Abstract
Animal epidemiological and clinical studies suggest that cholesterol is a risk factor for Alzheimer’s disease (AD). Nevertheless, the relation of cholesterol to mild cognitive impairment (MCI), influence of APOE genotype and its changes in lifespan is controversial. We investigated the potential impact of plasma total cholesterol (TC) on development of MCI and AD in the interdisciplinary longitudinal study on adult development and aging, a representative birth cohort (born 1930–1932), examined in 1993/1994 (VT1), 1997/1998 (VT2), and 2005/2007 (VT3). Of 500 participants at baseline, 381 survived and were examined at VT3. After exclusion of participants with lifetime prevalence of major psychiatric diseases or mild cognitive disorder due to a medical condition, 222 participants were included in the analysis. At VT3, 82 participants had MCI, 22 participants had AD, and 118 were in good health. Participants with MCI and AD at VT3 evidenced higher TC levels at VT1 than those who were healthy. Higher TC levels at baseline were associated with an increased risk for cognitive disorders at VT3 (highest vs. lowest quartile: OR 2.64, 95 % CI 1.12–6.23, p < 0.05). Over the 14 year follow-up, TC levels declined in those with MCI and AD, but remained stable in those who remained healthy. These findings were not modified by APOE genotype or use of cholesterol-lowering medications. Our findings demonstrate that higher TC levels are observed long before the clinical manifestation of MCI and AD in patients without psychiatric or somatic comorbidities and are independent of APOE genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholesterol is considered to be involved in the pathogenesis of mild cognitive impairment (MCI) and Alzheimer’s disease (AD). In view of that, clinical studies have demonstrated increased levels of cerebrospinal fluid 24-hydroxycholesterol in AD patients compared with healthy controls [1]. Likewise, epidemiological studies have established that higher total cholesterol (TC) in midlife is a risk factor for the development of AD [2–4] (for a review see [5, 6]), and that cholesterol-lowering agents, such as statins, may have a protective function [7, 8]. Interestingly, there is evidence that TC values decrease more rapidly in subjects experiencing subsequent cognitive decline or dementia compared with individuals who remain healthy. Declining cholesterol levels appear to accompany aging specifically in AD and are probably a manifestation of underlying dementia-related neuropathology [9, 10].
Experiments on cell cultures provide a feasible explanation for this association, indicating that the accumulation of cholesterol in neurons results in an accelerated cleavage of amyloid precursor proteins into amyloidogenic components [1]. This may lead to the formation of amyloid plaques in susceptible brain regions with consecutive neuronal degeneration. Thus, experiments on hypercholesterolemic rabbits [2] find an association with blood cholesterol and enhanced amyloid deposition in the brain. Additionally, cholesterol deposits in amyloid plaques may promote the stability thereof.
Most of the prospective studies reporting on cholesterol and cognitive impairment or dementia [2–4] have involved northern European populations, which are generally characterized by a high prevalence of the APOEε4 allele. This is of particular importance, since the presence of the APOEε4 allele confers an increased risk of AD. The APOE protein is involved in Aß metabolism, and the affinity of APOE for Aß is increased in the presence of lipids [11]. Animal studies demonstrate that a high cholesterol diet affects not only TC plasma levels but also modulates APOE expression, and APP and Aß secretion [12].
In the present study, we investigated the role of TC levels in the development of MCI and AD, as well as the potential impact of APOE genotype. Specifically, a German population-based sample born between 1930 and 1932 drawn from the interdisciplinary longitudinal study on adult development and aging (ILSE) was considered in the course of three examination waves—extending over 14 years.
Materials and methods
Participants
The ILSE is a prospective study of adult development in Germany based on two birth cohorts born in 1930–1932 and 1950–1952 [13, 14]. At baseline, in 1993–1995, participants were randomly selected and recruited from the community registers in the urban regions of Leipzig (Saxony) and Heidelberg/Mannheim (Palatine), for which inclusion is mandatory for citizens aged 16 years and older in Germany. This recruitment procedure yielded a representative sample of the communities included [15]. Participants were subsequently recontacted over a 14 year period, in 1997–2000 and 2005–2008.
