Abstract
The diacylglycerol kinase eta (DGKH) gene, first identified in a genome-wide association study, is one of the few replicated risk genes of bipolar affective disorder (BD). Following initial positive studies, it not only was found to be associated with BD but also implicated in the etiology of other psychiatric disorders featuring affective symptoms, rendering DGKH a cross-disorder risk gene. However, the (patho-)physiological role of the encoded enzyme is still elusive. In the present study, we investigated primarily the influence of a risk haplotype on amygdala volume in patients suffering from schizophrenia or BD as well as healthy controls and four single nucleotide polymorphisms conveying risk. There was a significant association of the DGKH risk haplotype with increased amygdala volume in BD, but not in schizophrenia or healthy controls. These findings add to the notion of a role of DGKH in the pathogenesis of BD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia (SCZ) and bipolar affective disorder (BD) are highly genetic disorders, with a heritability of up to 70 % as estimated on the basis of family and twin studies [3, 11, 20, 43]. Formal genetic but also molecular studies argue that at least a proportion of the genetic risk between both disorders is shared [9]. Genome-wide association studies (GWAS) already detected various common risk gene variants conveying a small contribution to the overall risk of these disorders; however, replication studies often failed to confirm the findings. Nevertheless, some genes emerged which could be replicated in several studies or across phenotypes, such as CACNA1C [31]. Another well-replicated risk gene for BD is DGKH, which codes for the enzyme diacylglycerol kinase eta (DGKη) [52]. DGKH was first identified as a BD risk gene in a GWAS carried out by Baum and associates [4]; later, it could be confirmed to be associated with BD risk in several case–control studies [36, 44, 50, 52], although there were also conflicting findings [37]. Our group has recently suggested that DGKH is also associated with adult attention-deficit/hyperactivity disorder (aADHD) as well as major depression, suggesting that its association is not specific for BD but rather a broader phenotype characterized by affective symptoms and/or emotional processing [44]. Further adding to the evidence that DGKH has a role in BD, a postmortem study found DGKH mRNA to be increased in the brain of bipolar subjects as well as in patients having suffered from schizophrenia [23].
On the molecular level, DGKη plays an important role in the inositol triphosphate second messenger pathway by catalyzing the metabolism of diacylglycerol (DAG) to phosphatidic acid. DAG is a necessary cofactor for many isoforms of protein kinase C (PKC). Therefore, DGKH regulates the activity of PKC isoforms which play a key role in various signaling pathways [28, 40]. Intriguingly, lithium, the first-line maintenance treatment for bipolar disorder [27, 49], also influences the inositol trisphosphate (IP3) second messenger pathway and PKC signaling [8]. On the systemic level, a DGKH risk variant was studied for its impact on brain functioning: Individuals at high familial risk for BD and carrying a DGKH risk haplotype (rs9315885, rs1012053, rs1170191, TAC) displayed differential brain activity within the left medial frontal gyrus, the left precuneus, and the right parahippocampal gyrus during a verbal fluency task in an MRI study [45]. To our knowledge, no study has, however, investigated whether genetic risk conveyed by DGKH has an influence on structural MRI measures. As outlined above, DGKH seems to be implicated in a wider range of disorders, but especially in BD and SCZ, confirmed by the results from Moya et al. [23]. Hence, the effect of DKGH variants on brain structure might also be found across those two disorders. One of the key regions implicated in emotional regulation and processing is the amygdala [7, 25, 39]. In SCZ and BD, structural and functional alterations of the amygdala have been reported [2, 5, 19] and risk genes may play a role in those abnormalities [47]. The same sample (including an additional group of obsessive–compulsive patients) has been previously analyzed regarding influence of CACNA1C risk genotypes on brain volume. In this previous analysis, the CACNA1C genotype showed a significant effect on relative amygdala volume in patients with schizophrenia and genotype specific effects on amygdala volume of hemisphere and diagnosis in patients with BD and schizophrenia [47]. As in this former analysis, the CACNA1C genotype did explain inter-individual differences in amygdala volume among schizophrenia patients, but only to a small extent among bipolar patients, we decided to perform an additional exploratory analysis regarding the DGKH gene. In the present study, we thus analyzed whether primarily a DGKH risk haplotype (rs994856/rs9525580/rs9525584 GAT) and/or risk polymorphisms in DGKH (rs994856, rs9525580, rs9525584, rs9315885), as identified and replicated in previous studies [4, 24, 44], are associated with changes in amygdala volume in patients with BD, schizophrenia, or healthy controls.
