Abstract
Functional abnormalities in regions associated with reward processing are apparent in people with depression, but the extent to which disease burden impacts on the processing of reward is unknown. This research examined the neural correlates of reward processing in patients with major depressive disorder and varying degrees of past illness burden. Twenty-nine depressed patients and twenty-five healthy subjects with no lifetime history of psychiatric illness completed the study. Subsets of fourteen patients were presenting for first lifetime treatment of a depressive episode, and fifteen patients had at least three treated episodes of depression. We used functional magnetic resonance imaging to study blood oxygen level-dependent signals during the performance of a contingency reversal reward paradigm. The results identified group differences in the response to punishers bilaterally in the orbitofrontal and medial prefrontal regions. In addition, areas such as the nucleus accumbens, anterior cingulate and ventral prefrontal cortices were activated greatest by controls during reward processing, less by patients early in the course of illness and least by patients with highly recurrent illness—suggesting that these areas are sensitive to the impact of disease burden and repeated episodes of depression. Reward processing in people with depression may be associated with diminished signaling of incentive salience, a reduction in the formation of reward-related associations and heightened sensitivities for negatively valenced stimuli, all of which could contribute to symptoms of depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anhedonia is a central feature of major depressive disorder (MDD) and is defined as a decrease in the ability to seek out and experience pleasurable activities [1]. As one of the earliest and most frequent precursors to adult-onset MDD [2], anhedonia is thought to potentiate existing genetic vulnerabilities to depression [3]. A dysregulation of the neural system involved in the processing of reward may contribute to the experience of anhedonia. Indeed in patients with MDD, suboptimal decision-making strategies, heightened sensitivities to negative feedback and punishment [4], and failures to modulate responses to rewards [5, 6] are closely aligned with the symptoms of anhedonia.
Functional imaging studies have identified a number of brain regions important in the processing of reward. At the core of this network is the nucleus accumbens (NAcc). Depressed individuals presenting with anhedonia have been shown to lack an increase in NAcc activity during the presentation of positive stimuli [7–9]. Further, depressed patients lack an ability to sustain activity in the NAcc indicating dysfunction in the ability to sustain pleasure over repeated presentations of positive stimuli when compared with healthy controls [10]. Frontal regions, such as the orbitofrontal cortex (OFC), appear to be important in assigning value to rewarding stimuli and in aspects of approach behavior and response inhibition [11, 12]. The ventral medial prefrontal cortex (VMPFC) plays a role in the contextual processing of reward [11, 13], the response to unexpected rewards [14], forming abstract representations of the reward value [15] and the assessment of reward outcomes [16]. Finally, the more dorsolateral frontal regions and the anterior cingulate cortex (ACC) engage in the integration of reward information to support response selection [17, 18].
Functional abnormalities in the regions associated with reward processing are apparent in people with depression [19], but whether disease burden influences the processing of reward is unknown. Accordingly, the objectives of the present study were (1) to examine the neural correlates of reward processing in patients with unipolar MDD and (2) to determine whether patients with a high past illness burden differ from patients with minimal past illness burden when processing reward. We therefore studied a group of patients with major depressive disorder and healthy controls and for further analysis subdivided the MDD group into those presenting for first treatment of an episode (FTE) of depression and those with multiple treated episodes (MTE) of depression.
Materials and methods
Participants and design
Patients were recruited from the Mood Disorders Clinic at St. Joseph’s Centre for Mountain Health Services in Hamilton. Subjects received a full explanation of the study protocol that was approved by the Research Ethics Board of St Joseph’s Healthcare Hamilton. Written informed consent was obtained. A psychiatrist confirmed a primary diagnosis of unipolar, nonpsychotic MDD according to DSM-IV criteria assessed during the SCID. A total of 29 patients were included in the study. Patients were further classified based on their illness duration. The FTE group (N = 14) consisted of patients presenting for the treatment of a current depressive episode with no prior treatment history of either pharmacotherapy or psychotherapy. The second group (MTE; N = 15) consisted of patients who had experienced at least three previous episodes of MDD and/or had an illness duration of at least 5 years, meaning that they received a formal diagnosis of MDD 5 years prior to participation in the study. Twenty-five control subjects were recruited from the community and were matched on age, sex and handedness for each patient group [healthy control for FTE (HCF) and healthy control for MTE (HCM)]. Healthy control subjects were free from medication and had no current symptoms or medical history of a mental health disorder. All participants were screened for exclusion criteria by a trained clinical research nurse. Exclusion criteria included history of head injury, unmanaged medical or neurological illness, alcohol or substance abuse, or history of electroconvulsive therapy or transcranial magnetic stimulation within the last 2 years. Depressive symptoms were assessed at the time of scanning using the Beck Depression Inventory (BDI [20]) and the Hamilton Rating Scale for Depression (HRSD [21]).