This study includes only those participants born 1930–1932 and who completed the 2005–2008 examination (n = 381). A description of this sample is as follows (see Fig. 1). At baseline, in 1993–1995 (VT1), 500 participants were examined. In the second examination (VT2) in 1997–1999, 449 persons were re-examined; and in 2005–2008 (VT3), 381 [76.2 % of the baseline sample, average age 74.3 (SD = 1.2) years] persons were examined. Of the 119 non-participants at VT3, 64 (53.8 %) had died, seven (5.9 %) no longer lived in the region, 39 (32.8 %) were ineligible, and nine (7.5 %) did not wish to participate.
Since MCI and AD were the primary outcomes of interest, additional exclusion criteria were applied at VT3, such that those who met criteria for other mental disorders such as vascular dementia, major depression, anxiety disorders, or mild cognitive disorder (MCI due to a medical condition as defined by ICD-10) were excluded. Thus, only those participants surviving from VT3—developing MCI or AD or remaining cognitively healthy—were included.
Given study exclusion criteria, the final sample at VT3 included 109 participants with MCI, 26 with AD, and 157 cognitively healthy individuals, without any psychiatric disorder. Of these, 70 participants refused APOE genotyping (39 without any psychiatric disorder, 27 with MCI and four with AD), leaving a final sample of 222 participants. Amount of missing data did not differ between diagnostic groups [χ2(2) = 1.16, p = 0.56].
The study was approved by the Ethical Committee of the University of Heidelberg. After a complete description of the study to the participants, written informed consent was obtained.
Psychiatric diagnoses at VT3
Psychiatric disorders were diagnosed using the German version of the Structured Clinical Interview for the DSM-III-R [16]. MCI was diagnosed according to the aging-associated cognitive decline criteria (AACD, International Psychogeriatric Association working Party, [17]) including (1) subjective impairment: A report by the individual or an informant that cognitive function has declined and (2) objective impairment: difficulties in any of the following cognitive domains, as indicated by neuropsychological test performance of at least one standard deviation below normal age and educational levels: memory and learning, attention and concentration, abstract thinking (problem solving, abstraction), language, and visuospatial functioning. Moreover, MCI patients were classified as amnestic or non-amnestic types, depending on the affected cognitive domain. AD and vascular dementia were diagnosed using the NINCDS–ADRDA and the NINDS–AIREN criteria, respectively [18, 19]. Additional methodological details have been described elsewhere [13, 14]. Particular care was taken to exclude participants with mild cognitive disorder (ICD-10), and major psychiatric disorders such as depression or substance abuse, since symptoms of these conditions overlap with dementia and other cognitive disorders. Clinical diagnoses were established by consensus of two psychiatrists (P.T., P.S.) under supervision of a specialist (J.S.) in geriatric psychiatry.
Laboratory measures
TC was analyzed with Advia® 2400 Chemistry System from Siemens Healthcare Diagnostics. Genomic DNA was extracted from whole blood using the High Pure PCR Template Preparation Kit (Roche Diagnostics, Mannheim, Germany) following the manufacturer’s instructions. APOE genotype was assessed using the LightCycler technology [20].
Survey measures
Participants were carefully screened for physical and mental health via questionnaires, extensive personal interviews, as well as medical and neuropsychological examinations at all visits (VT1, VT2, and VT3). The cognitive assessment included the mini-mental state examination (MMSE [21]), subtests of the Nürnberger–Alters–Inventar (NAI) [22] and the Leistungsprüfsystem [23], both of which are well-established and commonly used test batteries in Germany (for more details see [14]):
-
1.
Memory and learning: immediate word list recall and delayed word list recognition (NAI)
-
2.
Attention and concentration: Aufmerksamkeits–Belastungs-Test (d2 test [24].
-
3.
Abstract thinking: similarities subtest (Hamburg–Wchsler–Intelligneztest für Erwachsene) [25].
-
4.
Language-subtest of verbal fluency (Leistungsprüfungssystem).
-
5.
Visuospatioal functioning: subtest of visual imagination (Leistungsprüfungssystem).
Statistics
Diagnostic groups were compared using analyses of variance with repeated measures for time. Post hoc Tukey’s tests and χ2 tests were used where appropriate. To address potential effects of the APOE genotype, groups were additionally dichotomized according to the presence or absence of at least one e4 allele.
In order to assess the risk of MCI and AD associated with plasma TC, participants were divided into TC quartiles and odds ratios (OR) were calculated. Logistic regression analyses were performed to determine statistical significance at 95 % confidence intervals. In order to adjust for important potentially confounding variables, education, APOE genotype, socio-economic status, and gender were included into the logistic regression model.
