Abstract
Cannabis and synthetic cannabinoid formulations have now been legally approved in several countries for treatment of patients with Parkinson’s disease (PD). Hence, PD patients consult physicians more frequently for prescription of cannabinoids to alleviate symptoms that might not respond well to dopaminergic treatment. Despite the increasing volume of research generated in the field of cannabinoids and their effect on Parkinson’s disease, there is still paucity of sufficient clinical data about the efficacy and safety in PD patients. There is increasing understanding of the endocannabinoid system, and the distribution of cannabinoid receptors in basal ganglia structures might suggest potential benefit on parkinsonian symptoms. Concerning clinical research, only one of to date four conducted randomized placebo-controlled trials showed an effect on motor symptoms with alleviation of levodopa-induced dyskinesia. There are a growing number of uncontrolled trials and case reports that suggest beneficial effects of cannabinoids in PD patients. However, the variety of substances investigated, the varying routes of intake, differing doses and time courses make it difficult to compare data. We here provide an overview of the current literature in this field and discuss a pragmatic approach for the clinical use of cannabinoids in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to a change in German law in March 2017, medical cannabis can be prescribed and is reimbursed by public and private health insurers for patients with severe symptoms of Parkinson’s disease (PD), when previous therapies were unsuccessful or not tolerated, and a positive effect of cannabis on disabling symptoms is imaginable. Hereby, for the first time a substance has been approved without a specific indication and without any standard study demonstrating efficacy or safety. This led to an intense demand of patients for treatment with cannabinoids which is supported by a broad media interest and spectacular case reports on internet platforms showing tremendous improvement of parkinsonian symptoms such as dyskinesia or tremor after application of marijuana. Between June 2017 and April 2018, prescriptions of unprocessed marijuana leaves have increased more than fivefold up to 2.33 million Euro per month while prescriptions of standardized cannabinoid formulations are quite stable (Stellungnahme GKV-Spitzenverband Aug/2018).
The unusual approval of cannabinoids has led to a growing scientific interest in the potential therapeutic properties of cannabis for several, especially chronic diseases [for review see Mainka et al. (2018)]. In PD, high concentrations of cannabinoid receptors have been found in the basal ganglia (Giuffrida et al. 2005), and the influence of the endocannabinoid system on basal ganglia functioning and cortico-striatal processing has recently been investigated intensively in parkinsonian models. However, there is still lack of clinical data on the use of cannabinoids in PD patients.
This article aims to provide an overview of the cannabinoid system, its potential impact for treatment of parkinsonian symptoms and the present experimental and clinical data in PD.
Cannabinoids and the endocannabinoid system (ECS)
Cannabis sativa (marijuana) contains more than 100 phytocannabinoids (ElSohly and Gul 2014) with the psychotropic delta9-tetrahydrocannabinol (Δ9-THC) being the most abundant, the non-psychotropic cannabidiol (CBD) being the second most abundant component. Similar to the endocannabinoids produced by the human body such as anandamide or 2-arachidonylglycerol (2-AG), these phytocannabinoids act via the cannabinoid receptors 1 (CB-1R) and 2 (CB-2R) which are the two most important receptors of the endocannabinoid system (ECS). In analogy to anandamide, Δ9-THC in vitro activates both CB-1R and CB-2R. Due to CYP3A4 processing, some anandamide metabolites show higher affinity to CB-2R than CB-1R (Pratt-Hyatt et al. 2010). CBD in contrast does not elicit direct action on CB-R. Experimental evidence suggests agonism at, among others, 5-hydroxytryptamine receptors, transient receptor potential (TRP)-like channels and peroxisome proliferator-activated γ (PPARγ) receptors (Navarro et al. 2018). Moreover, recent data suggest a functional antagonism by modulating CB-R1 (Laprairie et al. 2015) and CB-R2 (Martinez-Pinilla et al. 2017) function. It might therefore lack detectable psychoactivity compared to Δ9-THC.