fMRI paradigm and procedure
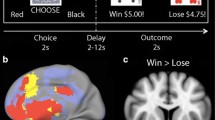
Our selection of an fMRI paradigm was guided by our interest in examining activation associated with both reward and punishment as well as the response to demands for reversal shifting. The task by O’Doherty et al. [16] was selected as it has previously identified the activation of the OFC during periods of monetary gains and losses in healthy individuals. Prior to the scan, subjects were given a brief orientation to the task. During the first part of the training, subjects were shown two unfamiliar fractal images (approximately 5 × 5 cm2), which appeared in random order on either side of an IBM ThinkPad screen. Subjects were asked to identify one of the two symbols consistently for five trials. Participants then received instructions for a “Money Game” (See Fig. 1). Subjects were told to select the image they felt would be the most rewarding choice over a number of trials, based on previous monetary outcomes.
Reward–punishment paradigm was based on O’Doherty et al. [16]. On each trial, the subject selects the fractal they feel is most rewarding. Once the most profitable fractal has been selected a number of times, the contingencies are reversed. The subject must detect the reversal and change their responses accordingly
The two fractals were predetermined to be either rewarding or punishing over the course of a number of trials (4–6 trials). The most rewarding fractal was associated with rewards or gains in the range of $80 to $250 (large gain), and punishment or loss values of $−60 to $−10 (small loss). The most punishing fractal was associated with large losses that ranged from $−250 to $−600, and small gains of $30 to $60. After making a selection, the dollar amount associated with the choice was presented on the screen along with a running total of the amount gained or lost from the start of the game. Unbeknownst to the subject, the gain: loss values for the reward and punishment fractals appeared in specific ratios (reward fractal—70:30; punishment fractal—40:60).
Subjects were told that after selecting the most profitable fractal, a number of times the conditions would switch (the original rewarding fractal now becoming the punishing fractal, and the original punishing fractal now becoming the rewarding fractal), and that they should adapt to this switch. The training phase continued until subjects were able to successfully complete two condition reversals. Prior to entering the scanner, the subjects were informed that the dollar amounts were fictional and that they would not receive any of money “earned” during the experiment. Instead, at the completion of the study, subjects received a $25 gift card to a local movie retail store.
Following training, each participant completed the task while undergoing an fMRI scan. The task was projected onto a mirrored visor located on top of the head coil within the scanner bore. Subjects made their responses by pressing one of the two buttons of a response box attached to their right hand. The presentation of stimuli and recording of responses were controlled by Eprime presentation software (http://www.pstnet.com).
During the fMRI portion of the experiment, the protocol was altered slightly. Initially, during the baseline portion of the experiment, subjects were asked to consistently identify the occurrence, left or right, of one of the two fractals. The fractals were the same ones used in the training phase, although subjects were now asked to identify the opposite fractal. Once the subject had correctly identified the specified fractal for 20 trials, the experiment progressed to the “Money Game”. Subjects received the following instructions: “You will now play the Money Game. Decide which fractal is more profitable, reverse your choice when needed.” After a selection, the dollar amounts associated with their choices and the running totals were shown on the screen, in the same position as the training phase, for 3 s. The screen was then cleared and a fixation point was shown for 4, 7, 10 or 13 s (jittered intertrial interval) prior to the next trial presentation. The “Money Game” continued for approximately 30 min in the scanner.