Materials and methods
Participants
Participants in this study were 18 healthy unrelated participants, 23 patients with first-episode schizophrenia, and 30 euthymic patients with bipolar I disorder (YMRS ≤10 and MADRS ≤11). Diagnosis according to DSM-IV criteria was obtained using the German version of the Structural Clinical Interview for DSM-IV applied by board-certified study psychiatrists (O.G., and H.S. and T.W.) from the Department of Psychiatry and Psychotherapy at Saarland University Hospital and confirmed by the treating psychiatrist. Participants were Caucasian Europeans, mostly Germans and recruited from the Saarland area. Most patients received a stable medication, including first- and second-generation antipsychotics, antidepressants, and/or mood stabilizers at the time of the study. Demographic and clinical data are presented in Table 1. Exclusion criteria for patients and controls were MRI contraindications, organic disorders of the central nervous system (e.g., infectious, toxic or cerebrovascular disease, traumatic brain injury and epilepsy), mental retardation, or severe German language difficulties. Exclusion criteria for healthy controls were past or present psychiatric, neurological or severe medical disorder, and a positive family history of psychiatric disorders. Severity of clinical symptoms was evaluated with Positive and Negative Symptom Scales (PANSS) in patients with SCZ and the Young Mania Rating Scale (YMRS) in BD patients as well as the Montgomery–Åsberg Depression Rating Scale (MADRS). A detailed description of the study sample investigated in the present work is available elsewhere [47]. The study was in accordance with the Declaration of Helsinki and approved by the local ethics committee. Prior to participation, patients and controls gave written informed consent.
MRI acquisition and volumetric measurement
MRI scans were produced with a 1.5 T Magnetom Sonata (Siemens, Erlangen). A T1-weighted, magnetization prepared rapid gradient echo sequence (MPRAGE, echo time: TE = 4 ms, repetition time: TR = 1,900 ms, inversion time: TI = 700 ms, flip angle = 15°, image matrix = 256 × 256 mm) to allow a clear distinction between gray and white matter, slice thickness = 1 mm, no gap, generating 176 consecutive sagittal slices with a voxel size of 1 mm3 was used. The images were re-orientated and aligned parallel to the anterior–posterior commissural axis, and the origin was set to the anterior commissure. Image processing and volumetric analysis was performed using the software packages MRIcro and Analyze (Freeware, Chris Rorden). MRI images were converted into MRIcro files for manual tracing of the amygdala in each slice. Multiplication of the traced area on each slice by slice thickness, and the sum of all volumes were calculated to generate the total volume of the amygdala for each hemisphere.
Additionally, Statistical Parametric Mapping (SPM99) and MATLAB were applied exclusively for the assessment of the gray matter (GM) volume from total brain. Using the SPM99 segmentation tool, brains were segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) to calculate total GM volume according to the voxel number.
A trained image analyst who was blind to the diagnosis or genotype of the participant used the MRIcro software to perform the manual tracing. Amygdala volume was measured by direct manual tracing of the boundaries as previously described in own previous work [42]. The intra-rater reliability assessed by intra-class correlation coefficients (ICC) was sufficiently high for amygdala (right side: ICC = 0.98, left side: ICC = 0.98) volume measurement.
Statistical analysis was performed on relative volumes corresponding to the volume of the right or left amygdala divided by total brain GM volume to adjust for differences in individual head size.
Genotyping
DNA was extracted from venous blood from EDTA tubes using a standard de-salting method. Genotyping was performed using Sequenom’s MassArray® system (Sequenom, San Diego, CA) coupled to a Bruker Autoflex mass spectrometer (Bruker Daltonics, Bremen, Germany) according to the instructions supplied by the manufacturer. All PCR reactions were done using the iPlex® chemistry (Sequenom, San Diego, CA) following the manufacturer’s standard operation procedure. Further details including primer sequences have been published previously [44].
