Abstract
Evidence accumulated in the last decade indicating that psychoactive substances negatively influence neurogenesis in the adult hippocampus has provided new insights into the neurobiology of drug addiction. Using a variety of experimental approaches and treatments, drugs such as psychomotor stimulants, opioids, alcohol and psychedelic compounds have been shown to alter one or several aspects of adult neurogenesis, including the rate of progenitor proliferation, the survival of newly generated cells, and the maturation and acquisition of cellular phenotype. This evidence, though critical from a neurotoxicological standpoint, cannot be linked unambiguously to the process of drug dependence at this stage of research. Drug addiction is a complex recurrent process involving the acquisition and maintenance of drug taking, followed by detoxification, abstinence and eventual relapse to drug seeking. The specific contribution of adult hippocampal neurogenesis to each of these processes is yet to be determined. This notwithstanding, the suggested role of the hippocampus in the storage and retrieval of declarative and contextual memories on the one hand, and in the regulation of mood and affect on the other, provides a fertile ground for further exploring the mutual relationships between postnatal hippocampal neurogenesis and addictive behaviours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug of abuse have the potential to produce addiction, a disorder of the brain typified by recurrent thoughts and actions aimed at compulsively taking the drug, loss of control over drug consumption and relapse to drug taking, even after detoxification and prolonged periods of abstinence. Drug addiction is a multifaceted process influenced by diverse contributing factors, including genetic predisposition and psychosocial influences [4, 30, 48, 55, 83]. Research over the last decades on the neuroscience of drug addiction has focused primarily on the mesolimbic and mesocortical dopamine pathways and their telencephalic projection targets as the principal neurobiological substrates mediating the effects of abused substances [40, 46, 69, 73, 92]. The implication of other neurotransmitter systems, such as excitatory amino acids and endogenous opioids, has become increasingly clear, as has the engagement of structures spanning beyond the ventral striatum, extended amygdala and frontocortical systems. The rapid strides made in recent years have contributed to identify wide-ranging drug-related plasticity correlates that occur in the nervous system of laboratory animals following acute and chronic drug exposure, both forced and self-initiated. Moreover, the advances of modern neuroimaging analysis have shed significant light over the brain abnormalities, cue-elicited activation patterns and neural pathways implicated in drug addiction in humans. The endeavours of basic and clinical researchers have stimulated theoretical approximations to the riddle of addiction. Current conceptualizations of drug addiction place the emphasis on different, yet complementary neurobiological aspects that underpin the acquisition, maintenance and extinction of drug taking behaviour, and ensuing relapse to drug seeking. In spite of significant progress, a complete picture of the biological basis of addiction has not emerged fully thus far.
The recent discovery that addictive drugs alter neurogenesis in the adult hippocampus has added a new dimension of complexity to the field of drug addiction research. Adult neurogenesis is believed to occur in at least two distinct regions of the brain; the subventricular zone (SVZ), a thin stripe of striatal tissue that borders the lateral ventricle, and the subgranular layer of the hippocampal dentate gyrus (DG) [3, 5]. These areas constitute throughout lifespan sources of newly generated cells, the majority of which become neurons that integrate into the preexisting circuitry. Post-embryonic neurogenesis has been demonstrated in a range of animal species, including amphibians, birds, reptiles and mammals, including non-human and human primates [6, 76]. Adult neurogenesis is well conserved throughout phylogeny and is therefore likely to be endowed with functional significance, the relevance of which is presently undetermined. Neurogenesis in the adult brain is a dynamic self-renewal process that involves proliferation, migration, differentiation and functional integration of new cells into either the olfactory bulb or the granular layer of the DG. Such phases of neurogenesis can be investigated separately by means of labelling and follow-up of proliferating cells through incorporation of labelled nucleotide precursors into DNA of dividing cells. A critical aspect to consider, which is often overlooked when studying the regulation of adult neurogenesis by addictive drugs and by other factors is the rate of survival of adult-generated cells. Indeed, only a subset of adult-born cells reaches maturity under normosensitive conditions. Numerous factors, both endogenous and exogenous, have been documented to regulate the different stages of adult neurogenesis. Humoral and neuroendocrine factors, neurotransmitters and growth factors, stress, physical activity, learning and memory, rearing conditions, environmental complexity and pharmacological agents, including abused substances, have all been implicated one way or the other in the regulation of neurogenesis in the adult CNS [14, 20, 42, 61]. This evidence critically suggests that adult neurogenesis is a process that is regulated tightly and that is exquisitely sensitive to variations in the neurochemical and external milieus. Moreover, such observations underscore the relevance of adult neurogenesis for providing plastic neuroadaptation to varying endogenous and exogenous conditions. The demonstrated fact that exposure to abused drugs affects negatively the self-renewal capacity of the adult hippocampus has yielded growing speculation on the potential role of adult neurogenesis in drug addiction. However, the evidence so far accrued in favour of such conjecture is correlational and, for the most part, circumstantial. In this review of recent findings, we consider evidence implicating hippocampal neurogenesis in the phenomenology of drug abuse and we propose a number of research strategies aimed at specifically addressing unresolved issues.