Image acquisition
The scanning session lasted approximately 1 hour during which subjects completed the reward processing task and a T1-weighted anatomical scan. Images were acquired using a GE 3T whole-body short bore scanner with eight parallel receiver channels (General Electric, Milwaukee, WI, USA). A three-dimensional volume SPGR pulse sequence with 124 contiguous slices (1.6 mm thick) was used to acquire anatomical images in the axial plane. Functional images were acquired with an optimized gradient-echo EPI sequence, and covering 32 axial slices (4 mm thick, no gap), beginning just below the most ventral part of the inferior temporal cortices (bilaterally) and encompassing the entire cerebrum (TR/TE = 3,000/35 ms, FOV = 24 cm, matrix = 64 × 64, flip angle 90°). Acquired images were transferred to a workstation, preprocessed and analyzed using BrainVoyager QX version 1.10.4 (Brain Innovation B.V., Maastricht, The Netherlands). The functional data sets were slice-time corrected, linear detrended, 3D-motion corrected and realigned, and normalized to Talairach space [22]. High-resolution T1-weighted three-dimensional (3D) anatomical MR data sets were transformed into Talairach space, used for co-registration and averaged to generate a composite image onto which functional activation results were projected.
Data analysis
An event-related deconvolution model for each participant was used to examine BOLD signal at every voxel. Using a random-effects multiple general linear model, the reward acquisition, punishment reversal, large gain, small gain, large loss and small loss conditions were set as the explanatory variables accounting for differences in blood oxygen level-dependent signals within and between groups. Reward acquisition included the contiguous series of trials in which both small punishers and larger rewards were delivered. The punishment reversal period was defined as the series of trials during which larger punishers and small rewards were delivered. To examine the response to changes in the magnitude of rewards, select trials in which large positive rewards were awarded were contrasted with trials in which small rewards were allocated. Finally, to examine responses to changes in the magnitude of punishers, trials in which large punishers were delivered were contrasted with trials associated with small punishers.
Imaging data were analyzed first at the level of the entire group of subjects with MDD (N = 29) compared against healthy controls (N = 25). From that analysis for each condition of interest, areas of significant activation were used to generate an activation map. This contrast specific activation map was then used to localize regions of interest in the subsequent analysis of the FTE versus healthy controls (HCF), MTE versus healthy controls (HCM), and FTE versus MTE comparisons. Contrasts were corrected for multiple comparisons using the false discovery rate methodology (threshold set to 0.05) [23], and the average statistical value for the resulting regions of interest is reported.
Results
Demographic, clinical and behavioral measures
There were no significant differences in age between the FTE group (26.4 ± 7.4) and their matched controls (HCF) (28.4 ± 7.9), or the MTE group (47.7 ± 9.4) and their matched controls (HCM) (46.3 ± 11.4). The MTE subjects included in the study had mean illness duration of 23.9 ± 12.7 years and had extensive documented past treatment histories prior to inclusion in this study. The FTE subjects included in the study identified the onset of depressive symptoms on an average of 8.3 ± 4.9 years prior to presentation, although none had ever sought treatment prior to participating in this study. FTE subjects had a significantly lower past burden of illness compared to MTE subjects [F(1,28) = 18.67, p < 0.001]. Despite the more extensive illness history, MTE subjects had less severe symptoms scores at the time of assessment with mean BDI score of 13.4 ± 5.7 and a mean HRDS score of 9.4 ± 4.8, compared to FTE subjects who had mean BDI score of 26.3 ± 12.7 [F(1,28) = 15.86, p < 0.001] and a mean HRDS score of 17.9 ± 7.0 [F(1,28) = 14.84, p < 0.001]. Eighty percent of MTE patients were treated with antidepressants (seven SSRIs, four SNRIs, two atypical antidepressants, one tetracyclic antidepressant, one MAOI). Of the patients on SSRIs, the majority were concurrently receiving medications that act on norepinephrine system and only one was prescribed SSRI in isolation. Three FTE patients had received minimal exposure to antidepressant treatment prior to scanning; all had less than 1 week’s lifetime exposure to psychotropic medication. Ten MTE patients had Hamilton Rating Scale for Depression [21] scores in the mild to moderate range, and five MTE patients were euthymic at the time of the scan. For ethical reasons, medication status was not an exclusion criteria and these participants were kept on their current medication regime during the study. See Table 1 for demographic information.
