBD showed a pattern of activation of Fos immunoreactive neurons like that of clozapine (atypical antipsychotic) and different from haloperidol (typical antipsychotic), activating limbic areas but not motor areas [50]. Moreover, CBD’s antipsychotic effect seems to involve the endocannabinoid system through inhibition of the enzyme fatty acid amide hydrolase (FAAH) and subsequent increases in anandamide levels, and/or activation of vanilloid TRPV1 and serotonin 5-HT1A receptors [51].
Anxiety and depression
Anxiety, depression, and apathy are among the most prevalent psychiatric symptoms in PD, being present in more than 30–40% of patients [1, 44]. Like psychosis, the pathophysiology of depressive and anxiety symptoms in PD is also not fully understood, but appears to involve neurochemical/neurotoxic alterations in mood circuits rich in glutamatergic, GABAergic, and serotonergic neurons caused by dopaminergic neurotoxicity. The efficacy of first-line antidepressant and anxiolytic medications (usually selective serotonin reuptake inhibitors or SSRIs) is low in PD patients and may lead to a worsening of motor symptoms [1, 44]. Therefore, there is a critical need for new pharmacological treatments for anxiety and depression in PD.
Several preclinical studies, modelling different anxiety and depressive symptoms and disorders have shown that CBD has anxiolytic, panicolytic, anticompulsive, and antidepressive effects [53,54,55,56]. Preclinical studies have shown that CBD induces significant decreases in autonomic arousal, conditioned fear expression, and long-term anxiogenic effects related to stress, and significant enhancement of fear extinction and reconsolidation blockade [53,54,55,56]. Regarding human studies, the anxiolytic effects of single CBD doses (300–600 mg) were demonstrated both in healthy volunteers [57,58,59,60] and in patients diagnosed with social anxiety disorder [61, 62]. To the best of our knowledge, there is no clinical trial that administered CBD to depressed patients with or without PD.
Although the anxiolytic effects of CBD have been reported in preclinical and clinical studies, the number of clinical trials is small and limited to socially anxious patients, and these studies have small sample sizes and a short duration [63]. In the case of depression, no clinical studies were performed. Therefore, large-scale trials are needed both to assess the possible antidepressive effects of CBD and the its long-term efficacy and safety.
The mechanisms of action involved in the anxiolytic and antidepressive effects of CBD appear to involve mainly agonism at serotonergic 5-HT1A and cannabinoid CB1 receptors [53,54,55,56, 63]. A key feature of the CB1 receptor-mediated anxiolytic and antidepressive effects is its association with hippocampal neurogenesis, which is hypothesized to depend on elevation of anandamide levels. Moreover, other mechanisms also seem to be involved, such as anti-inflammatory and antioxidant actions mediated by the vanilloid TRPV1 receptor [53,54,55,56, 63].
Sleep disturbances
PD patients also have an increased incidence of sleep disturbances, such as reduced quality of sleep, insomnia, restless legs syndrome, and rapid eye movement sleep behaviour disorder (RBP) [1, 43, 44]. As with other non-motor symptoms in PD, the pathophysiology of sleep disturbances in PD is not fully understood, but seems to be related to dopaminergic neurotoxicity and subsequent neurochemical alterations in brain areas involved in the regulation of the sleep-wake cycle controlled by cholinergic, GABAergic, and serotonergic neurons. The pharmacological management of sleep disturbances in this population is limited, being handled often with benzodiazepines and less frequently with melatonin and cholinesterase inhibitors. Benzodiazepines are associated with adverse reactions such as somnolence and impaired motor coordination, which may worse sleep and motor symptoms in PD patients, and they are also associated with tolerance, abuse/dependence, and withdrawal symptoms. Melatonin administration may induce somnolence and psychosis, which may lead to a worsening of sleep and psychotic symptoms, while cholinesterase inhibitors may induce gastrointestinal disturbances, anorexia, and bradycardia [1, 43, 44].