Drug studies and essential aspects on adult neurogenesis in the hippocampus
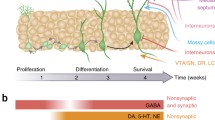
Putative progenitor cells in the DG are located in the subgranular zone, which is situated at the border between the granule layer of densely packed neurons and the hilus. Proliferating cells are usually labelled with exogenously-administered precursors such as [3H]thymidine and the analogue compound, 5′-bromo-2-deoxyuridine (BrdU). Other labelling procedures have become available, such as antibodies raised against proteins expressed selectively in the cell cycle. These include the histone-H3 phosphorylated on serine 10 (pHisH3), the proliferating cell nuclear antigen (PCNA) and the Ki-67 antigen. Once born, post-mitotic cells migrate a short distance into the granular layer of the DG, where they mature, differentiate, establish synaptic contact with neighbouring granule neurons and incoming axons from the perforant path, and extend axons into the CA3 hippocampal subfield (Fig. 1) [27, 41, 94]. The majority (ca. 80%) of proliferating cells becomes neurons, and a subset expresses markers of glial phenotype. The peripheral administration of labelling nucleotides such as BrdU allows the determination of proliferation rates shortly (ca. 2 h) after injection. Alternatively, injections of BrdU can be administered at a given time point allowing the animal to survive for varying periods of time (i.e., 2 weeks), in which case the determination of BrdU-positive cells in the DG yields a measure of the survival rate of the cells born at the time of BrdU labelling. Such protocol also allows the follow-up of newborn cells with the analysis of their maturation dynamics and the process of phenotype acquisition. Several other markers have been utilized to study these processes, including polysialylated neural cell adhesion molecule (PSA-NCAM), a marker of immature, differentiating cells, and doublecortin, a phosphoprotein associated with cytoskeletal microtubules, whose expression correlates with axonal outgrowth and synaptogenesis [62, 71]. When considering studies on the influence of drugs of abuse on hippocampal neurogenesis, or studies on the effects of other manipulations for the same matter, it is critical to establish adequate protocols for labelling dividing cells, such that proliferation and survival can be clearly differentiated, allowing therefore adequate interpretations. Further, the use of different markers of differentiation and maturation is advisable, as critical information can be obtained concerning the transition of the adult-generated cells through the different stages of morphological and functional development. Adult hippocampal neurogenesis includes a fine balance between proliferation and death. Such do-or-die scenario is further complicated by the complex dynamics of cell growth, differentiation and fate. In this context, when examining the influences of drugs on adult neurogenesis, it is essential that future work focuses on the process of neurogenesis as a whole, paying particular attention to the impact of the drug on the functions mediated by the newborn cells and by the DG by extension.
Diagram depicting different stages of development of newborn neurons in the adult hippocampus. Cells are born in the subgranular layer of the DG (1) and begin to migrate into the granular layer (2), where cells differentiate and expand short dendritic and axonal processes (3). Immature neurons establish synaptic contact with incoming axons from the perforant path (4) and fully mature into the granular layer, innervating the CA3 subfield of the hippocampus via the mossy fiber pathway (5)
Regulation of adult neurogenesis by psychoactive drugs
The effects of abused drugs on adult neurogenesis have been extensively documented. In this section, we will briefly review the evidence gathered so far on this topic; detailed reviews can be found elsewhere [15, 22, 23, 65, 70]. A wide variety of drug treatments and methods of drug administration have been used, combined with different approaches to study cytogenesis, survival, maturation and differentiation of neural progenitors in the DG. Most of the studies suggest a detrimental influence of drug exposure on dentate neurogenesis.