Statistical analysis
All tests were performed with SPSS statistical software (IBM™SPSS Statistics 20). Significance level was set at α = 0.05, all tests were two-tailed. Dependent variable was relative amygdala volume (left, right). Independent factors were diagnosis, DGKH risk haplotype GAT and four SNPs in the DGKH gene selected from previous studies (rs994856, rs9525580, rs9525584, rs9315885). Only the analyses on risk haplotype GAT were of primary interest. Data were tested on normal distribution with Kolmogorov–Smirnov test, χ 2 tests were used to check Hardy–Weinberg equilibrium (df = 2) and the distribution of sex (df = 1) and diagnosis (df = 2) across the GAT haplotypes. Demographic variables were compared between the genetic groups with χ 2 tests and analysis of variance (ANOVA). The influence of demographic variables and medicament effects on dependent variables was analyzed with ANOVA and Pearson’s correlation. An age-adjusted duration of illness was constructed as follows: we calculated the individual age at the beginning of the study minus individual age at onset of illness plus mean age at onset of all schizophrenic patients or of all bipolar patients, respectively. For the controls, the individual age at the beginning of the study was taken. This adjustment, enabling us to control for age and disease duration simultaneously, was necessary since there is no available disease duration data for controls [30].
Repeated measures ANCOVA with hemisphere as within-subject factor (left, right) and diagnosis, genotype, and sex as between-subject factors and age-adjusted disease duration as a covariate was computed for relative amygdala volumes. In case of significance, as subgroup analyses, genotypes were analyzed separately for left and right volumes on the one hand and for schizophrenia patients and patients with bipolar disorder on the other hand.
Power analysis
Post hoc power analysis for main hypothesis was computed with G*Power 3.1 [12]. A repeated measures ANOVA model, between-subject factor DGKH (two groups: no GAT/1 or more GAT), a medium effect size as observed for the DGKH effects on relative amygdala volumes (partial η 2 = 0.08), α = 0.05, two measurements (right/left hemisphere), and a correlation between the measurements (right, left) of r = 0.5 were assumed. A sufficient power of 1 − ß = 0.81 was achieved.
Results
Demographic variables
Amygdala relative volumes were normally distributed (relative amygdala volume: left: Z = 0.46, p = 0.98, right: Z = 0.38, p = 0.99), and data were in the Hardy–Weinberg equilibrium (p > 0.15 for every SNP). Independent sample t tests (α-level 0.05; two-tailed; degrees of freedom = 70) were used to test whether genotype groups differed according to age or years of education (Table 1) and no significant difference was found between groups. χ 2 tests on independence were used to check whether the distribution was different for sex and handedness between genetic groups and demonstrated no significant difference for hand preference, but a difference by trend regarding sex. The GAT haplotype occurred more frequently in male participants by trend (p = 0.064) (Table 1).
There were significant influences of factor diagnosis on left amygdala volume (highest volume in controls, lowest volume in schizophrenia patients, F = 5.9, df = 2, 65, p = 0.004). Furthermore, there were significant interactions between diagnosis and sex for the left (F = 3.9, df = 2, 65, p = 0.026) and for the right (F = 4.4, df = 2, 65, p = 0.016) amygdala. Male controls featured the highest volume, while in the female groups bipolar patients had the highest volume. Main analyses were adjusted for diagnosis, sex, and age-adjusted duration of illness. There were no significant influences of handedness or duration of education on amygdala volume. Patients with BD who were taking lithium had increased right amygdala volumes in comparison with patients not taking lithium (+12 %, F = 4.3, df = 1, 28, p = 0.047). There were no other significant influences of factor lithium on amygdala volume. In the schizophrenia group, there were no significant correlations between chlorpromazine equivalents (CPZ) and amygdala volumes.