Recent preclinical studies in rodents show that CBD administration can produce both sedative-hypnotic effects and increases in wakefulness, depending on dose, route of administration, and duration of the experiment [64,65,66,67,68]. For instance, moderate and high doses of CBD increased the total percentage of sleep and REM sleep latency in rats, while the moderate dose decreased REM sleep latency [64]. Human studies investigating the effects of CBD on sleep are scarce [43, 65, 69,70,71,72]. In healthy volunteers, CBD (600 mg) induced sedative effects [69], but it did not produce any effects on cognitive, subjective or polysomnography measures with a lower dose (300 mg) [70], while it increased wakefulness when co-administered at a lower dose (15 mg/day) with THC (15 mg/day) [71]. In subjects with insomnia, CBD (40–160 mg/day) reduced dream recall, and the higher dose also increased total sleep time and reduced the frequency of awakenings [71]. In the specific case of PD patients, a case series of four PD patients with a diagnosis of RBD that participated in an RCT involving the administration of 75 or 300 mg CBD for 6 weeks showed that CBD was well tolerated and induced a rapid and persistent (up to 4 weeks) reduction in symptom frequency [43]. However, after interruption of the CBD treatment the symptoms returned. Thus, large-scale trials are needed to assess the efficacy of CBD on sleep disorders and its long-term safety.
The mechanisms of action involved in the effects of CBD on the sleep-wake cycle appear to depend on the ECS, which is involved in the regulation of the circadian cycle [64,65,66,67,68,69]. Indeed, preclinical evidence shows that the CB1 receptor is expressed in brain areas directly related to the regulation of the sleep–wake cycle and that its activation by anandamide and other antagonists increases the duration of slow-wave and REM sleep and reduces wakefulness, while administration of antagonists increases wakefulness and reduces slow-wave and REM sleep [64,65,66,67,68,69]. Therefore, the CBD effects on sleep could also depend on its interaction with the CB1 receptor, possibly by inhibition of anandamide uptake and hydrolysis and subsequent activation of CB1 receptors (high doses, hypnotic-like effects), or by antagonism and this receptor (low doses, wakefulness-like effects).
Cognitive dysfunction
Cognitive dysfunction—including deficits on attention, information processing, verbal fluency, and episodic memory—and dementia are among the most significant non-motor symptoms of PD, occurring in up to 40% of patients. Neurocognitive dysfunction and dementia seem to be caused by impaired cholinergic and glutamatergic activity after dopaminergic neurotoxicity, especially at later stages of the disease. Thus, cognitive symptoms are usually treated with cholinesterase inhibitors and N-methyl-d-aspartate (NMDA) receptor antagonists, respectively, which have limited efficacy and may induce significant adverse reactions [1, 44].
The neuroprotective effects of CBD have been reported in several animal models, including PD models [72, 73]. Indeed, it is relevant to highlight the fact that all preclinical studies reviewed above involving CBD administration are models of neurotoxic effects, induced by toxins (6-OHDA, MPP+, paraquat, lactacystin, and reserpine) [13, 20, 21, 36, 39, 40] or modeling a neurological disease [37]. Except for one study [20], all the other studies showed a neuroprotective effect of CBD [13, 21, 36, 37, 39, 40]. The mechanisms of action responsible for the neuroprotective effects of CBD on nigrostriatal dopaminergic neurons seem to be related to its anti-inflammatory and antioxidant actions and its capacity to attenuate microglia activation in the substantia nigra [63, 72,73,74]. In preclinical models of PD specifically, CBD reduced free radicals, gliosis, iNOS, tau and amyloid deposition and increased complex IV, autophagy, and the ratio of reduced/oxidized glutathione in mice [37], and co-administration of CBD and capsazepine also in mice significantly reduced COX-2 and NF-κB expression [39].