During task performance, the FTE patients had significantly faster response times than controls in reaction to both the reward acquisition phase (FTE mean = 915.9 ms, HCF mean = 1,053.5 ms; F = (1,26) = 4.63, p < 0.04]) and the punishment reversal phase (FTE mean = 940.4 ms, HCF mean = 1,050.8 ms; F(1,25) = 6.40, p < 0.02). There were no significant differences between these two groups on the number of successful switches in reward contingencies (FTE mean = 12.4, HCF mean = 12.6, [F(1,27) = 0.247, p < 0.62]). When examining MTE patients and their matched controls HCM, there was a significant difference in the latencies averaged across all trial types [F(1,27) = 5.41, p < 0.028]. MTE patients had a significantly delayed reaction time (mean = 1,219.7 ms) in comparison with matched controls (mean = 1,059.9 ms). However, there was no significant difference in reaction time for the reward acquisition phase between these two groups (MTE mean = 1,202.5 ms, HCM mean = 1,050.0 ms) or the punishment reversal (MTE mean = 1,230.0 ms, HCM mean = 1,078.1 ms) periods specifically. Additionally, there was a trend toward a fewer number of successful switches in reward contingency condition in the MTE group in comparison with controls (MTE mean = 10.7, HCM mean = 11.9; F(1,28) = 3.87, p < 0.06).
Imaging data
Reward versus punishment
Between-group activation patterns were determined for the contrast of reward acquisition versus punishment reversal. Reward acquisition was defined as the consistent selection of the correct rewarding fractal for 4–6 trials. The punishment reversal phase was defined as the trials when the subject selected the old rewarding fractal and continued to do so until the new rewarding fractal was acquired. This contrast allowed for the examination of all rewarding conditions in comparison with all punishing conditions.
In the first level of analysis, significant differences were observed between the larger MDD group and controls (full group) in a number of areas. Control subjects had increased activation in comparison with MDD patients bilaterally in the NAc, anterior cingulate cortex (ACC: BA 32) and also the right hippocampus (Fig. 2). Using an activation map containing these same regions, we then examined this contrast between controls and FTE and MTE patients, respectively. HCM control subjects had increased activation in comparison with MTE subjects bilaterally in the NAc, ACC (BA 25 & 32) and hippocampus. Additional significant findings for this contrast revealed differences in the ventral medial prefrontal cortex (VMPFC; BA 10) activation between MTE patients and HCM subjects, MTE patients compared to FTE patients and FTE patients compared to HCF. Thus, activation for this region showed a graded response across the groups, such that MTE > FTE > healthy controls. Finally, differential engagement of the ACC and superior, middle and dorsomedial frontal regions distinguished HCM from HCF participants. These findings suggest that the NAc and VMPFC findings that differentiated FTE from MTE patients were a function of disease-related rather than age-related processes.
Full group differences in the activation of the nucleus accumbens and anterior cingulate cortices during reward acquisition relative to punishment/contingency reversal. FDR-corrected statistical maps are superimposed on averaged anatomical group images. Images are presented according to radiological convention
Magnitude of reward or punishment
Between-group analyses evaluated the neural responses associated with variable magnitudes of rewarding or punishing stimuli. The effects of the magnitude of rewards were assessed by contrasting large rewards and small rewards. Analysis of the larger patient group revealed significantly increased activation bilaterally of the NAc and inferior frontal cortices and ACC (BA 47 on the right, and BA 32 on the left, respectively) in controls versus MDD patients. Examination of the response to the magnitude of rewards in the FTE and MTE patient groups revealed similar activation in these same areas. Areas of significant activation revealed in pairwise contrasts across the three groups in the large gain versus small gain comparison revealed changes the ACC and bilateral NAc. Here, controls (HCF and HCM, respectively) had greater activation than both FTE and MTE patients. In addition, FTE patients in turn had greater activation of these regions than MTE patients. The contrast between HCF and HCM did not reveal age-related changes in the ACC or NAc. As such, the pattern was considered an activation gradient across the study groups; healthy controls > FTE patients > MTE patients. Other areas of significant differences between groups are reported in Table 2.
Losses of large or small magnitude were contrasted to determine the effect of the magnitude of punishment. Patients had significantly less activation than controls to large penalties (large losses > small losses) in a number of regions. In particular, patients had less activation of the bilateral lateral orbital frontal regions (BA 13) and less activation than controls in the VMPFC (BA 10). The same contrasts were then employed to examine the effect of illness burden in depressed patients. Controls had increased activation in the VMPFC and lateral inferior frontal cortex in comparison with both FTE and MTE patient groups, although the comparison with MTE patients did not survive correction for multiple comparisons. Finally, FTE patients had increased neural responses in the VMPFC (BA 10) in comparison with MTE patients, although this comparison did not remain after correction for multiple comparisons. These results created a trend toward a graded response between the three groups such that healthy controls > FTE subjects > MTE subjects in BA 10 when responses to large and small losses were compared. Other areas of significant differences between groups are reported in Table 2.