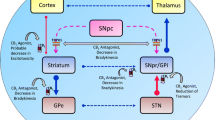
CB-1R is primarily located in the nervous system. High concentrations have been found in the hippocampus, the association cortex, cerebellum, basal ganglia [especially in the medial part of the internal pallidum (GPi)], the dorsal roots of the spinal cord, and peripheral nerves, with almost no traceability in the thalamus and brain stem. CB-2R is mainly expressed in the gastrointestinal tract, in lymphatic tissue and peripheral nervous system but also in the CNS, primarily in neurons of the dorsal nucleus of the vagus nerve, the nucleus ambiguus, the spinal trigeminal nucleus, and on microglia. CB-1R and CB-2R are G protein-coupled receptors, which inhibit the activity of the adenyl-cyclase via the G0/Gi unit, thereby influencing the release of neurotransmitters such as glutamate, dopamine and acetylcholine. Other transmitter pathways including serotonergic, GABAergic (γ-aminobutyric acid), NMDA (N-methyl-d-aspartate) and opioid systems are also modulated via indirect mechanisms. Amongst the other G protein-coupled receptors of the ECS, it was recently shown that the TRPV1-receptor (transient receptor potential cation channel subfamily V member 1) is also expressed in the basal ganglia (Stampanoni et al. 2017). Overall, the ECS regulates synaptic transmission via feedback mechanisms to avoid excitatory and inhibitory excesses. Furthermore, the ECS activates the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway via the Gβγ complex, a pathway that has regulatory properties regarding cell development, cell differentiation and apoptosis. The structural analysis of cannabinoid receptors ultimately paved the way for the development of synthetic cannabinoids. Today, several cannabinoid-based preparations are available for medicinal use (for reviews see Gandor and Ebersbach 2017; Mainka et al. 2018).
Experimental studies suggesting potential implications of cannabinoids for treatment of motor complications and neuroprotection in experimental models of Parkinson’s disease
Cannabinoids modulate basal ganglia function on two levels which are especially relevant for levodopa-induced dyskinesia (LID), i.e. the glutamatergic/dopaminergic synaptic neurotransmission and the cortico-striatal plasticity. Furthermore, activation of the ECS might induce neuroprotective effects related to direct receptor-independent mechanisms (Carroll et al. 2012), activation of anti-inflammatory cascades in glial cells via CB-2R (Klein 2005; Lastres-Becker et al. 2005), and anti-glutamatergic anti-excitotoxic properties (Fernandez-Ruiz et al. 2010a).
Effect of cannabinoids on synaptic neurotransmission
Early animal studies demonstrated an effect of cannabinoids on the catecholaminergic and dopaminergic systems (Garriott et al. 1967; Howes and Osgood 1974). CB-1R and the endocannabinoid agonists anandamide and 2-AG occur in high concentrations in the dopaminergic system, including the striatum (Herkenham et al. 1991), where they modulate dopaminergic transmission as a retrograde feedback system on presynaptic glutamatergic and GABAergic nerve endings (Pertwee and Ross 2002). Cannabinoid agonists are thought to enhance GABA-induced signal transduction in the basal ganglia’s indirect loop by inhibiting GABA uptake into the lateral part of the GPi. Activation of CB-1R at glutamatergic synapses suppresses the excitatory drive onto NMDA and AMPA receptors on dopaminergic neurons resulting in suppression of excitation. Both mechanisms may contribute to an anti-dyskinetic effect (for review see Covey et al. 2017). Especially the medial part of the GPi, the main thalamo-cortical output region within the basal ganglia expresses high concentration of CB-1R (Sierra et al. 2015).
CB-2R have been found on human nigrostriatal dopaminergic neurons (Garcia et al. 2015), which suggests direct modulating properties of the ECS on dopaminergic transmission (Stampanoni et al. 2017). Nigrostriatal neurons, however, do not express CB-1R (Julian et al. 2003), but are influenced by the endocannabinoid system (Fernandez-Ruiz 2009; Fernandez-Ruiz et al. 2010b) via CB-1R expressing GABAergic, glutamatergic, and opioidergic neurons (Gubellini et al. 2002; van der Stelt and Di Vincenzo 2003; Centonze et al. 2007). In addition, some endocannabinoids have been found to interact with TRP channels (Starowicz et al. 2007), which are expressed on dopaminergic neurons (Mezey et al. 2000).
Activation of CB-1R in mesencephalic brain areas has furthermore been described to increase acetylcholine release and thereby reduce the local cholinergic deficit in PD (Chagas et al. 2014a). In addition, interaction of cannabinoids with the serotonergic system might also influence LID: striatal dopaminergic denervation leads to a shift of levodopa conversion into dopamine from dopaminergic into serotonergic neurons, which results in a non-physiologic pulsatile dopamine release (false transmitter) (Espay et al. 2018).
The activating properties of a ligand with low CB-receptor affinity, such as Δ9-THC depend mainly on the CB-receptor concentration on a target structure. Since CB-receptor concentration varies widely between different brain areas, this might explain the different properties CB-ligands have on the brain. Furthermore, the relative sensitivity of GABAergic and glutamatergic neurons to CB1-R ligands differ between species (Pertwee 2008).