GAT haplotype influence on amygdala volume
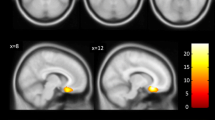
Considering the effects of the risk haplotype, repeated measures ANCOVA for relative amygdala volumes revealed a significant effect for factor DGKH GAT haplotype (F = 4.6, df = 1, 57, p = 0.035, effect size partial η 2 = 0.08). A main effect for hemisphere was observed (F = 50.4, df = 1, 57, p < 0.0005, volume right >left). Following separate analysis for right and left side showed significant effects of diagnosis for the left amygdala (F = 7.2, df = 2, 57, p = 0.002, highest volume in controls and lowest in schizophrenic patients). There was no significant interaction between sex and genotype on amygdala volume. There were no significant effects of the DGKH genotype on the left amygdala volume (F = 1.8, df = 1, 57, p = 0.19). For more details, see Fig. 1. Regarding the right amygdala, there were significant effects of the DGKH haplotype on volume (F = 6.3, df = 1, 57, p = 0.015, partial η 2 = 0.10), in that risk haplotype carriers had increased volumes. For details, see Fig. 2.
Relative left amygdala volume for bipolar disorder (BD), schizophrenia (SCZ), and healthy controls (CTRL). ALL includes BD and SCZ without CTRL. The graph displays mean values and standard deviations. GAT haplotype carriers in the BD group had a significantly greater amygdala volume (adjusted for total brain volume) compared to subjects carrying no GAT haplotype. *p < 0.05
Relative right amygdala volume for bipolar disorder (BD), schizophrenia (SCZ), and healthy controls (CTRL). The graph displays mean values and standard deviations. GAT haplotype carriers in the CTRL group, BD, and SCZ as one group (ALL) had a significantly greater amygdala volume (adjusted for total brain volume), compared to subjects carrying no GAT haplotype. *p < 0.05
A sub-analysis on BD and schizophrenia alone compared to healthy controls (ANOVA, between-subject factors DGKH, sex; inner-subject factor hemisphere, covariate age/disease duration; followed by side-separated analysis) revealed an association of the DGKH GAT haplotype with increased amygdala volume in the bipolar patients (F = 4.3, df = 1, 24, p < 0.05, partial η 2 = 0.15), but not in schizophrenia patients and controls (Figs. 1, 2). Additionally, there were significant sex (F = 11.0, df = 1, 24, p = 0.004) and hemisphere effects (F = 35.8, df = 1, 24, p < 0.0005) in bipolar patients. The relative volume of the right amygdala was larger than on the left side and females had an increased volume as compared to males. Subsequent univariate analysis showed significant effects of DGKH genotype on left amygdala (F = 7.8, df = 1, 24, p = 0.010, partial η 2 = 0.24) and also significant sex effects (left: F = 7.3, df = 1, 24, p = 0.013, right: F = 9.2, df = 1, 24, p = 0.006). These data did not change when lithium effects were controlled for.
Besides a significant effect for SNP rs9525580 on relative amygdala volume, left side (F = 5.6, df = 1, 52, p = 0.021, partial η 2 = 0.10, increased for C allele carriers), single marker analysis revealed no significant DGKH effects (see Table 2).
Discussion
In the present study, we demonstrated that a DGKH risk haplotype, identified in a previous study [44], influences amygdala volume in BD, but not in schizophrenia patients or healthy controls. Only one of the additionally examined single nucleotide polymorphisms (rs994856, rs9525580, rs9525584, rs9315885) was significantly associated with amygdala volume, rs9525580, supposedly being the variant conveying the effect on amygdala volume. In our previous study, rs9525580 was one of the two single markers that have been shown to be associated with bipolar disorder, but also with adult ADHD [44]. However, in the actual analysis, this was only a secondary and exploratory finding. Thus, if rs9525580 is the “true,” causal variant has yet to be replicated in future studies.