Discussion
Our results suggest that patients with depression have functional abnormalities in brain regions involved with the processing of reward. In particular, patients had less activation than controls in the NAc, VMPFC and ACC under conditions of reward acquisition and with changes in the magnitude of gain (Fig. 2). Moreover, patients responded to losses by engaging the lateral OFC and VMPFC regions to a lesser extent than controls.
These findings were refined by further examination of the patients based on whether they had a minimal or extensive history of illness. It is important to note that treatment history for the MTE patients also includes past and current medication use. Recent evidence suggests that SSRIs can reduce striatal activation under reward conditions, whereas drugs that impact on the norepinephrine system accentuate this response [24]. While these effects may have been minimized in our MTE group by the concurrent administration of medications from these two drug classes, we cannot address this question under the current design. The current data therefore pertain to a “clinically” relevant sample of MDD patients with extensive illness histories. Our results point to several brain regions that showed a gradient of responses that was dependent on treatment history, including the OFC and NAc. Control subjects had the greatest degree of activation in these regions, with less activation in FTE patients and least by MTE patients.
Understanding the dysregulation in the reward network may provide insight into the neural underpinnings of anhedonia, a common but poorly studied feature of MDD. These results suggest that altered reward circuitry in MDD may initially involve diminished signaling of incentive salience by the NAc. Alterations in the NAc and OFC might then lead to the formation of fewer anticipatory reward-related associations. This reduction could promote symptoms of anhedonia, as fewer experiences would be tagged as rewarding. Changes in OFC function may also result in diminished capacity to gauge punishers and a generalized heightened sensitivity for negative affective-laden stimuli. Further, the outcome of reduced affective signaling and the generation of fewer reward-related associations may directly impact on VMPFC capacities to associate changes in reward contingencies with self-relevant information. Whether changes in VMPFC activity reflect a lessening of higher-order integrative processing or a self-protective psychological response, such changes could contribute further to symptoms of anhedonia. Below, we examine the group differences in the response to rewards and punishers and explore further the functional roles of key regions important in the neuropathology of MDD.
Responses to changes in reinforcement contingencies and punishers and the VMPFC
We observed group differences in the recruitment of the VMPFC when trials during which the subjects chose rewarding stimuli (reward acquisition) were contrasted with trials in which the response contingencies had reversed (punishment reversal). Here, recruitment of the VMPFC was greatest in MTE patients, less in FTE patients and the least in healthy controls. It is possible that these group differences are driven by either heightened engagement of the VMPFC under rewarding conditions or reduced engagement (or deactivation) of this region during contingency reversal periods and punishment. Consequently, to clarify the directionality of these VMPFC findings, we examined the activation of this region (within group) during both the reward acquisition and punishment reversal periods as they related to baseline rest period of the paradigm (Table 3; Fig. 3). Consistent with the literature, all three groups demonstrated activation within the VMPFC during reward acquisition [11, 13].
In contrast, during the punishment reversal phase, the VMPFC was activated by controls, undistinguished from baseline in FTE patients and deactivated by MTE patients. These results suggest that group effects in the reward acquisition/punishment reversal comparison discussed above were not so much a consequence of differences in the responses to the receipt of rewards but more so reflective of (1) the processes involved in signaling changes in reinforcement contingencies and (2) the response to loss. While not mutually exclusive, it may be that both factors contribute to the altered reward processing and anhedonia in MDD, and as such will be explored further below.
During the contingency reversal periods, the subject finds that the stimulus that has come to be associated with gain is now suddenly paired with loss and that the stimulus that was previously associated with loss is now more rewarding. Of note, therefore, are studies of reward processing that have suggested a role for the VMPFC in response to unexpected rewards [14] and in the contextual processing of reward [11, 13]. Current theories propose that this region is active in the assembly and integration of abstract representations of reward value [15]. Thus, the attenuated activation of this region in MDD during the contingency reversal period may signify diminished responses to novel rewards and to changes in reward contingencies.