SAS software (version 9.01; SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Demographic and clinical characteristics of the diagnostic groups are summarized in Table 1.
Diagnostic groups (AD, MCI, healthy controls) did not differ on the basis of sex, statin use, and APOEε4 allele. Moreover, no significant differences between diagnostic groups emerged with respect to body mass index (BMI) for VT1 (F = 1,182; n.s.), VT2 (F = 1,786; n.s.), and VT3 (F = 1,226; n.s.), smoking behavior for VT1 (χ2 = 1.090, df = 2, n.s.), VT2 (χ2 = 0.276, df = 2, n.s.), and VT3 (χ2 = 0.224, df = 2, n.s.), hypertension at VT1 (Fisher’s exact test: n.s.), VT2 (Fisher’s exact test: n.s.) or diabetes mellitus for VT1 (χ2 = 0.289, n.s.) and VT2 (Fisher’s exact test: n.s.). AD cases were slightly older than the cognitively healthy. Length of formal school education was shorter in those with AD or MCI (F = 13.23, p < 0.0001). Mean MMSE scores differed between groups (F = 100.10, p < 0.0001) with the MCI group ranking in between participants with AD and the cognitively healthy. 19.5 % of MCI cases were classified as amnestic (scoring >1SD below average on NAI word list), 65.9 % were classified as non-amnestic.
TC levels at baseline differed between diagnostic groups with MCI and AD patients having higher TC levels than controls (F = 3.179, p = 0.044). Post hoc analysis revealed a significant difference between MCI and healthy controls (p = 0.046). Average TC levels declined during follow-up in MCI and AD, but were almost stable in participants who remained cognitively healthy at VT3. These findings were confirmed by a repeated measures ANOVA that yielded a main effect of time (F = 15.51, df = 2/438, p < 0.0001) and an interaction effect of diagnosis by time (F = 3.88, df = 4/438, p < 0.005), while diagnosis alone was not informative (F = 0.90, df = 2/219, p = 0.4, n.s.).
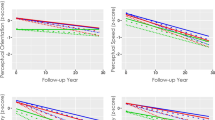
Diagnostic groups were also dichotomized according to presence of an APOEε4 allele (Table 2; also see Fig. 2). TC levels were significantly higher among those with any e4 allele (p < 0.05). A repeated measures ANOVA with time as within-subject factor revealed significant main effects for APOE and time, as well as a significant time by diagnosis interaction (F = 14.39, df = 1/216, p < 0.005; F = 13.68, df = 2/432, p < 0.001; and F = 5.40, df = 4/432, p < 0.005, respectively). No other main or interaction effect emerged (diagnosis: F = 0.77, df = 2/216; diagnosis × APOE: F = 0.50, df = 2/216; time × APOE: F = 2.06, df = 2/432; time × diagnosis × APOE: F = 2.34, df = 4/432).
Longitudinal course of total cholesterol means by diagnosis and APOE genotype. VT visit time, CH cognitive healthy, MCI mild cognitive impairment, AD Alzehimer’s disease, + participants with APOEε4 allele, − participants without APOEε4 allele. VT1 (1993–1995) mean age = 62.4 (SD = 2.4), VT2 (1995–1998) mean age = 66.7 (SD = 1.1), VT3 (2005–2008) mean age = 74.3 (SD = 1.2)
A logistic regression revealed an increased risk of developing a cognitive disorder for the higher quartile of TC levels in comparison with the lower (OR 2.64, 95 % CI 1.12–6.23, p < 0.05) and second lower quartile (OR 3.15, CI 1.29–7.70, p < 0.05). TC levels at VT2 and VT3 were not associated with cognitive disorders.
Discussion
In our study, TC levels at age 60 were associated with a diagnosis of MCI or AD 14 years later. Moreover, in the course of 14 years, a distinct trajectory in TC levels among those who develop MCI or AD in comparison with subjects that remain healthy was observed, such that TC remained stable in the cognitively healthy, whereas decline was observed among those developing MCI or AD. Interestingly, these associations were independent of potential modulators of the cholesterol–dementia relationship, such as APOE genotype and statin use, as well as other potentially confounding variables, including cardiovascular risk factors (e.g., smoking, hypertension, BMI, and diabetes).