Alcohol has been one of the most studied drugs in recent years. Initial reports indicated that acute binge alcohol exposure decreased cell proliferation in the DG [66]. The same authors found that impaired proliferation after binge alcohol treatment was followed by a burst in proliferation one week after alcohol exposure, and by an increase in doublecortin protein expression in the DG two weeks after drug treatment [67]. These data suggested spontaneous compensation and reversibility of the effects of alcohol on cell proliferation after withdrawal. The effects reported with moderate doses of alcohol have been mixed. BrdU labelling was diminished in mice self-administering alcohol, an effect which was counteracted by exercise in a running wheel [16]. In this instance, however, alcohol and BrdU treatments were administered concurrently and therefore mixed effects on proliferation and survival complicate the interpretation of the findings. Moderate doses of alcohol administered to rats as liquid diet for a period of 6 weeks decreased the survival rate of newly generated granule cells, without altering the proliferation rate of neural progenitors [36]. These authors implicated oxidative stress as a factor contributing to cell death, as the antioxidant ebselen prevented alcohol-induced toxicity to hippocampal cells. Also, using a variety of BrdU labelling protocols, others have found that long-term (10 weeks) voluntary ethanol self-administration in a two-bottle free-choice model in mice enhanced cytogenesis in the DG, with new cells surviving and differentiating normally [1]. Thus, these data suggested that the effects of alcohol on adult dentate neurogenesis might be highly dependent on dosage, intake patterns and length of exposure.
In the hippocampus, nicotine enhances excitatory synaptic transmission [33] and modulates the release of several transmitters, including dopamine, serotonin and norepinephrine [79]. The effects of nicotine on adult neurogenesis in the DG have been investigated. Nicotine self-administration in rats reduced expression of PSA-NCAM and increased cell death in the DG [2], as did exogenously administered nicotine [78], thus suggesting a negative impact of nicotine on adult dentate neurogenesis.
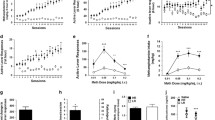
The psychomotor stimulants methamphetamine and cocaine have been shown to influence negatively neurogenesis in the DG. Single exposure to methamphetamine transiently diminished cell proliferation in the DG [84]. In turn, chronic cocaine exposure decreased cell proliferation but did not interfere with phenotype acquisition of the newborn cells [93]. These findings have been confirmed and extended. Both short- and long-term cocaine treatments reduced cytogenesis in the DG, but neither the survival nor the dendritic maturation of the newly generated cells became affected by the treatments (Fig. 2) [19]. The effects of cocaine exposure on adult dentate neurogenesis could result from dopamine D1 and/or D2 receptor activation. Studies in vitro indicated that dopamine D1-like receptor activation reduced the entry of progenitor cells from the G(1)- to the S-phase of the cell cycle [68], whereas D2-like activation inhibited neural stem cell proliferation [44]. However, studies in vivo should be performed to identify the neuropharmacological basis of the actions of cocaine on adult dentate neurogenesis.
Chronic cocaine exposure decreases cell proliferation in the rat DG. Photomicrographs show BrdU labelling (two upper panels) and doublecortin expression (lower panel) in the rat DG following 24-day cocaine (20 mg/kg per day) or saline treatments. Note that proliferating BrdU-positive cells (brown spots) are situated in the subgranular layer of the DG. Cocaine exposure reduced the number of proliferating cells by 35%. By contrast, cocaine treatment did not affect the survival of cells labelled with BrdU prior to the treatments nor did it influence their maturation dynamics. Note the extensive arborisation patterns of doublecortin-immunoreactive cells in the DG of rats treated with either cocaine or saline. See [19] for detailed description of the effects of cocaine on cell proliferation, and on survival and maturation of neural precursors in the DG
Further evidence on the effects of other addictive compounds on adult hippocampal neurogenesis has been presented. Studies with opiate drugs in rats indicated that morphine decreased cell proliferation and long-term cell survival following repeated, but not acute exposure [21]. In the same study, it was reported that heroin self-administration diminished cell proliferation. The molecular mechanisms mediating the deleterious effects of opiates on postnatal hippocampal neurogenesis, as with most abused drugs, have not been elucidated. The evidence currently available on the effects of cannabis (marijuana, cannabinoids) is controversial. On the one hand, it has been shown that the synthetic cannabinoid HU210 and the endocannabinoid AEA promoted proliferation, but not differentiation, of cultured embryonic hippocampal progenitors possibly through sequential activation of CB1 receptors, G(i/o) proteins, and ERK signalling [38]. However, another report indicated that delta(9)-tetrahydrocannabinol (THC), the main active ingredient in marijuana, was without effect on cell proliferation in the adult murine DG after acute, sequential or chronic escalating dosing [45]. Further work is needed to clarify these inconsistencies.