Moreover, CBD also has potential to improve cognitive function such as learning and memory in animal models [40, 73], and these effects appear to me mediated by increases in endocannabinoid (anandamide) tone, activation of CB1/2 receptors, and subsequent hippocampal neurogenesis [63, 73]. Recent (unpublished) results from our group indicate that facilitation of 5-HT1A-mediated neurotransmission can also be involved. In humans, CBD does not seem to affect psychomotor functions or cognition [41, 42, 52, 70, 75, 76]. This is especially relevant for PD patients, since motor and cognitive symptoms have a central role in the disease. However, the neuroprotective effects of CBD have not been clearly demonstrated in humans, especially in clinical populations [41, 42, 52, 75,76,77]. In PD patients specifically, none of the trials reviewed above showed evidence neither of improvements in cognition [41, 42] nor in biomarkers of neuroprotection [42]. Moreover, these trials share the limitations of small number of volunteers and short duration. Thus, large-scale trials are needed to better investigate the possible beneficial effects of CBD on cognition and neuroprotection.
Pain
One of the most prevalent non-motor symptoms in patients with PD is chronic pain, which affects between 60 and 80% of patients [78,79,80]. A recent study of almost 2000 PD patients suggests that this chronic pain cannot be explained only by peripheral factors, but central causes seem to play a much more important role than was previously believed [81]. Several preclinical studies have demonstrated the effect of CBD on pain relief [82,83,84]. A recent review did not find double-blind studies in humans testing the effects of CBD alone on pain relief [85], but a case study with seven patients who had chronic pain after kidney transplantation showed that CBD was well tolerated and showed an analgesic effect [86]. It should be noteworthy that the best evidence for the effects in chronic pain has been provided by THC rather than CBD, although THC negative impact in cognition and motor coordination may limit its use as a monotherapy in PD.
Decline in quality of life
There is a direct relationship between the above mentioned non-motor PD symptoms (psychosis, anxiety, depression, sleep disorders, cognitive dysfunction, and pain) and the quality of life (QOL) of PD patients. These symptoms are major predictors in the decline of QOL [1, 42, 44]. Despite this fact, few studies assessed the effects of pharmacological treatments on the QOL of PD patients, and the results are inconsistent.
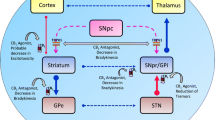
As explained in detailed above, a RCT with PD patients showed that, compared to placebo, CBD (300 mg/day) administration was associated with significant improvements in the QOL (as assessed by the Parkinson’s Disease Questionnaire-39) [42]. Improvements were observed in the questionnaire total score and in the individual factors “activities of daily living” and “stigma”. The authors suggested that these effects could be related to the beneficial effects of CBD on other non-motor symptoms (psychosis, anxiety, depression, sleep disorders, and cognitive dysfunction). Thus, CBD would be different from traditional pharmacological treatments that usually target specific sites of action to treat particular symptoms, since this compound would act more globally and simultaneously on several symptoms and by different mechanisms of action that are not completely understood [63, 73]. Apparently, because the pathogenesis of PD (and other diseases in general) is often multi-factorial, this multi-target characteristic of CBD is what could make it more beneficial than other drugs in these patients. The possible mechanisms of action involved in the therapeutic effects of CBD in the non-motor symptoms of PD are illustrated in Fig. 1.
Safety, tolerability, and level of evidence
The literature on the human safety and tolerability of CBD report few significant adverse reactions associated with both the acute and chronic administration of CBD in a wide range of doses (up to 1.500 mg/day) [41,42,43, 51, 52, 57,58,59,60,61,62, 70, 75, 76]. The most common of these effects are tiredness, diarrhea, and changes on appetite/weight, and CBD does not seem to induce tolerance or significant effects on cognition [41,42,43, 51, 52, 70, 75, 76]. In the specific case of PD, in all trials reviewed, above CBD was well tolerated and no adverse reactions were reported [41,42,43].