Effect of cannabinoids on cortico-striatal plasticity
Experimental in vivo PD studies indicate that the ECS modulates cortico-striatal synaptic plasticity. In conditions with LID, CB-1R agonists induce an anti-dyskinetic effect by reducing abnormal dopamine induced cortico-striatal long-term potentiation (LTP) and promoting long-term depression (LTD), which makes glutamatergic synapses less excitable to future stimulation. Inhibition of CB-1R reverses this anti-dyskinetic effect [for review see Stampanoni et al. (2017)].
An increase in ECS activity was detected both in a PD animal model and in human tissue analyses from PD patients (Fernandez-Ruiz et al. 2011), showing an upregulation of cannabinoid receptors (Gomez-Galvez et al. 2016; Lastres-Becker et al. 2001), an accumulation of cannabinoid receptor agonists (Di Marzo et al. 2000; van der Stelt et al. 2005) and a reduction in their degradation (Gubellini et al. 2002). This adaptation of the ECS was reversed by chronic levodopa substitution in an animal model (Maccarrone et al. 2003).
Effect of cannabinoids on neuroprotection
Cannabinoids have been reported to provide neuroprotection not only in cell tissue, but also in rodent PD models. One study provided evidence that Δ9-THC acted as a neuroprotective substance in rats with hemiparkinsonism. Chronic administration of this cannabinoid after they were subjected to unilateral lesions of the nigrostriatal dopaminergic neurons with 6-hydroxydopamine produced a significant recovery in the impairment of dopaminergic transmission caused by the toxin, suggesting a reduction of dopaminergic cell death (Lastres-Becker et al. 2005). In another study, the CB-2R agonist HU-308 and the inhibitor of the endocannabinoid inactivation (IoEI) AM404, but not the CB-1R agonist ACEA or the IoEI UCM707 reversed 6-OHDA-induced dopamine (DA) depletion or tyrosine hydroxylase deficit (Garcia-Arencibia et al. 2007). Both study groups suggested potential receptor-independent antioxidant or anti-inflammatory properties of those cannabinoids that provide neuroprotection against the progressive degeneration of nigrostriatal dopaminergic neurons. The activation of CB-2R, but not CB-1R, might exhibit some neuroprotective potential in PD which is potentially based on an anti-inflammatory effect (Garcia-Arencibia et al. 2007; Gomez-Galvez et al. 2016). This assumption is supported by a rodent PD model study, where CB-2R agonism protected against MPTP-induced nigrostriatal degeneration by inhibiting microglial activation/infiltration (Price et al. 2009).
Effect of cannabinoids on motor function in animal studies and humans
With regard to the effect of cannabinoids on motor function, studies in animal models of PD yielded heterogeneous and partially conflicting results. CB-1R agonists have been found to reduce akinesia, motor impairment (Fernandez-Espejo et al. 2004; Kreitzer and Malenka 2007; van Vliet et al. 2008) and tremor (Sanudo-Pena and Walker 1997), potentially due to a receptor-independent mechanism (Kreitzer and Malenka 2007; Fernandez-Espejo et al. 2004). In contrast, another group reported an increase in bradykinesia after administration of Δ9-THC or the cannabinoid agonist levonantradol in primates (Meschler et al. 2001). During the same study, a CB-1R antagonist failed to alleviate MPTP-induced parkinsonian symptoms. Another study in rodents, however, found reduced akinesia in 6-hydroxydopamine lesioned rats after administration of the CB-1R antagonist rimonabant (Kelsey et al. 2009). Furthermore, antidyskinetic effects have been described after administration of both CB-1R agonists (Fox et al. 2002; Kelsey et al. 2009) and antagonists (van der Stelt et al. 2005; van Vliet et al. 2008).
Clinical studies in patients with Parkinson’s disease
Numerous case series and single case reports concluded that cannabinoids might have potential beneficial effects on PD motor symptoms such as akinesia, tremor or dyskinesia. In contrast, data from randomized placebo-controlled trials (RCTs) on effects on PD motor symptoms are rare and less encouraging. So far, 4 RCTs evaluating the effects of CBD, THC/CBD, nabilone, and rimonabant with altogether 49 PD patients have been published. Of those, 3 RCTs failed to show a significant effect of cannabinoids on parkinsonian motor symptoms or LID when applied as add-on therapy. Overall, CB-1R antagonists did not reduce bradykinesia (Mesnage et al. 2004), and the effect on tremor is not clear (Consroe 1998; Sieradzan et al. 2001; Carroll et al. 2004). However, one RCT reports reduced LID after administration of the CB-1R agonist nabilone (Sieradzan et al. 2001). Several case series and reports suggest improvement of non-motor symptoms with cannabinoids, but there is lack of RCTs addressing these effects.