Response to negative feedback is particularly accentuated in depressed patients when the feedback carries emotional significance [25]. Patients with depression can be distinguished from controls by a “double dissociation” of VMPFC responses to positive and negative affective stimuli [26]. Reduced VMPFC activation to sad stimuli in MDD may reflect a negative bias in visual emotion processing, corresponding to a heightened sensitivity for sad visual stimuli. Drevets et al. [27] have observed that patients with depression have enhanced sensitivity to negative feedback during the performance of a reversal learning task. Compared to controls, patients’ demands for reversal shifting were associated with attenuated activity in the dorsomedial and ventrolateral prefrontal cortices. Reduced engagement of prefrontal regions in depression may have signaled failures to suppress emotional responses and to modulate activation in limbic structures following negative feedback. Accordingly, in the current study, an attenuated VMPFC response to punishing stimuli in depressed patients may be the result of heightened sensitivity to the negative affective content of such stimuli and indicative of a failure to regulate neuronal responses to this feedback.
The VMPFC is also associated with the processing of experiential and emotionally self-relevant information. For example, an fMRI study by Schmitz and Johnson [28] presented participants with both positive and negative trait adjectives and found increased activation within a network that included the VMPFC when subjects matched descriptive words with self-referent statements. Areas of the medial PFC are implicated in processes that involve the maintenance of a representation of self in order to direct future behavioral responses to both reward and emotional contingencies [29]. Collectively, these studies suggest that this region is engaged when individuals judge outcomes according to experiential and emotionally laden self-referent information. Depression may therefore involve reduced capacity to associate changes in reward contingencies with self-relevant information. In particular, diminished responses to novel rewards and reduced self-referential processing may be core to the symptoms of anhedonia.
Punishment and the orbitofrontal cortices
Response to differences in the magnitude of loss was reduced in depressed patients compared with controls. Attenuated responses were apparent in the ventrolateral regions of the orbitofrontal cortices. Contrasts between the MDD subgroups and their controls, however, revealed only trends toward greater recruitment of this region by control subjects. Collectively, these findings are consistent with evidence that points to alterations of the OFC in MDD, including postmortem studies [30], structural imaging studies [31] and a functional imaging study that used a go/no-go task with emotional words as stimuli [32].
Heightened ventrolateral OFC activation in our control subjects is consistent with studies identifying this region as important in the processing of punishment [16, 33]. Similarly, localized lateral OFC findings led O’Doherty and colleagues to suggest medial–lateral OFC dissociation for rewards and punishers, although subsequent work [33, 34] noted activation in this region during both punishing and rewarding outcomes. In a study of reversal learning by O’Doherty et al. [33], activity in the lateral OFC predicted subsequent shifts in contingencies. These findings suggest that the more lateral regions may be important for the generation of stimulus reinforcement associations that represent anticipatory value [33, 35]. Accordingly, the ventrolateral findings in the present study may indicate that there are diminished capacities to gauge the magnitude of loss and develop expectations as to the probable outcomes of subsequent choices in patients with MDD.
The processing of the magnitude of rewards
Another cross-group graded pattern of activation was evident when we looked specifically at reward trials and examined the effects that the size of the reward had on brain activation. Contrasting trials with large gains to trials with small gains revealed activation of the NAc that was greatest for control subjects, attenuated for FTE patients, and least by patients with extensive past illness (Fig. 4). Group differences in NAc activation were also observed in the comparison of reward acquisition with punishment reversal trials. In this instance, controls had greater NAc activation than MTE patients.
The NAc is an integral part of the mesolimbic reward system and reward processing [13, 14, 36–38]. The NAc may be particularly involved in anticipating reward and responding to unexpected outcomes, but this hypothesis is controversial. Others have suggested that this area may be active in signaling incentive salience and important in processes that assign motivational value and amplify cue triggered “wanting” of rewards [39].