Our findings confirm a previous report by Solomon et al. [4] suggesting a bidirectional relationship between TC levels and MCI/AD diagnosis such that high TC levels in midlife is a risk factor for subsequent dementia, while an observed decrease of TC levels after midlife may reflect ongoing disease processes. Thus, our results support findings [10] describing a more pronounced decline in TC among those who develop AD at least 15 years before a clinical diagnosis [26].
Study methodologies differ in this field, which may influence observations, as well as interpretation thereof. Some [2, 3, 27, 28]—but not all [26, 29]—studies show a relationship between higher TC values and subsequent development of MCI or dementia. Most studies relating TC to dementia are cross sectional or have included participants across a broad age range. This limits conclusions related to temporality of the association, since TC levels do not only change with age, but also with secular effects (e.g., birth cohort) [30]. It is well documented that TC levels increase with age, rising to a plateau before the age of 70 and subsequently decrease in older age (for an example in the German population see [31]). Since our study cohort is comprised of persons from the general German population born between 1930 and 1932, we have, by design, adjusted for age.
In addition, differences in diagnostic criteria exist. In a Finnish study [4], for example, only individuals scoring 24 or below on the MMSE were referred for subsequent diagnostic evaluation with respect to MCI or AD. This screening algorithm may have led to a selection of individuals who were in more advanced stages of MCI or dementia but excluded most cases of preclinical dementia. In our study, every participant underwent thorough neuropsychological testing, as did participants in the Gothenburg birth cohort studies [26, 32]. This made it possible to identify subjects in early stages of MCI who often score above 24 on the MMSE. In addition, we improved diagnostic accuracy via personal medical assessment of each participant focusing on medical and neuropsychiatric morbidities. Thus, given our inclusion- and exclusion criteria, our baseline sample was cognitively healthy. We were therefore able to chart the trajectory of TC levels in relation to MCI/AD overtime. In addition, participants with mild cognitive disorder due to a general medical condition (i.e., cardiovascular disease or severe metabolic disorder) or any psychiatric comorbidity (e.g., history of major depression or alcohol abuse) were excluded to assess the relationship of TC among participants with a specific MCI syndrome, and at risk for developing an AD type of dementia. This was accomplished in concordance with the Consensus of the International Psychogeriatric Association [17] which emphasizes that the differential diagnosis between MCI, dementia, and ICD-10 “mild cognitive disorder” should be considered the most important.
The prevalence of the APOEε4 allele was higher in those with AD versus those developing MCI or remaining cognitively healthy. That this difference did not reach statistical significance may be due to lack of power, to the low prevalence of AD in this age group and the relatively low APOEε4 prevalence. The Finnish population, however, has a higher APOEε4 allele prevalence, as well as higher average TC levels and lower MMSE scores [33]. These characteristics may well account for the strong TC-dementia observations in Finnish studies. Solomon et al. [4] found APOEε4 prevalence of 35 % in those without dementia or MCI, but over 50 % in those with dementia. Other European and American community-based studies yielded APOEε4 prevalences that are comparable with those observed in ILSE among cognitively healthy elderly: 11.2 % in a French study [34] and 12.6 % in white Americans [35]. These population differences emphasize potential difficulties in extrapolating results from one population to another even within Europe, and underscores the importance of conducting epidemiological studies in individual European countries in relationship to vascular factors in dementia etiology.
Selection effects have to be discussed as a potential confounding factor, since we solely focused on survivors from the ILSE. In Mielke et al. [32] the differences in TC levels reached significance only in the survivor analysis, and no significant differences appeared between the groups in the whole sample analysis. Nevertheless, our follow-up quote of 76 % of initial subjects in the 14 years with a strict and complete clinical examination of all study subjects in all visits expands the clinical significance of our findings.
To our knowledge, this is the first study describing the longitudinal relationship of APOE and TC in relation to clinically diagnosed MCI and AD. Considered together, APOE genotype and TC levels may be independent risk factors [2] for AD at the population level. One epidemiological study [36] found high TC levels to be a risk factor only among non-APOEε4 carriers, however, this study dichotomized their sample according to TC levels without reporting whether APOEε4 carriers had elevated TC levels in comparison with non-carriers [37–39]. Gender and statin use have to be considered as potential confounding factors. However, diagnostic groups showed only marginal, non-significant differences with respect to these variables; in addition, the proportion of subjects who received statins were rather low and did not exceed 33 %. Studies describing a protective effect of statins in cognitive disorders (AD and MCI) are misunderstanding, with some showing a protective effect [7, 40] and other showing no clear relation [41, 42]. Since most of them focused on dementia and very few in MCI as an outcome, more prospective studies are needed to address this topic.