Regarding the level of evidence for the possible therapeutic use of CBD on non-motor symptoms of PD, according to the recommendations of the Oxford Centre for Evidence-Based Medicine (OCEBM) [87], systematic reviews of RCTs would provide the most reliable studies (Level 1), followed by individual RCTs (Level 2), cohort studies (Level 3), case series (Level 4), and preclinical studies (Level 5). In the case of CBD and non-motor symptoms of PD, there are evidences of its possible efficacy from preclinical studies (Level 5), case series (Level 4), and individual RCTs (Level 2). However, it is important to note that in the case of some of the non-motor symptoms (such as anxiety), clinical trials were not performed specifically in PD patients, and for some symptoms, no clinical trials have been performed at all (depression). Moreover, in those trials, where the non-motor symptoms have been investigated in PD patients (for instance, psychosis, sleep disturbances, cognitive dysfunction, and QOL), the number of studies and their sample sizes are small, and most trials used single doses of CBD for short periods of time. Therefore, although CBD seems to have a good safety and tolerability profile for PD patients and the evidence for its efficacy reaches the Level 2 of the OCEBM recommendations, there is still insufficient data to recommend CBD as a pharmacological treatment for these symptoms. Large-scale clinical trials with longer duration are needed to overcome the current limitations of the available studies on CBD and non-motor symptoms of PD.
Importantly, in June 2018, the US Food and Drug Administration has approved CBD for treating seizures associated with Lennox-Gastaut and Dravet syndromes in patients aged 2 years or older, and the European Medicines Agency is also evaluating this possibility [88]. These decisions confirm the safety of CBD and its therapeutic potential and will open new possibilities for exploring other therapeutic properties of CBD. Moreover, CBD is sold as a dietary supplement in several countries, where it is used for various medical conditions. An online survey published in July 2018 with people who were regularly using CBD showed that 1483 respondents (from a total of 2400) used CBD to treat at least one medical condition. The four medical conditions most cited were: chronic pain, anxiety, depression, and sleep disorders, and only 4.3% reported no improvement with CBD treatment [89]. This observation strongly suggests the utility of CBD for non-motor symptoms of PD.
Conclusions
Non-motor symptoms of PD are highly prevalent and debilitating, and available treatments have limited efficacy and are associated with significant adverse reactions. The ECS is involved in the pathophysiology of PD, suggesting that cannabinoids could perhaps have therapeutic effects on PD symptoms. However, to the best of our knowledge, there are only four RCTs involving the administration of CB1 receptor agonists (nabilone, cannabis extract) or antagonists (rimonabant) to PD patients, and only one of them (n = 7) reported significant beneficial effects (significant reductions on levodopa-induced dyskinesia). Importantly, all cannabinoids were well tolerated.
At least seven preclinical studies investigated the effects of CBD on basic models of PD, with one study reporting non-significant results on the motor effects. Moreover, three clinical studies in PD patients showed significant beneficial effects of CBD administration, all involving non-motor symptoms (psychosis, sleep disturbances, low QOL, problems with daily activities, and stigma). Preclinical studies also show that CBD has antidepressant, anxiolytic, and neuroprotective/pro-cognitive effects, and the sum of these effects (together with positive the effects on psychosis and sleep disturbances) seems to be responsible for improving QOL and well-being. Thus, CBD is a multi-target and promising drug for treating non-motor symptoms of PD. However, from the three studies, only one was an RCT with a small sample size (n = 25) and with short duration (6 weeks). Therefore, double-blind, placebo-controlled RCTs with larger and different stage of the disease samples are necessary to elucidate the efficacy and mechanisms involved in the therapeutic potential of CBD on the motor and non-motor symptoms of PD. Moreover, it is still required to investigate CBD safety, interaction with antiparkinsonian drugs, long-term effects and to determinate its adequate therapeutic window for each comorbid condition. Finally, it is crucial that clinical studies involving CBD administration use a pure and pharmaceutical-grade compound, and the population should be advised about the possible hazards of consuming unregulated products labeled as CBD. Between October 2017 and January 2018, more than 50 people in Utah were intoxicated by a synthetic cannabinoid labeled as CBD [90]. The main symptoms were altered mental status, nausea, vomiting, seizures, and shaking. This report shows that products labeled as CBD need to be regulated to minimize possible risks [91, 92].