A recent meta-analysis of brain volumetric studies in bipolar patients provides evidence for a reduction in whole brain volume, enlargement of left and right ventricles, and an increased volume of the globus pallidus in bipolar patients. When comparing BD to schizophrenia, increased right amygdala volume in BD was described by Arnone and colleagues. No difference to controls, however, was detected in this meta-analysis [2]. Findings with respect to volumetric measurements of the amygdala in BD are, however, inconsistent, which might account for this negative finding. While some studies reported increased volume as compared to healthy controls [29], others argued for a volume reduction [10, 46] or even no difference at all [17, 26, 34, 35, 41, 51]. The inconsistencies in those previous findings could be due to different samples sizes, comorbidities of the participants or heterogeneity of the medication (especially lithium, as suggested by Hajek and associates), and endophenotypes [15]. Also seems age to play an important role regarding amygdala volumes in bipolar patients. A recent mega-analysis, which examined meta-analysis as well as unpublished date of structural MRI studies of bipolar patients, provided evidence that bipolar patients had increased right lateral ventricular, left temporal lobe, and right putamen volumes. Differences in amygdala and hippocampal volumes were detected between healthy controls and first-episode bipolar patients; the latter had reduced cerebral, amygdala, and hippocampus volumes, but there was a significantly increase in bipolar patients who received lithium in both hippocampus and amygdala volumes [18]. The influence of lithium could be replicated in the present study, at least for its effects on amygdala volume, as bipolar patients taking lithium showed a larger relative amygdala volume concordant with previous studies [13, 16, 42]. Including lithium medication as a factor did, however, not change the significant influence of the DGKH GAT haplotype on amygdala volume. In the present study, it could interestingly be shown that genetic variants of DGKH, whose gene product plays a role in a lithium-sensitive pathway, cause a similar effect on amygdala volume as lithium itself. Lithium may increase amygdala volume due to neurotrophic effects, e.g., by increasing the expression of neurotrophic factors [14, 22], but the potential molecular mechanism of DGKH variants on brain volume is still elusive.
The physiological role of DGKH, as gene product of DKGH, is not fully understood. DGK eta belongs to a family of lipid kinases and catalyzes the metabolization of DAG to phosphatidic acid [28]. DAG is produced by cleavage of PIP2 into IP3 and DAG by phospholipase C. DAG in turn activates PKC which phosphorylates and thereby regulates proteins of various pathways [38]. Among others, PKC phosphorylates Disheveled, an inhibitor of GSK3β, the latter of which is known to be inhibited by lithium [32]. Lithium also reduces PKC activity by inhibiting inositol monophosphates [6]. The converging pathway that leads to an increase in amygdala volume due to lithium treatment and due to the DGKH risk haplotype could involve PKC signaling. The specific molecular mechanisms how genetic variation within DGKH contributes to the pathogenesis of BD in general, however, still remain unclear. It has been speculated that genetically induced decreased DGK eta activity cause increased PKC activity as mentioned above and subsequent downstream pathways may have an impact on further pathomechanisms of BD [21, 33]. To our best knowledge, only a single study demonstrated increased expression of DGKH in the prefrontal cortex of bipolar patients [23], while expressional analysis of other brain regions do not yet exist. PKC signaling has been suspected to play a role in the pathophysiology of bipolar disorder and PKC inhibiting agents have been shown to have anti-manic properties, at least in animal models [1]. Given that DGK eta in its physiological role and lithium both induce a reduction of PKC signaling, this strongly argues for alterations of this pathway as a basis of the influence on amygdala volume. In what manner the described DGKH haplotype could affect amygdala volume specifically in bipolar patients only can be speculated on because of the still unclear physiological role of DAG eta in the human brain and the unknown molecular mechanisms of its involvement in the pathogenesis of bipolar disorder. Knockdown of DGKη in HELA cells lead to a proliferation defect because the enzyme seems to act as a regulatory component of the Ras/B-Raf/C-Raf/MEK/ERK signaling cascade [48]. In mice, Dgkh expression seems to be altered in raphe and amygdala dependent on prenatal environment of their mothers (Post and Reif, in preparation). It might thus be speculated that altered DGKH gene function due to genetic (rather than regulatory) variation might in particular affect the amygdala as well. The molecular mechanisms, how alterations in DGKH affect amygdala volume, however, remain elusive due to a dearth of data on the function of this protein. Given that the DAG pathway plays a pivotal role in neuronal development, a contribution of DGKH to amygdala plasticity seems, however, plausible.