Hernández-Rabaza et al. (2006) studied the influence of binge exposure to 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”), a drug with stimulant and hallucinogenic properties, on progenitor proliferation and precursor survival and maturation in the rat DG. The results demonstrated that binge dosing with MDMA does not affect cytogenesis in the adult DG but compromises the survival of neural precursors producing a decrease in the range of 40% in cell survival measured two weeks after the MDMA challenges (Fig. 3). These authors showed that the surviving cells had normal patterns of arborisation and dendritic maturation [35]. The negative effects of MDMA on adult hippocampal neurogenesis might be associated with serotonin dysregulation, redox activation or endocrine factors.
Binge administration of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) impairs the survival of neural precursors in adult rat DG. BrdU pulses were given prior to binge MDMA treatment (eight injections of 5 mg/kg, one every 6 h). Photomicrographs demonstrate a decrease in BrdU labelling in the subgranular (SG, 1) and granular (G, 2) layers of the DG. The surviving cells, however, showed normal pattern of morphological maturation (see [35])
In summary, most addictive drugs, especially when administered chronically, alter proliferation or survival dynamics of neural progenitors, thus impairing in one way or the other the process of neuronal repopulation of the adult DG. The functional implications of such impairment are unknown to this date. Considerable efforts have been made to identify and characterize the functions of adult neurogenesis. With reference to the hippocampus, perhaps the most provoking evidence now links adult neurogenesis with learning and memory processes, on the one hand, and with the regulation of stress and affective behaviour, on the other hand (see this volume Czéh and Lucassen, Vollmayr et al.). In addition, adult neurogenesis has its most self-evident function as endogenous mechanism for self-renewal and repair. Both learning and affective dysregulation are driving forces that guide the acquisition and consolidation of addictive behaviours. Furthermore, relapse is the most formidable complication in drug addiction. One of the most salient features that occurs during withdrawal or abstinence from compulsive drug use is the ability of drug-associated environmental cues (e.g., drug-associated locations and paraphernalia) and unobservable internal states (e.g., disphoria and stress) to elicit renewed drug desire. Learning, memory and stress are now regarded as critical processes underlying compulsive drug use and relapse. Impaired adult neurogenesis in the DG could be involved in at least some of these processes, either by promoting aberrant associations between external cues, internal states or contexts with previously reinforced drug experiences, by producing dysphoria, stress or anxiety, or a by combination of these precipitants. What is the evidence implicating adult hippocampal neurogenesis in learning and memory processes and in the control of mood and affect?
Adult hippocampal neurogenesis, memory and addiction
When asked about the functions of adult hippocampal neurogenesis, one has several considerations to make. First, in an exercise of simplicity, we can refer to the functions of the DG. This approach is useful because the DG is, after all, the restricted site of neurogenesis in the adult hippocampus. However, by equating the functions of adult hippocampal neurogenesis to those of the DG we are ignoring the fact that new neurons are being continuously incorporated to the granule layer, and that this does not take place, or does at a minimum rate, in structures such as the neocortex or the basal ganglia. Yet, it might be useful to compare the neurological and cognitive deficits produced by ablation of hippocampal neurogenesis (e.g., with brain irradiation or antimitotic drugs) with those produced by damage to the entire DG (e.g., with intragyral injections of colchicine). Could the new cells, before becoming fully mature, play a different function to that accomplished by developmentally generated neurons? A possible role for the new neurons in the hippocampus could be to play a subset of the functions of the DG or to amplify them. We can also study the functions of the newborn cells. One interesting idea that has emerged is that the newborn cells could be undergoing plasticity changes before they reach full maturity, their functions thus being different to those of fully mature granule neurons. Some evidence indicated that adult-generated cells exhibit, unlike most developmentally generated neurons, lower thresholds for induction of LTP and lack of sensitivity to GABAergic inhibition [77, 80, 89]. Moreover, new neurons are able to produce action potentials [87]. Such a unique functional profile could make adult-born granule neurons particularly suited to undergo modification following subtle excitatory variations. A system that undergoes plastic changes is more capable of learning than a fixed, stable system. Such plasticity suggests a role for adult neurogenesis in learning and memory.