References
World Health Organization (WHO) (2006) Neurological disorders: public health challenges. WHO Press, Geneva
Giacoppo S, Mandolino G, Galuppo M et al (2014) Cannabinoids: new promising agents in the treatment of neurological diseases. Molecules 19:18781–18816
Stampanoni Bassi M, Sancesario A, Morace R et al (2017) Cannabinoids in Parkinson’s disease. Cannabis Cannabinoid Res 2:21–29
Di Marzo V, Hill MP, Bisogno T et al (2000) Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J 14:1432–1438
Lastres-Becker I, Cebeira M, de Ceballos ML et al (2001) Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur J Neurosci 14:1827–1832
Silverdale MA, McGuire S, McInnes A et al (2001) Striatal cannabinoid CB1 receptor mRNA expression is decreased in the reserpine-treated rat model of Parkinson’s disease. Exp Neurol 169:400–406
Brotchie JM (2003) CB1 cannabinoid receptor signalling in Parkinson’s disease. Curr Opin Pharmacol 3:54–61
Farkas S, Nagy K, Jia Z et al (2012) The decrease of dopamine D2/D3 receptor densities in the putamen and nucleus caudatus goes parallel with maintained levels of CB1 cannabinoid receptors in Parkinson’s disease: a preliminary autoradiographic study with the selective dopamine D2/D3 antagonist [3H]raclopride and the novel CB1 inverse agonist [125I]SD7015. Brain Res Bull 87:504–510
Gómez-Gálvez Y, Palomo-Garo C, Fernández-Ruiz J et al (2016) Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 64:200–208
Maneuf YP, Crossman AR, Brotchie JM (1997) The cannabinoid receptor agonist WIN 55,212-2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson’s disease. Exp Neurol 148:265–270
Fox SH, Henry B, Hill M et al (2002) Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord 17:1180–1187
González S, Mena MA, Lastres-Becker I et al (2005) Cannabinoid CB(1) receptors in the basal ganglia and motor response to activation or blockade of these receptors in parkin-null mice. Brain Res 1046:195–206
Lastres-Becker I, Molina-Holgado F, Ramos J et al (2005) Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol Dis 19:96–107
Cao X, Liang L, Hadcock JR et al (2007) Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther 323:318–326
Morgese MG, Cassano T, Cuomo V et al (2007) Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson’s disease: role of CB(1) and TRPV1 receptors. Exp Neurol 208:110–119
García-Arencibia M, Ferraro L, Tanganelli S et al (2008) Enhanced striatal glutamate release after the administration of rimonabant to 6-hydroxydopamine-lesioned rats. Neurosci Lett 438:10–13
van Vliet SA, Vanwersch RA, Jongsma MJ et al (2008) Therapeutic effects of Delta9-THC and modafinil in a marmoset Parkinson model. Eur Neuropsychopharmacol 18:383–389
Price DA, Martinez AA, Seillier A et al (2009) WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci 29:2177–2186
Walsh S, Gorman AM, Finn DP et al (2010) The effects of cannabinoid drugs on abnormal involuntary movements in dyskinetic and non-dyskinetic 6-hydroxydopamine lesioned rats. Brain Res 1363:40–48
Chung YC, Bok E, Huh SH et al (2011) Cannabinoid receptor type 1 protects nigrostriatal dopaminergic neurons against MPTP neurotoxicity by inhibiting microglial activation. J Immunol 187:6508–6517
García C, Palomo-Garo C, García-Arencibia M et al (2011) Symptom-relieving and neuroprotective effects of the phytocannabinoid ∆9-THCV in animal models of Parkinson’s disease. Br J Pharmacol 163:1495–1506
Carroll CB, Zeissler ML, Hanemann CO et al (2012) ∆9-tetrahydrocannabinol (∆9-THC) exerts a direct neuroprotective effect in a human cell culture model of Parkinson’s disease. Neuropathol Appl Neurobiol 38:535–547
Martinez A, Macheda T, Morgese MG et al (2012) The cannabinoid agonist WIN55212-2 decreases L-DOPA-induced PKA activation and dyskinetic behavior in 6-OHDA-treated rats. Neurosci Res 72:236–242