Uncontrolled studies: surveys and prospective observational studies (“case series”)
In an anonymous survey of 339 Czech PD patients, 25% reported to take cannabis buds orally (“half a teaspoon of fresh or dried leaves” with a meal). Of these 85 patients, 46% reported general improvement of their PD symptoms with reduction of resting tremor (31%), decrease in bradykinesia (45%), reduction of muscle rigidity (38%) and improvement of LID (14%). 5% of patients experienced worsening of symptoms (Venderova et al. 2004).
In a Web-based self-reported assessment of PD and MS patients with a high recruitment rate (96.1%), 454 of 595 (76.3%) subjects were PD patients. 36.6% of them either inhaled or, to a least extent, consumed cannabis orally, mainly for medicinal use (73.7%) and for longer than 12 months (72.3%). The cannabis using PD patients reported high efficacy of cannabis (6.2 [SD 1.8] on a scale from 0 to 7) with lower levels of disability, specifically in domains of mood, memory, and fatigue. Furthermore, 47.8% reported reducing prescription medication since beginning cannabis use (Kindred et al. 2017).
An observational study in 22 Israeli patients showed a significant motor improvement (UPDRS III) in 30% of subjects after smoking cannabis (0.5 g, unspecified composition) with improvement of the UPDRS subitems resting tremor, rigidity, and bradykinesia, and the non-motor aspects sleep and pain (Lotan et al. 2014).
In contrast, tremor did not improve in a case series with 5 PD patients smoking a single cigarette with a comparatively high (first) dose of 1 g marijuana (2.9% THC) (Frankel et al. 1990).
In an open-label study in 6 patients with PD-associated psychosis, CBD powder dissolved in oil and administered orally in a capsule (mean dose of 400 mg/day) significantly reduced psychiatric positive symptoms, such as illusions and hallucinations, and negative symptoms, such as withdrawal and depression, assessed with the Brief Psychiatric Rating Scale (BPRS). Furthermore, the total UPDRS score assessed as secondary outcome measurement significantly improved at a 4-week follow-up (Zuardi et al. 2009).
In an open-label study, Zuardi et al. treated six PD patients with psychiatric plus symptoms, such as illusions and hallucinations, and minus symptoms, such as withdrawal and depression, with CBD over a period of 4 weeks and reported a significant reduction in psychotic symptoms, as measured with the Parkinson Psychosis Questionnaire (PPQ) and the Brief Psychiatric Rating Scale (BPRS) (Zuardi et al. 2009).
In a small uncontrolled study, 4 PD patients with REM sleep behaviour disorder received 75 or 300 mg 99.9% purified CBD orally per day (dissolved in corn oil and placed in gelatin capsules) and reported reduced or completely eliminated agitation, beating, kicking and nightmares (Chagas et al. 2014a).
Randomized, double-blind, placebo-controlled trials
In a randomized, double-blind, placebo-controlled crossover trial, Caroll et al. studied the potential effect of a standardized whole-plant extract with a defined THC content, a THC-to-CBD ratio of about 2:1 and body-weight adapted dosage on dyskinesia in 17 PD patients. Despite the double-blind design, 71% of patients correctly identified their respective treatment arm. Cannabis was applied as oral cannabis extract and well tolerated, but showed no alleviation or worsening of parkinsonian symptoms. There was no evidence for a treatment effect on LID as assessed by the UPDRS and Rush Dyskinesia Rating Scale, or on any of the secondary outcome measures such as other UPDRS motor scores, PDQ-39, pain or sleep quality (Carroll et al. 2004).
Chagas et al. randomized 21 PD patients to receive CBD daily in doses of either 75 mg, 300 mg, or placebo, with each group containing 7 patients. CBD was provided in powdered form, dissolved in corn oil and placed in gelatin capsules. After 6 weeks, motor function (UPDRS motor score) and quality of life (PDQ-39) were assessed and compared to baseline. The improvement in PDQ-39 sum score was significantly higher in patients treated with 300 mg/day of CBD, while UPDRS scores did not differ between groups (Chagas et al. 2014b).
Sieradzan et al. evaluated the effect of the CB-1R and CB-2R agonist nabilone (administered orally as capsule of same colour and taste compared to placebo) on levodopa-induced dyskinesia in 7 patients in a crossover design. A total dose of 0.03 mg/kg body weight was administered in 2 portions 12 h and 1 h before an acute levodopa challenge, which then was repeated 14 days later when groups had been crossed over. Severity, but not duration of dyskinesia improved significantly in the nabilone group. However, no change in the severity of PD symptoms and no difference in motor improvement after the acute levodopa challenge were observed. In the nabilone group, 5 of 7 patients experienced mild sedation, dizziness, hyperacusis, disorientation, and scenic visual hallucinations (Sieradzan et al. 2001).