Several methodological limitations need to be considered for the interpretation of the results of this study. First, the numbers of participants in the different subgroups are rather small. Second, there were a higher number of male risk haplotype carriers. As the genotype effect in the BD group was more pronounced in the female patients, the influence of the risk genotype could be interacting with sex. Larger samples to study this effect are needed. The third limitation is the issue of multiple testing. For this study, the sole primary hypothesis was that the DGKH GAT haplotype affected relative amygdala volume. All other analyses, especially on the single DGKH SNPs, were of secondary interest. For this reason, no Bonferroni adjustment of the type I error probability was applied although other analyses than presented here were performed on the same data set [47]. An adjustment of the error probability would decrease the test power, thereby precluding identification of existing mean differences (i.e., type-II error). Due to the explorative study design, the findings presented here should thus be considered an entry point for further investigations. Further studies incorporating larger samples including drug-naïve and adolescent patients will help to verify our findings.
In summary, this work demonstrates a specific effect of a DGKH haplotype on amygdala volume in BD and therefore supports the role of the DGKH gene in the pathogenesis of this disease. As lithium medication also leads to increase in amygdala volumes, alterations in PKC signaling could be hypothesized to be the common molecular basis of the influence of the genetic variant and lithium medication. Further research regarding the molecular consequences of DGKH gene variants, eventually leading to alterations in brain volumes, is thus warranted.
References
Abrial E, Lucas G, Scarna H, Haddjeri N, Lambas-Senas L (2011) A role for the PKC signaling system in the pathophysiology and treatment of mood disorders: involvement of a functional imbalance? Mol Neurobiol 44:407–419
Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM (2009) Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry 195:194–201
Barnett JH, Smoller JW (2009) The genetics of bipolar disorder. Neuroscience 164:331–343
Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, Georgi A, Schumacher J, Schwarz M, Abou Jamra R, Hofels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ (2008) A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry 13:197–207
Blond BN, Fredericks CA, Blumberg HP (2012) Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disord 14:340–355
Bone R, Springer JP, Atack JR (1992) Structure of inositol monophosphatase, the putative target of lithium therapy. Proc Natl Acad Sci USA 89:10031–10035
Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN (2013) Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. doi:10.1093/cercor/bht154
Chen G, Masana MI, Manji HK (2000) Lithium regulates PKC-mediated intracellular cross-talk and gene expression in the CNS in vivo. Bipolar Disord 2:217–236
Cross-Disorder Group of the Psychiatric Genomics Consortium; Genetic Risk Outcome of Psychosis (GROUP) Consortium (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379
DelBello MP, Adler CM, Strakowski SM (2006) The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectr 11:298–311
Domschke K, Reif A (2012) Behavioral genetics of affective and anxiety disorders. Curr Top Behav Neurosci 12:463–502
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM (2008) Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport 19:221–224
Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S (2001) Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology 158:100–106
Hajek T, Cullis J, Novak T, Kopecek M, Hoschl C, Blagdon R, O’Donovan C, Bauer M, Young LT, Macqueen G, Alda M (2012) Hippocampal volumes in bipolar disorders: opposing effects of illness burden and lithium treatment. Bipolar Disord 14:261–270
Hajek T, Kopecek M, Hoschl C, Alda M (2012) Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. J Psychiatry Neurosci 37:333–343
Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Hoschl C (2009) Amygdala volumes in mood disorders—meta-analysis of magnetic resonance volumetry studies. J Affect Disord 115:395–410
Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, Kieseppa T, Altshuler LL, Fornito A, Malhi GS, McIntosh AM, Yurgelun-Todd DA, Labar KS, Sharma V, MacQueen GM, Murray RM, McDonald C (2011) Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry 69:326–335
Honea R, Crow TJ, Passingham D, Mackay CE (2005) Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 162:2233–2245
Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM (2009) Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373:234–239
Manji HK, Lenox RH (1999) Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry 46:1328–1351
Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK (2000) Lithium-induced increase in human brain grey matter. Lancet 356:1241–1242
Moya PR, Murphy DL, McMahon FJ, Wendland JR (2010) Increased gene expression of diacylglycerol kinase eta in bipolar disorder. Int J Neuropsychopharmacol 13:1127–1128
Ollila HM, Soronen P, Silander K, Palo OM, Kieseppa T, Kaunisto MA, Lonnqvist J, Peltonen L, Partonen T, Paunio T (2009) Findings from bipolar disorder genome-wide association studies replicate in a Finnish bipolar family-cohort. Mol Psychiatry 14:351–353
Pessoa L, Adolphs R (2010) Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci 11:773–783
Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP (2008) Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 47:1289–1298
Pfennig A, Bschor T, Falkai P, Bauer M (2013) The diagnosis and treatment of bipolar disorder: recommendations from the current s3 guideline. Dtsch Arztebl Int 110:92–100
Sakane F, Kanoh H (1997) Molecules in focus: diacylglycerol kinase. Int J Biochem Cell Biol 29:1139–1143
Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, McKinney BA, Bodurka J, Kent Teague T, Drevets WC (2012) Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain Behav Immun 31:161–171. doi:10.1016/j.bbi.2012.10.007
Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WF, Bogerts B, Schmitz C, Falkai P (2009) Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 117(4):395–407. doi:10.1007/s00401-008-0430-y
Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Ripke S, Santangelo S, Sullivan PF (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379
Stambolic V, Ruel L, Woodgett JR (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6:1664–1668
Steckert AV, Valvassori SS, Mina F, Lopes-Borges J, Varela RB, Kapczinski F, Dal-Pizzol F, Quevedo J (2012) Protein kinase C and oxidative stress in an animal model of mania. Curr Neurovasc Res 9:47–57
Swayze VW 2nd, Andreasen NC, Alliger RJ, Ehrhardt JC, Yuh WT (1990) Structural brain abnormalities in bipolar affective disorder. Ventricular enlargement and focal signal hyperintensities. Arch Gen Psychiatry 47:1054–1059
Swayze VW 2nd, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC (1992) Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 31:221–240
Takata A, Kawasaki H, Iwayama Y, Yamada K, Gotoh L, Mitsuyasu H, Miura T, Kato T, Yoshikawa T, Kanba S (2011) Nominal association between a polymorphism in DGKH and bipolar disorder detected in a meta-analysis of East Asian case-control samples. Psychiatry Clin Neurosci 65:280–285
Tesli M, Kahler AK, Andreassen BK, Werge T, Mors O, Mellerup E, Koefoed P, Melle I, Morken G, Wirgenes KV, Andreassen OA, Djurovic S (2009) No association between DGKH and bipolar disorder in a Scandinavian case-control sample. Psychiatr Genet 19:269–272
Topham MK, Epand RM (2009) Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim Biophys Acta 1790:416–424
Townsend J, Altshuler LL (2012) Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord 14:326–339
Tu-Sekine B, Goldschmidt H, Petro E, Raben DM (2013) Diacylglycerol kinase theta: regulation and stability. Adv Biol Regul 53:118–126
Usher J, Leucht S, Falkai P, Scherk H (2010) Correlation between amygdala volume and age in bipolar disorder—a systematic review and meta-analysis of structural MRI studies. Psychiatry Res 182:1–8
Usher J, Menzel P, Schneider-Axmann T, Kemmer C, Reith W, Falkai P, Gruber O, Scherk H (2010) Increased right amygdala volume in lithium-treated patients with bipolar I disorder. Acta Psychiatr Scand 121:119–124
van Dongen J, Boomsma DI (2013) The evolutionary paradox and the missing heritability of schizophrenia. Am J Med Genet B Neuropsychiatr Genet 162B:122–136