Gutiérrez-Valdez AL, García-Ruiz R, Anaya-Martínez V et al (2013) The combination of oral L-DOPA/rimonabant for effective dyskinesia treatment and cytological preservation in a rat model of Parkinson’s disease and L-DOPA-induced dyskinesia. Behav Pharmacol 24:640–652
Benbadis SR, Sanchez-Ramos J, Bozorg A et al (2014) Medical marijuana in neurology. Expert Rev Neurother 14:1453–1465
Koppel BS, Brust JC, Fife T et al (2014) Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 82:1556–1563
Arjmand S, Vaziri Z, Behzadi M et al (2015) Cannabinoids and tremor induced by motor-related disorders: friend or foe? Neurotherapeutics 12:778–787
Catlow B, Sanchez-Ramos J (2015) Cannabinoids for the treatment of movement disorders. Curr Treat Options Neurol 17:370
Fernández-Ruiz J, Romero J, Ramos J (2015) Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. Handb Exp Pharmacol 231:233–259
Sieradzan KA, Fox SH, Hill M et al (2001) Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology 57:2108–2111
Fox SH, Kellett M, Moore AP et al (2002) Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord 17:145–149
Carroll CB, Bain PG, Teare L et al (2004) Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology 63:1245–1250
Mesnage V, Houeto JL, Bonnet AM et al (2004) Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol 27:108–110
Venderová K, Růzicka E, Vorísek V et al (2004) Survey on cannabis use in Parkinson’s disease: subjective improvement of motor symptoms. Mov Disord 19:1102–1106
Finseth TA, Hedeman JL, Brown RP 2nd et al (2015) Self-reported efficacy of cannabis and other complementary medicine modalities by Parkinson’s disease patients in Colorado. Evid Based Complement Altern Med 874849:6
Balash Y, Bar-Lev Schleider L, Korczyn AD et al (2017) Medical cannabis in Parkinson disease: real-life patients’ experience. Clin Neuropharmacol 40:268–272
Lotan I, Treves TA, Roditi Y et al (2014) Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol 37:41–44
García-Arencibia M, González S, de Lago E et al (2007) Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res 1134:162–170
Casarejos MJ, Perucho J, Gomez A et al (2013) Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy. J Alzheimers Dis 35:525–539
Gomes FV, Del Bel EA, Guimaraes FS (2013) Cannabidiol attenuates catalepsy induced by distinct pharmacological mechanisms via 5-HT1A receptor activation in mice. Prog Neuropsychopharmacol Biol Psychiatry 46:43–47
dos-Santos-Pereira M, da-Silva CA, Guimarães FS et al (2016) Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: possible mechanism of action. Neurobiol Dis 94:179–195
Peres FF, Levin R, Suiama MA et al (2016) Cannabidiol prevents motor and cognitive impairments induced by reserpine in rats. Front Pharmacol 7:343
Zuardi AW, Crippa JA, Hallak JE et al (2009) Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol 23:979–983
Chagas MH, Zuardi AW, Tumas V et al (2014) Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 28:1088–1098
Chagas MH, Eckeli AL, Zuardi AW et al (2014) Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther 39:564–566
Vanle B, Olcott W, Jimenez J et al (2018) NMDA antagonists for treating the non-motor symptoms in Parkinson’s disease. Transl Psychiatry 8:117
Zuardi AW, Crippa JA, Hallak JE et al (2012) A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des 18:5131–5140
Schubart CD, Sommer IE, Fusar-Poli P et al (2014) Cannabidiol as a potential treatment for psychosis. Eur Neuropsychopharmacol 24:51–64
Iseger TA, Bossong MG (2015) A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res 162:153–161
Fakhoury M (2016) Could cannabidiol be used as an alternative to antipsychotics? J Psychiatr Res 80:14–21