In an exploratory, randomized, double-blind, placebo-controlled study, Mesnage et al. evaluated among others the effects of the CB-1R antagonist rimonabant on the severity of motor symptoms and LID after administration of a single dose of levodopa in 4 PD patients. Rimonabant was well tolerated, but did not improve UPDRS motor scores or the UPDRS dyskinesia score (Mesnage et al. 2004).
Table 1 gives an overview of surveys, case series, open-label studies and randomized controlled trials evaluating the effects of cannabinoids or cannabinoid antagonists in Parkinson’s disease.
Table 2 gives an overview on single motor- and non-motor symptoms assessed in trials evaluating the effects of cannabinoids or cannabinoid antagonists in Parkinson’s disease.
Tolerability, medical risks and contraindications of prescribing cannabinoids in PD patients
Medical cannabinoids containing THC can cause central, gastro-intestinal and cardiovascular unwanted effects, such as somnolence, dizziness, nausea, vomiting, tachycardia, hypotension, dry mouth, diarrhoea, loss of balance or fatigue, as well as psychiatric symptoms such as disorientation, confusion, hallucinations, and altered mood. Tolerance to these side effects usually develops within a short time. Withdrawal symptoms of therapeutically used cannabis have hardly ever been a problem (Grotenhermen and Muller-Vahl 2012; Whiting et al. 2015). While THC is responsible for the psychotropic effects of cannabis, clinical studies did not reveal psychotic side effects for CBD (Chagas et al. 2014b). CBD does not seem to induce psychiatric symptoms, but may have some anti-psychotic effects in PD patients (Zuardi et al. 2009).
Because of the high prevalence of psychotic symptoms in PD patients, the psychotropic effects of THC are of special interest (Chang and Fox 2016). Sieradzan et al. reported psychotropic side effects such as scenic visual hallucinations in 5 of 7 PD patients treated with the synthetic THC analogue nabilone (Sieradzan et al. 2001). In a study by Lotan et al. 6 of included 28 patients (21%) dropped out due to side effects including psychotic symptoms following cannabis application (Lotan et al. 2014). Furthermore, PD patients often show an insufficient adherence to the prescribed medication with a drop-out rate of 10–67%, depending on the study (Malek and Grosset 2015). Prevalence of dopaminergic dysregulation syndrome consisting of an addictive use of dopaminergic substances is 3.4–4.1% in PD patients (Giovannoni et al. 2000; Pezzella et al. 2005). Since abuse mostly occurs with fast-acting substances such as soluble levodopa or apomorphine s.c. (Roland et al. 2016), inhalative cannabis should be handled with special care in PD patients because they provide a significantly faster onset of action compared to manufactured drugs or extracts. However, long-term data for safety and tolerability of medically used inhalative cannabinoids are lacking. It must furthermore be emphasized that in THC-based preparations similar to those available for non-medical use, the psychotropic adverse effects outweigh potential therapeutic effects.
Considering the cardiovascular side effects, cannabinoids can lead to an orthostatic drop in blood pressure and even orthostatic syncopes (Sidney 2002). This might have a special impact on PD patients, since orthostatic hypotension caused by sympathetic cardiac denervation is a frequent non-motor-symptom in PD (Barone et al. 2009). In accordance, Sieradzan et al. described an orthostatic drop in systolic blood pressure in all seven PD patients after nabilone intake with one drop-out due to symptomatic orthostatic hypotension (Sieradzan et al. 2001).
Although medical guidelines defining contraindications for using cannabis are missing, there is consensus that cannabinoids should not be prescribed in patients with severe personality disorders, schizophrenia and other psychotic disorders, or in patients with a history of substance abuse (Izzo et al. 2009; Ablin et al. 2016). Cannabis consumption goes along with increased sympathetic activity resulting in an increased myocardial oxygen demand. In accordance, symptoms of myocardial hypoxia occur earlier in patients with pre-existing angina pectoris (Prakash et al. 1975; Aronow and Cassidy 1974), and the risk of myocardial infarction is increased by 1- to 4.8-fold in cannabis users (Jouanjus et al. 2014; Mittleman et al. 2001). Therefore, a strict indication is recommended in patients with cardiovascular disease (Health Canada 2017). Cannabinoids may promote fatty liver disease and should be avoided in patients with chronic hepatitis C (Hezode et al. 2008). In patients with impaired liver or kidney function, the effect of THC and CBD may be increased or prolonged. Pregnant women and those considering becoming pregnant should be advised to avoid using marijuana or other cannabinoids; nursing mothers should not be prescribed cannabinoids because Δ9-THC accumulates in breast milk and metabolites have been found in infant faeces, indicating that THC is absorbed and metabolized by the infant (The American College of Obstetricians and Gynecologists Committee 2015; Health Canada 2017).