In conclusion, higher TC levels at age 60 are associated with the development of MCI and AD at age 75 years (highest vs. lowest quartile: OR 2.64, 95 % CI 1.12–6.23, p < 0.05). While TC levels decline and stabilize during this period, these effects are not accounted for by APOE genotype, birth cohort, statin treatment, or other cardiovascular risk factors. These findings support the hypothesis that preventive measures targeted on TC in relationship to cognition may have benefit before the seventh decade of life.
References
Schonknecht P, Lutjohann D, Pantel J, Bardenheuer H, Hartmann T, von Bergmann K, Beyreuther K, Schroder J (2002) Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer’s disease compared to healthy controls. Neurosci Lett 324(1):83–85
Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H (2002) Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 137(3):149–155
Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64(2):277–281. doi:10.1212/01.WNL.0000149519.47454.F2
Solomon A, Kareholt I, Ngandu T, Winblad B, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M (2007) Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology 68(10):751–756. doi:10.1212/01.wnl.0000256368.57375.b7
Kivipelto M, Solomon A (2006) Cholesterol as a risk factor for Alzheimer’s disease—epidemiological evidence. Acta Neurol Scand Suppl 185:50–57. doi:10.1111/j.1600-0404.2006.00685.x
Anstey KJ, Lipnicki DM, Low LF (2008) Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry 16(5):343–354. doi:10.1097/JGP.0b013e31816b72d4
Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med 5:20. doi:10.1186/1741-7015-5-20
Li G, Larson EB, Sonnen JA, Shofer JB, Petrie EC, Schantz A, Peskind ER, Raskind MA, Breitner JC, Montine TJ (2007) Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology 69(9):878–885. doi:10.1212/01.wnl.0000277657.95487.1c
Mielke MM, Zandi PP, Shao H, Waern M, Ostling S, Guo X, Bjorkelund C, Lissner L, Skoog I, Gustafson DR (2010) The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology 75(21):1888–1895. doi:10.1212/WNL.0b013e3181feb2bf
Stewart R, White LR, Xue QL, Launer LJ (2007) Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol 64(1):103–107. doi:10.1001/archneur.64.1.103
Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, Ladu MJ, Rostagno A, Frangione B, Ghiso J (2000) Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid beta peptides. Biochem J 348(Pt 2):359–365
Howland DS, Trusko SP, Savage MJ, Reaume AG, Lang DM, Hirsch JD, Maeda N, Siman R, Greenberg BD, Scott RW, Flood DG (1998) Modulation of secreted beta-amyloid precursor protein and amyloid beta-peptide in brain by cholesterol. J Biol Chem 273(26):16576–16582
Schroder J, Kratz B, Pantel J, Minnemann E, Lehr U, Sauer H (1998) Prevalence of mild cognitive impairment in an elderly community sample. J Neural Transm Suppl 54:51–59
Schonknecht P, Pantel J, Kruse A, Schroder J (2005) Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects. Am J Psychiatry 162(11):2071–2077
Martin P, Martin M (2000) Design und Methodik der Interdisziplinären Längsschnittstudie des Erwachsenenalters. In: Martin P (ed) Aspekte der Entwicklung im mittleren und höheren Erwachsenenalter. Steinkopff, Darmstadt, pp 17–27
Wittchen H, Zaudig M, Schramm E, Spengler P, Mombour W, Klug J, Horn R (1991) Strukturiertes klinisches Interview für DSM-III-R. Beltz-Test, Göttingen
Levy R (1994) Aging-associated cognitive decline. Working party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr 6(1):63–68
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34(7):939–944
Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A et al (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS–AIREN international workshop. Neurology 43(2):250–260
Aslanidis C, Schmitz G (1999) High-speed apolipoprotein E genotyping and apolipoprotein B3500 mutation detection using real-time fluorescence PCR and melting curves. Clin Chem 45(7):1094–1097
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Oswald W, Fleischmann V (1991) Nürnberger Alters–Inventar. Universität Erlangen-Nürnberg, Erlangen
Sturm W, Willmes K, Horn W (1993) Leistungsprüfsystem für 50-90jährige. Hogrefe, Göttingen
Brickenkamp R (1978) Test d2: Aufmerksamkeits–Belastungs-test. Hofgrefe, Göttingen
Tewes W (1991) HAWIE-R: Hamburg–Wchsler–Intelligneztest für Erwachsene, revision. Huber, Bern
Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, Skoog I (2005) High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 64(10):1689–1695. doi:10.1212/01.WNL.0000161870.78572.A5
Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A (2005) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62(10):1556–1560. doi:10.1001/archneur.62.10.1556
Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A (1998) Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology 17(1):14–20
Reitz C, Tang MX, Luchsinger J, Mayeux R (2004) Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol 61(5):705–714. doi:10.1001/archneur.61.5.70561/5/705
Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF (1999) Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology 53(1):189–196
Richter V, Rassoul F, Hentschel B, Kothe K, Krobara M, Unger R, Purschwitz K, Rotzsch W, Thiery J, Muradian K (2004) Age-dependence of lipid parameters in the general population and vegetarians. Z Gerontol Geriatr 37(3):207–213. doi:10.1007/s00391-004-0232-3
Mielke M, Zandi P, Shao H, Waern M, Östling S, Guo X, Björkelund C, Lissner L, Skoog I, Gustafson D (2010) The 32 year relationship between cholesterol and dementia from mid- to late-life. Neurology 75(21):1888–1895
Gustafson DR, Melchior L, Eriksson E, Sundh V, Blennow K, Skoog I (2010) The ACE insertion deletion polymorphism relates to dementia by metabolic phenotype, APOEepsilon4, and age of dementia onset. Neurobiol Aging 31(6):910–916. doi:10.1016/j.neurobiolaging.2008.07.015
Schachter F, Faure-Delanef L, Guenot F, Rouger H, Froguel P, Lesueur-Ginot L, Cohen D (1994) Genetic associations with human longevity at the APOE and ACE loci. Nat Genet 6(1):29–32. doi:10.1038/ng0194-29
Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R (1998) The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 279(10):751–755
Hall K, Murrell J, Ogunniyi A, Deeg M, Baiyewu O, Gao S, Gureje O, Dickens J, Evans R, Smith-Gamble V, Unverzagt FW, Shen J, Hendrie H (2006) Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology 66(2):223–227. doi:10.1212/01.wnl.0000194507.39504.17
Mahley RW, Rall SC Jr (2000) Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1:507–537. doi:10.1146/annurev.genom.1.1.507
Sing CF, Davignon J (1985) Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. Am J Hum Genet 37(2):268–285
Wehr H, Parnowski T, Puzynski S, Bednarska-Makaruk M, Bisko M, Kotapka-Minc S, Rodo M, Wolkowska M (2000) Apolipoprotein E genotype and lipid and lipoprotein levels in dementia. Dement Geriatr Cogn Disord 11(2):70–73
Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD (2008) Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology 71(5):344–350. doi:10.1212/01.wnl.0000319647.15752.7b
Rockwood K (2006) Epidemiological and clinical trials evidence about a preventive role for statins in Alzheimer’s disease. Acta Neurol Scand 185:71–77. doi:10.1111/j.1600-0404.2006.00688.x
Beydoun MA, Beason-Held LL, Kitner-Triolo MH, Beydoun HA, Ferrucci L, Resnick SM, Zonderman AB (2011) Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health 65(11):949–957. doi:10.1136/jech.2009.100826
Acknowledgments
The interdisciplinary longitudinal study on adult development and aging (ILSE) was supported by the “Research Program of the State of Baden-Württemberg” and the “Federal Ministry for Family, Senior Citizen, Women, and Youth, Germany.” P. Toro and J. Schröder received additional support by the “Marsilius Kolleg,” center of advanced studies, University of Heidelberg, Germany. This work was supported by the European Commission under the 7th Framework Programme of the European Union “Therapeutic and preventive impact of nutritional lipids on neuronal and cognitive performance in aging, Alzheimer’s disease and vascular dementia” coordinated by Prof Dr. Tobias Hartmann.
Conflict of interest
P. Toro, CH. Degen, D. Gustafson, M.Pierer, P. Schönknecht and J. Schröder declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toro, P., Degen, C., Pierer, M. et al. Cholesterol in mild cognitive impairment and Alzheimer’s disease in a birth cohort over 14 years. Eur Arch Psychiatry Clin Neurosci 264, 485–492 (2014). https://doi.org/10.1007/s00406-013-0468-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-013-0468-2