Gururajan A, Malone DT (2016) Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res 176:281–290
Guimarães VM, Zuardi AW, Del Bel EA et al (2004) Cannabidiol increases Fos expression in the nucleus accumbens but not in the dorsal striatum. Life Sci 75:633–638
Leweke FM, Piomelli D, Pahlisch F et al (2012) Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2:e94
McGuire P, Robson P, Cubala WJ et al (2018) Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry 175:225–231
Schier AR, Ribeiro NP, Silva AC et al (2012) Cannabidiol, a Cannabis sativa constituent, as an anxiolytic drug. Rev Bras Psiquiatr 34:S104–S110
Blessing EM, Steenkamp MM, Manzanares J et al (2015) Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 12:825–836
Zlebnik NE, Cheer JF (2016) Beyond the CB1 receptor: is cannabidiol the answer for disorders of motivation? Annu Rev Neurosci 39:1–17
Soares VP, Campos AC (2017) Evidences for the anti-panic actions of cannabidiol. Curr Neuropharmacol 15:291–299
Zuardi AW, Cosme RA, Graeff FG et al (1993) Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol 7:82–88
Crippa JA, Zuardi AW, Garrido GE et al (2004) Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 29:417–426
Bhattacharyya S, Morrison PD, Fusar-Poli P et al (2010) Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35:764–774
Zuardi AW, Rodrigues NP, Silva AL et al (2017) Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol 8:259
Bergamaschi MM, Queiroz RH, Chagas MH et al (2011) Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36:1219–1226
Crippa JA, Derenusson GN, Ferrari TB et al (2011) Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol 25:121–130
Mandolini GM, Lazzaretti M, Pigoni A et al (2018) Pharmacological properties of cannabidiol in the treatment of psychiatric disorders: a critical overview. Epidemiol Psychiatr Sci 27:327–335
Chagas MH, Crippa JA, Zuardi AW et al (2013) Effects of acute systemic administration of cannabidiol on sleep-wake cycle in rats. J Psychopharmacol 27:312–316
Gates PJ, Albertella L, Copeland J (2014) The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med Rev 18:477–487
Murillo-Rodríguez E, Sánchez D, Tejeda-Padrón A et al (2014) Potential effects of cannabidiol as a wake-promoting agent. Curr Neuropharmacol 12:269–272
Babson KA, Sottile J, Morabito D (2017) Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep 19:23
Linares IMP, Crippa JAS, Chagas MHN (2017) Beneficial effects of cannabis and related compounds on sleep. In: Preedy V (ed) Handbook of cannabis and related pathologies: biology, pharmacology, diagnosis, and treatment, 1st edn. Academic Press, London, pp 877–882
Carlini EA, Cunha JM (1981) Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol 21:S417–S427
Linares IMP, Guimaraes FS, Eckeli A et al (2018) No acute effects of cannabidiol on the sleep-wake cycle of healthy subjects: a randomized, double-blind, placebo-controlled, crossover study. Front Pharmacol 9:315
Nicholson AN, Turner C, Stone BM et al (2004) Effect of delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol 24:305–313
Peres FF, Lima AC, Hallak JEC et al (2018) Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol 9:482
Campos AC, Fogaça MV, Scarante FF et al (2017) Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 8:269
Little JP, Villanueva EB, Klegeris A (2011) Therapeutic potential of cannabinoids in the treatment of neuroinflammation associated with Parkinson’s disease. Mini Rev Med Chem 11:582–590
Bergamaschi M, Queiroz RH, Zuardi AW et al (2011) Safety and side effects of cannabidiol, a Cannabis sativa. Curr Drug Saf 6:237–249
Kerstin I, Grotenhermen F (2017) An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res 2:139–154