Conclusion
Changes in legislation allow for the treatment of severely affected PD patients with medical cannabis or cannabinoids when standard therapy remains insufficient to adequately control PD symptoms.
However, based on the currently available data from only four double-blind randomized placebo-controlled trials with altogether not more than 49 patients, no profound treatment recommendation can be given at present.
Moreover, trials were extremely heterogeneous regarding applied subtype of cannabinoids, route and time course of administration, drug concentration and assessment period. Only one RCT showed improvement of motor symptoms with reduction of LID. Even considering results of all available non-RCTs, i.e. one open-label study, three case series and two surveys, cannabinoids showed little to no effect on motor symptoms and only some minor influence on non-motor symptoms in PD patients.
However, a growing body of preclinical research demonstrates the influence of cannabis and cannabinoids on the dopaminergic system. Thus, further evidence-based clinical studies on the efficacy and tolerability of cannabinoids in patients with Parkinson’s disease are needed to elicit potential therapeutic effects and allow for an evidence-based treatment recommendation.
References
Ablin J, Ste-Marie PA, Schafer M, Hauser W, Fitzcharles MA (2016) Medical use of cannabis products: lessons to be learned from Israel and Canada. Schmerz 30:3–13
Aronow WS, Cassidy J (1974) Effect of marihuana and placebo-marihuana smoking on angina pectoris. N Engl J Med 291:65–67
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, Pontieri FE, Quatrale R, Ramat S, Scala R, Volpe G, Zappulla S, Bentivoglio AR, Stocchi F, Trianni G, Dotto PD (2009) The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 24:1641–1649
Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, Parkin SG, Fox P, Wright D, Hobart J, Zajicek JP (2004) Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology 63:1245–1250
Carroll CB, Zeissler ML, Hanemann CO, Zajicek JP (2012) Delta(9)-tetrahydrocannabinol (Delta(9)-THC) exerts a direct neuroprotective effect in a human cell culture model of Parkinson’s disease. Neuropathol Appl Neurobiol 38:535–547
Centonze D, Rossi S, Prosperetti C, Gasperi V, De CV, Bari M, Tscherter A, Febbraro F, Bernardi G, Maccarrone M (2007) Endocannabinoids limit metabotropic glutamate 5 receptor-mediated synaptic inhibition of striatal principal neurons. Mol Cell Neurosci 35:302–310
Chagas MH, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, Camilo MR, Bergamaschi MM, Schenck CH, Hallak JE, Tumas V, Crippa JA (2014a) Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther 39:564–566
Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, dos Santos AC, Teixeira AL, Hallak JE, Crippa JA (2014b) Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 28:1088–1098
Chang A, Fox SH (2016) Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs 76:1093–1118
Consroe P (1998) Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis 5:534–551
Covey DP, Mateo Y, Sulzer D, Cheer JF, Lovinger DM (2017) Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology 124:52–61
Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM (2000) Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J 14:1432–1438
ElSohly M, Gul W (2014) Constituents of Cannabis sativa. In: Pertwee RG (ed) Handbook of cannabis. Oxford University Press, New York, p 1093
Espay AJ, Morgante F, Merola A, Fasano A, Marsili L, Fox SH, Bezard E, Picconi B, Calabresi P, Lang AE (2018) Levodopa-induced dyskinesia in Parkinson disease: current and evolving concepts. Ann Neurol 84:797–811
Fernandez-Espejo E, Caraballo I, Rodriguez DF, Ferrer B, El Banoua F, Flores JA, Galan-Rodriguez B (2004) Experimental parkinsonism alters anandamide precursor synthesis, and functional deficits are improved by AM404: a modulator of endocannabinoid function. Neuropsychopharmacology 29:1134–1142
Fernandez-Ruiz J (2009) The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol 156:1029–1040
Fernandez-Ruiz J, Garcia C, Sagredo O, Gomez-Ruiz M, de Lago E (2010a) The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets 14:387–404
Fernandez-Ruiz J, Hernandez M, Ramos JA (2010b) Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16:e72–e91
Fernandez-Ruiz J, Moreno-Martet M, Rodriguez-Cueto C, Palomo-Garo C, Gomez-Canas M, Valdeolivas S, Guaza C, Romero J, Guzman M, Mechoulam R, Ramos JA (2011) Prospects for cannabinoid therapies in basal ganglia disorders. Br J Pharmacol 163:1365–1378
Fox SH, Henry B, Hill M, Crossman A, Brotchie J (2002) Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord 17:1180–1187