Few studies have explored NAc activity in MDD. One study using PET to examine striatal dopamine uptake found a decrease in NAc dopamine metabolism in a group with marked affective flattening [40]. The dysfunction in dopamine metabolism in this area could be reflective of the decreased volume seen in the NAc in MDD [41]. Recently, Knutson [7] used a monetary incentive delay task that required unmedicated MDD patients and matched healthy controls to respond to rapidly presented cues in anticipation of gains or losses. Unexpectedly, they found no group differences in NAc activation when subjects were anticipating gain; however, a trend was observed with reduced MPFC and NAc responses when depressed subjects were monitoring gain outcomes, consistent with findings of altered NAc activation in response to rewards in subjects with MDD. Healthy subjects may recruit this area to anticipate rewards more effectively than depressed subjects. Notably, the pattern of results suggest that early in the course of MDD, there may be abnormalities in the NAc function that are exacerbated with repeated episodes or chronic illness. Difficulties in linking reward information with outcomes in the NAc are consistent with reduced salience and attention to reward-learning stimuli and the clinical picture of anhedonia. For patients with more extensive illness histories, antidepressant medication may help to address mood symptoms, but may have less impact on the broader cognitive schemas involving incentive salience that become more entrenched with time [42].
Strengths and limitations
The strength of the current study is the relatively large number of subjects who completed the functional task (fifty-four in total). This allowed the patient group to be broken into subsets of patients while maintaining a reasonable number of patients in each subgroup. While intentionally varying on burden of illness, the sample was an otherwise well-characterized and homogenous group of nonpsychotic, unipolar outpatients who were free of major comorbidity.
The present study has several limitations. Notably, some patients were taking antidepressant medication, and it is possible that the observed differences in activation were influenced by treatment. In addition, four of the MTE patients were receiving antipsychotic medications, and as such, altered dopaminergic function in these patients may have impacted on the engagement of striatal regions. Additionally, because of the cross-sectional nature of the design, it is impossible to confirm whether the accrued burden of illness in the MTE group led to or resulted in part from the altered patterns of reward processing that were observed in this group compared to those with minimal illness burden. Longitudinal functional imaging studies that repeat the same task in patients across various points in illness history are lacking, likely because of the complexity involved in this approach. Additionally, although this study used matched control groups, there was a significant age difference between the MTE and FTE groups, and examining age effects in controls does not adequately disentangle burden from age. Future work will need to examine this question longitudinally. Further, the contingency reversal paradigm also has limitations. At the completion of the study, the compensation participants received for their time and effort was a gift card and was awarded irrespective of their performance on the task. Other studies have tied study compensation to performance, which may serve as a stronger incentive. Although prior studies have suggested that the NAc plays a role in the anticipation of rewards [43], the protocol employed in the current study was not designed to differentiate between reward anticipation and reward outcome processing. It will be important for future work to address the function of the NAc in reward anticipation in MDD.
Conclusions
Regional differences in reward processing were apparent between depressed patients and controls during the performance of a contingency reversal reward paradigm. The pattern of results suggests that the recruitment of the NAc in MDD is attenuated during the processing of reward, and as a consequence, both the signaling of incentive salience and the capacity to form reward-related associations of anticipatory value may be reduced. Across groups, the recruitment of areas such as the NAc, ACC and ventral prefrontal cortices during reward processing was greatest in controls, less by first treatment patients and least by multiple treatment patients—suggesting that these areas are sensitive to the impact of disease burden and repeated episodes of depression.
The responses to punishment and changes in reward contingencies in MDD are marked by reduced recruitment of the VMPFC and lateral OFC. Based on the extant literature, we have suggested that the VMPFC findings point to reduced capacities to regulate responses to negative affective stimuli and reduced abilities to associate self-relevant information with changes in reward contingencies. In the aggregate, these data highlight fundamental shifts in the processing emphasis of depressed patients during the experience of reinforcing or punishing events. The results also emphasize that the capacity of relevant neural systems to be activated in either a primary or compensatory manner may be importantly determined by the burden of illness that patients experience.