Solowij N, Broyd SJ, Beale C et al (2018) Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open-label clinical trial. Cannabis Cannabinoid Res 3:21–34
Beiske AG, Loge JH, Ronningen A et al (2009) Pain in Parkinson’s disease: prevalence and characteristics. Pain 141:173–177
Nègre-Pagès L, Regragui W, Bouhassira D et al (2008) Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord 23:1361–1369
Politis M, Wu K, Molloy S et al (2010) Parkinson’s disease symptoms: the patient’s perspective. Mov Disord 25:1646–1651
Silverdale MA, Kobylecki C, Kass-Iliyya L et al (2018) A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2018.06.001
Costa B, Trovato AE, Comelli F et al (2007) The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol 556:75–83
Ward SJ, Ramirez MD, Neelakantan H et al (2011) Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female c57bl6 mice. Anesth Analg 113:947–950
Genaro K, Fabris D, Arantes ALF et al (2017) Cannabidiol is a potential therapeutic for the affective-motivational dimension of incision pain in rats. Front Pharmacol 8:391
Mücke M, Phillips T, Radbruch L et al (2018) Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 3:CD012182
Cuñetti L, Manzo L, Peyraube R et al (2018) Chronic pain treatment with cannabidiol in kidney transplant patients in Uruguay. Transplant Proc 50:461–464
Howick J, Chalmers I, Glasziou P et al (2011) The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653. Accessed 31 July 2018
Wise J (2018) FDA approves its first cannabis based medicine. BMJ 361:k2827
Corroon J, Philips JA (2018) A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res 3:152–161
Horth RZ, Crouch B, Horowitz BZ et al (2018) Notes from the field: acute poisonings from a synthetic cannabinoid sold as cannabidiol—Utah, 2017–2018. MMWR Morb Mortal Wkly Rep 67:587–588
Acknowledgements
The authors are thankful to Dr. José Diogo de Souza and Mr. Luíz Avanzo for the preparation of the Figure. JAC, JEH, AWZ, and FSG are recipients of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) 1A productivity fellowships. Research was supported in part by Grants from (1) Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP); (2) Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); (3) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); (4) Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA, Brazil); (5) Center for Interdisciplinary Research on Applied Neurosciences (NAPNA), University of São Paulo, São Paulo, Brazil (NAPNA); and (6) National Institute for Translational Medicine (INCT-TM; CNPq/FAPESP, Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JAC, JH, FSG, and AWZ are co-inventors (Mechoulam R, Crippa JA, Guimaraes FS, Zuardi A, Hallak, JE, and Breuer A) of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023” Def. US no. Reg. 62193296; 29/07/2015; INPI on 19/08/2015 (BR1120150164927). The University of São Paulo has licensed the patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1). The University of São Paulo has an agreement with Prati-Donaduzzi (Toledo, Brazil) to “develop a pharmaceutical product containing synthetic cannabidiol and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson’s disease, and anxiety disorders”. JAC and JEH have received travel support from and are medical advisors of BSPG-Pharm. JAC has a Grant from University Global Partnership Network (UGPN)—Global priorities in cannabinoid research excellence. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Crippa, J.A.S., Hallak, J.E.C., Zuardi, A.W. et al. Is cannabidiol the ideal drug to treat non-motor Parkinson’s disease symptoms?. Eur Arch Psychiatry Clin Neurosci 269, 121–133 (2019). https://doi.org/10.1007/s00406-019-00982-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-019-00982-6