Frankel JP, Hughes A, Lees AJ, Stern GM (1990) Marijuana for Parkinsonian tremor. J Neurol Neurosurg Psychiatry 53:436
Gandor F, Ebersbach G (2017) Cannabinoids in the treatment of Parkinson’s disease. Neurol Int Open 1:307–311
Garcia MC, Cinquina V, Palomo-Garo C, Rabano A, Fernandez-Ruiz J (2015) Identification of CB(2) receptors in human nigral neurons that degenerate in Parkinson’s disease. Neurosci Lett 587:1–4
Garcia-Arencibia M, Gonzalez S, de Lago E, Ramos JA, Mechoulam R, Fernandez-Ruiz J (2007) Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res 1134:162–170
Garriott JC, King LJ, Forney RB, Hughes FW (1967) Effects of some tetrahydrocannabinols on hexobarbital sleeping time and amphetamine induced hyperactivity in mice. Life Sci 6:2119–2128
Giovannoni G, O’Sullivan JD, Turner K, Manson AJ, Lees AJ (2000) Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry 68:423–428
Giuffrida R, Vingerhoets FJ, Bogousslavsky J, Ghika J (2005) Pain in Parkinson’s disease. Rev Neurol (Paris) 161:407–418
Gomez-Galvez Y, Palomo-Garo C, Fernandez-Ruiz J, Garcia C (2016) Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 64:200–208
Grotenhermen F, Muller-Vahl K (2012) The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int 109:495–501
Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M (2002) Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci 22:6900–6907
Health Canada (2013) Information for health care professionals—Cannabis (marihuana, marijuana) and the cannabinoids. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/marihuana/med/infoprof-eng.pdf. Accessed 13 May 2017
Herkenham M, Lynn AB, De Costa BR, Richfield EK (1991) Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res 547:267–274
Hezode C, Zafrani ES, Roudot-Thoraval F, Costentin C, Hessami A, Bouvier-Alias M, Medkour F, Pawlostky JM, Lotersztajn S, Mallat A (2008) Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology 134:432–439
Howes J, Osgood P (1974) The effect of delta9-tetrahydrocannabinol on the uptake and release of 14C-dopamine from crude striatal synaptosoma; preparations. Neuropharmacology 13:1109–1114
Izzo AA, Borrelli F, Capasso R, Di Vincenzo M, Mechoulam R (2009) Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30:515–527
Jouanjus E, Lapeyre-Mestre M, Micallef J (2014) Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc 3:e000638
Julian MD, Martin AB, Cuellar B, Rodriguez DF, Navarro M, Moratalla R, Garcia-Segura LM (2003) Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience 119:309–318
Kelsey JE, Harris O, Cassin J (2009) The CB(1) antagonist rimonabant is adjunctively therapeutic as well as monotherapeutic in an animal model of Parkinson’s disease. Behav Brain Res 203:304–307
Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, Shaffer WR, Rudroff T (2017) Cannabis use in people with Parkinson’s disease and multiple sclerosis: a web-based investigation. Complement Ther Med 33:99–104
Klein TW (2005) Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 5:400–411
Kreitzer AC, Malenka RC (2007) Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature 445:643–647
Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172:4790–4805
Lastres-Becker I, Cebeira M, de Ceballos ML, Zeng BY, Jenner P, Ramos JA, Fernandez-Ruiz JJ (2001) Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur J Neurosci 14:1827–1832
Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernandez-Ruiz J (2005) Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol Dis 19:96–107
Lotan I, Treves TA, Roditi Y, Djaldetti R (2014) Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol 37:41–44
Maccarrone M, Gubellini P, Bari M, Picconi B, Battista N, Centonze D, Bernardi G, Finazzi-Agro A, Calabresi P (2003) Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem 85:1018–1025
Mainka T, Stork J, Hidding U, Buhmann C (2018) Cannabis in Parkinson’s disease: hype or help? Fortschr Neurol Psychiatr 86:106–116
Malek N, Grosset DG (2015) Medication adherence in patients with Parkinson’s disease. CNS Drugs 29:47–53
Martinez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, Ferreiro-Vera C, Oyarzabal J, Canela EI, Lanciego JL, Nadal X, Navarro G, Borea PA, Franco R (2017) Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol 8:744
Meschler JP, Howlett AC, Madras BK (2001) Cannabinoid receptor agonist and antagonist effects on motor function in normal and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP)-treated non-human primates. Psychopharmacology 156:79–85
Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F, Le Fur G, Damier P, Welter ML, Agid Y (2004) Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol 27:108–110
Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A (2000) Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA 97:3655–3660
Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE (2001) Triggering myocardial infarction by marijuana. Circulation 103:2805–2809
Navarro G, Reyes-Resina I, Rivas-Santisteban R, Sanchez DMV, Morales P, Casano S, Ferreiro-Vera C, Lillo A, Aguinaga D, Jagerovic N, Nadal X, Franco R (2018) Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem Pharmacol 157:148–158
Pertwee RG (2008) The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153:199–215
Pertwee RG, Ross RA (2002) Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids 66:101–121
Pezzella FR, Colosimo C, Vanacore N, Di Rezze S, Chianese M, Fabbrini G, Meco G (2005) Prevalence and clinical features of hedonistic homeostatic dysregulation in Parkinson’s disease. Mov Disord 20:77–81
Prakash R, Aronow WS, Warren M, Laverty W, Gottschalk LA (1975) Effects of marihuana and placebo marihuana smoking on hemodynamics in coronary disease. Clin Pharmacol Ther 18:90–95
Pratt-Hyatt M, Zhang H, Snider NT, Hollenberg PF (2010) Effects of a commonly occurring genetic polymorphism of human CYP3A4 (I118 V) on the metabolism of anandamide. Drug Metab Dispos 38:2075–2082
Price DA, Martinez AA, Seillier A, Koek W, Acosta Y, Fernandez E, Strong R, Lutz B, Marsicano G, Roberts JL, Giuffrida A (2009) WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci 29:2177–2186
Roland CL, Lake J, Oderda GM (2016) Prevalence of prescription opioid misuse/abuse as determined by international classification of diseases codes: a systematic review. J Pain Palliat Care Pharmacother 30:258–268
Sanudo-Pena MC, Walker JM (1997) Role of the subthalamic nucleus in cannabinoid actions in the substantia nigra of the rat. J Neurophysiol 77:1635–1638
Sidney S (2002) Cardiovascular consequences of marijuana use. J Clin Pharmacol 42:64S–70S
Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM (2001) Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology 57:2108–2111
Sierra S, Luquin N, Rico AJ, Gomez-Bautista V, Roda E, Dopeso-Reyes IG, Vazquez A, Martinez-Pinilla E, Labandeira-Garcia JL, Franco R, Lanciego JL (2015) Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct 220:2721–2738
Stampanoni BM, Sancesario A, Morace R, Centonze D, Iezzi E (2017) Cannabinoids in Parkinson’s disease. Cannabis Cannabinoid Res 2:21–29
Starowicz K, Nigam S, Di Vincenzo M (2007) Biochemistry and pharmacology of endovanilloids. Pharmacol Ther 114:13–33
The American College of Obstetricians and Gynecologists Committee (2015) No. 637. Marijuana use during pregnancy and lactation. Obstet Gynecol 126:234–238
van der Stelt M, Di Marzo V (2003) The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol 480:133–150
van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, Brotchie JM (2005) A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J 19:1140–1142
van Vliet SA, Vanwersch RA, Jongsma MJ, Olivier B, Philippens IH (2008) Therapeutic effects of Delta9-THC and modafinil in a marmoset Parkinson model. Eur Neuropsychopharmacol 18:383–389
Venderova K, Ruzicka E, Vorisek V, Visnovsky P (2004) Survey on cannabis use in Parkinson’s disease: subjective improvement of motor symptoms. Mov Disord 19:1102–1106
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J (2015) Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313:2456–2473
Zuardi AW, Crippa JA, Hallak JE, Pinto JP, Chagas MH, Rodrigues GG, Dursun SM, Tumas V (2009) Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol 23:979–983
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Prof. Carsten Buhmann, MD: fees for advisory board participation: UCB Pharma, Zambon. Lecture fees: AbbVie Pharma, BIAL Pharma, Desitin, GE Healthcare, Grünenthal Pharma, Licher GmbH, Medtronic, Novartis, TAD Pharma, UCB Pharma, Zambon Pharma; Dr. Tina Mainka: royalties: Urban Fischer in Elsevier Verlag. Honoraria for lectures: GE Healthcare. Dr. Florin Gandor, MD: fees for advisory board participation: AbbVie Pharma. Lecture fees: MERZ Pharma, BIAL Pharma; Prof. Georg Ebersbach, MD: consultancy fees: AOK Nordost. Fees for advisory board participation: AbbVie Pharma, Grünenthal Pharma, Neuroderm Inc., Stada Pharma, Neurocrine Inc. Lecture fees: AbbVie Pharma, BIAL Pharma, Britannia Pharma, Desitin Pharma, Licher GmbH, UCB Pharma, Zambon Pharma, royalties: Kohlhammer Verlag, Thieme Verlag.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buhmann, C., Mainka, T., Ebersbach, G. et al. Evidence for the use of cannabinoids in Parkinson’s disease. J Neural Transm 126, 913–924 (2019). https://doi.org/10.1007/s00702-019-02018-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02018-8