References
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn., text revision. American Psychiatric Association, Washington, DC
Wilcox HC, Anthony JC (2004) Child and adolescent clinical features as forerunners of adult-onset major depressive disorder: retrospective evidence from an epidemiological sample. J Affect Disord 82:9–20
Loas G (1996) Vulnerability to depression: a model centered on anhedonia. J Affect Disord 41:39–53
Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel ES (1997) Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J Neurol Neurosurg Psychiatry 63:74–82
Pizzagalli D, Iosifescu D, Hallett LA, Ratner KG, Fava M (2009) Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatric Res 43:76–87
Must A, Szabó Z, Bódi N, Szász A, Janka Z, Kéri S (2006) Sensitivity to reward and punishment and the prefrontal cortex in major depression. J Affect Disord 90:209–215
Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH (2008) Neural responses to monetary incentives in major depression. Biol Psychiatry 63:686–692
Harvey PO, Pruessner J, Czechowska Y, Lepage M (2007) Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry 12:767–775
Schaefer HS, Putnam KM, Benca RM, Davidson RJ (2006) Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol Psychiatry 60:974–986
Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG et al (2009) Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA 106(52):22445–22450
Elliott R, Newman JL, Longe OA, William Deakin JF (2004) Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. NeuroImage 21:984–990
Dolan RJ (2007) Keynote address: revaluing the orbital prefrontal cortex. Ann N Y Acad Sci 1121:1–9
Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001) Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport 12:3683–3687
Ramnani N, Elliott R, Athwal BS, Passingham RE (2004) Prediction error for free monetary reward in the human prefrontal cortex. NeuroImage 23:777–786
Elliott R, Dolan RJ, Frith CD (2000) Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex (New York, NY: 1991) 10:308–317
O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C (2001) Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 4:95–102
Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D (2003) A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage 18:263–272
Ullsperger M, von Cramon DY (2004) Neuroimaging of performance monitoring: error detection and beyond. Cortex 40:593–604
Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC (2008) Neural basis of abnormal response to negative feedback in unmedicated mood disorders. NeuroImage 42:1118–1126
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6:278–296
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme Medical Publishers, New York
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15:870–878
McCabe C, Mishor Z, Cowen PJ, Harmer CJ (2010) Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67:439–445
Murphy FC, Michael A, Robbins TW, Sahakian BJ (2003) Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med 33:455–467
Keedwell P, Andrew C, Williams S, Brammer M, Phillips M (2005) A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry 58:495–503
Drevets WC (2007) Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci 1121:499–527
Schmitz TW, Johnson SC (2006) Self-appraisal decisions evoke dissociated dorsal-ventral aMPFC networks. NeuroImage 30:1050–1058
Stuss DT, Levine B (2002) Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol 53:401–433
Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ (2007) GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32:471–482
Steffens DC, McQuoid DR, Welsh-Bohmer KA, Krishnan KR (2003) Left orbital frontal cortex volume and performance on the benton visual retention test in older depressives and controls. Neuropsychopharmacology 28:2179–2183
Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ (2002) The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry 59:597–604
O’Doherty J, Critchley H, Deichmann R, Dolan RJ (2003) Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci 23:7931–7939
Elliott R, Newman JL, Longe OA, Deakin JF (2003) Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci 23:303–307
Fellows LK (2004) The cognitive neuroscience of human decision making: a review and conceptual framework. Behav Cognit Neurosci Rev 3:159–172
Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS (2004) Choice selection and reward anticipation: an fMRI study. Neuropsychologia 42:1585–1597
Kuhnen CM, Knutson B (2005) The neural basis of financial risk taking. Neuron 47:763–770
Spicer J, Galvan A, Hare TA, Voss H, Glover G, Casey B (2007) Sensitivity of the nucleus accumbens to violations in expectation of reward. NeuroImage 34:455–461
Berridge K (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431
Bragulat V, Paillère-Martinot ML, Artiges E, Frouin V, Poline JB, Martinot JL (2007) Dopaminergic function in depressed patients with affective flattening or with impulsivity: [18F]fluoro-l-dopa positron emission tomography study with voxel-based analysis. Psychiatry Res 154:115–124
Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B (1999) Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatr Clin Neurosci 11:71–78
Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD (2008) Abnormal temporal difference reward-learning signals in major depression. Brain 131(8):2084–2093
Knutson B, Cooper JC (2005) Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol 18:411–417
Acknowledgments
This research was funded by a Young Investigator Award from the Ontario Mental Health Foundation awarded to Geoffrey Hall.
Conflict of interest
All authors have no financial interests or potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hall, G.B.C., Milne, A.M.B. & MacQueen, G.M. An fMRI study of reward circuitry in patients with minimal or extensive history of major depression. Eur Arch Psychiatry Clin Neurosci 264, 187–198 (2014). https://doi.org/10.1007/s00406-013-0437-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-013-0437-9