Weber H, Kittel-Schneider S, Gessner A, Domschke K, Neuner M, Jacob CP, Buttenschon HN, Boreatti-Hummer A, Volkert J, Herterich S, Baune BT, Gross-Lesch S, Kopf J, Kreiker S, Nguyen TT, Weissflog L, Arolt V, Mors O, Deckert J, Lesch KP, Reif A (2011) Cross-disorder analysis of bipolar risk genes: further evidence of DGKH as a risk gene for bipolar disorder, but also unipolar depression and adult ADHD. Neuropsychopharmacology 36(10):2076–2085. doi:10.1038/npp.2011.98
Whalley HC, Papmeyer M, Romaniuk L, Johnstone EC, Hall J, Lawrie SM, Sussmann JE, McIntosh AM (2012) Effect of variation in diacylglycerol kinase eta (DGKH) gene on brain function in a cohort at familial risk of bipolar disorder. Neuropsychopharmacology 37:919–928
Wijeratne C, Sachdev S, Wen W, Piguet O, Lipnicki DM, Malhi GS, Mitchell PB, Sachdev PS (2013) Hippocampal and amygdala volumes in an older bipolar disorder sample. Int Psychogeriatr 25:54–60
Wolf C, Mohr H, Schneider-Axmann T, Reif A, Wobrock T, Scherk H, Kraft S, Schmitt A, Falkai P, Gruber O (2013) CACNA1C genotype explains interindividual differences in amygdala volume among patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 264(2):93–102. doi:10.1007/s00406-013-0427-y
Yasuda S, Kai M, Imai S, Takeishi K, Taketomi A, Toyota M, Kanoh H, Sakane F (2009) Diacylglycerol kinase eta augments C-Raf activity and B-Raf/C-Raf heterodimerization. J Biol Chem 284:29559–29570
Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, O’Donovan C, Macqueen G, McIntyre RS, Sharma V, Ravindran A, Young LT, Milev R, Bond DJ, Frey BN, Goldstein BI, Lafer B, Birmaher B, Ha K, Nolen WA, Berk M (2013) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 15:1–44
Yosifova A, Mushiroda T, Kubo M, Takahashi A, Kamatani Y, Kamatani N, Stoianov D, Vazharova R, Karachanak S, Zaharieva I, Dimova I, Hadjidekova S, Milanova V, Madjirova N, Gerdjikov I, Tolev T, Poryazova N, O’Donovan MC, Owen MJ, Kirov G, Toncheva D, Nakamura Y (2011) Genome-wide association study on bipolar disorder in the Bulgarian population. Genes Brain Behav 10:789–797
Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G (2010) Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Front Hum Neurosci 4:189
Zeng Z, Wang T, Li T, Li Y, Chen P, Zhao Q, Liu J, Li J, Feng G, He L, Shi Y (2011) Common SNPs and haplotypes in DGKH are associated with bipolar disorder and schizophrenia in the Chinese Han population. Mol Psychiatry 16:473–475
Acknowledgments
This study was supported by the DFG (Grant RTG 1253/1, RE1632/5-1 to AR), the BMBF (DZHI, Project 01EO1004, to AR), and the IZKF (Project Z3-24, to SKS). I. Reck and C. Gagel are credited for excellent technical assistance.
Conflict of interest
S. Kittel-Schneider, B. Malchow, H. Scherk, C. Wolf, S. Trost, M. Backens, D. Zilles, W. Reith, and T. Schneider-Axmann declare no conflicts of interest. A. Hasan has been invited to scientific conferences by Janssen-Cilag, Pfizer, and Lundbeck. He received paid speakership by Desitin. O. Gruber was an honorary speaker for AstraZeneca, Bristol-Meyers Squibb, Janssen-Cilag, and Otsuka and has been invited for scientific congresses by AstraZeneca, Janssen-Cilag, and Pfizer. A. Schmitt was honorary speaker for TAD Pharma and Roche and has been member of advisory boards for Roche. P. Falkai has been member of the advisory boards for Janssen-Cilag, BMS, Lundback, Pfizer, Lilly, and AstraZeneca and received an educational grant from AstraZeneca and honoraria as lecturer from Janssen-Cilag, BMS, Lundbeck, Pfizer, Lilly, and AstraZeneca. T. Wobrock received honoraria and has participated in speaker bureaus for Alpine Biomed, AstraZeneca, Bristol-Myers Squibb, Lilly, Essex, Organon, Janssen-Cilag, Pfizer, Sanofi-Synthelabo/Aventis and Lundbeck and a research grant from AstraZeneca. A. Reif has received research grants from Astra Zeneca.
Author information
Authors and Affiliations
Corresponding author
Additional information
O. Gruber and A. Reif have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kittel-Schneider, S., Wobrock, T., Scherk, H. et al. Influence of DGKH variants on amygdala volume in patients with bipolar affective disorder and schizophrenia. Eur Arch Psychiatry Clin Neurosci 265, 127–136 (2015). https://doi.org/10.1007/s00406-014-0513-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-014-0513-9