Perhaps one of the most important functions of the hippocampal formation is the storage, consolidation and retrieval of declarative, spatial and associative long-term memory [11, 81]. Consequently, the hypothesis that adult neurogenesis in the DG is involved in learning and memory has undergone intense scrutiny. Evidence in favour and against has been put forward. A variety of approaches have been used, including selective depletion of new neurons by means of irradiation, antimitotic drugs, and measurement of proliferation and survival rates of newborn neurons after learning of hippocampal dependent tasks [52]. An ample range of tasks has been assayed, including trace conditioning, spatial memory performance, contextual fear conditioning and working memory tasks. Taken together, the observations so far accrued do not allow establishing a definite link between adult hippocampal neurogenesis and learning. Further refinement of the behavioural assays is clearly required for the field to advance. It is worth pointing out that the hippocampus is now regarded as a modularly organized network of functionally segregated subfields. Behavioural studies involving discrete lesions of specific hippocampal sectors, including selective lesions of the DG, CA3 or CA1 subfields [28, 49–51] have clearly dissociated some of the mnemonic functions of each of these areas. Furthermore, current computational models [9, 74] support functional heterogeneity within the hippocampus. Thus, future studies on adult hippocampal neurogenesis should address how newborn neurons contribute to the behavioural functions specifically mediated by the DG, and not necessarily those that have been classically ascribed to the hippocampus and temporal lobe systems. Of the potential functions mediated by the DG, and especially those that depend on the integrity of its self-renewal capability, researchers should identify those that might be relevant to addiction. As reviewed below, the hippocampus plays a significant role in the acquisition, consolidation and expression of conditioned behaviours associated with drug experiences and in relapse to drug seeking. The field of stem cell research and neurogenesis would benefit from the use of fine grain behavioural analysis in animal models of drug addiction, such that the specific contribution of adult dentate neurogenesis to the distinct cognitive processes underlying addictive behaviour is clearly established.
Addiction and the recollection of drug memories
Current neurobiological perspectives on substance addiction emphasize the notion of drugs as reinforcers which impinge on learning and memory systems [13, 63, 72, 91], producing pathological activation and inactivation of reward pathways and usurpation of executive control over motivated actions. Distinct memory traces are distributed in the brain by means of anatomically segregated, but partially overlapping, mnemonic systems. A tripartite division was postulated which included the hippocampal formation, the amygdala and the dorsal striatum [57]. Within this account, the hippocampus is held to be responsible for the acquisition and retrieval of declarative memory and stimulus-stimulus associations. The amygdala system is believed to mediate Pavlovian associations between stimuli and contingencies, both reinforcing and aversive. Last, the dorsal striatum mediates implicit, dopamine-modulated habit-based learning. The ventral striatum and the mesolimbic dopamine pathways are situated at the crossroad of such memory systems, thus interfacing memory with motivation and drive. Memories of addictive experiences in the form of discrete cues and episodes could be stored in the distinct learning and memory modules. With repeated drug use, the experience-dependent recruitment of these pathways could guide the consolidation and gradual automation of drug seeking actions, and their association and conditioned reinstatement by exteroceptive cues, such as discrete and contextual stimuli, by interoceptive cues, such as stress and affective dysregulation, and by drug re-exposure.
Considerable converge has been demonstrated between the molecular events that lead to drug-induced long-term neuronal adaptation with those mediating learning-dependent neuronal plasticity. Both memory-associated processes and drug-induced neuroplasticity implicate the same signal transduction pathways, and correlate with identified morphological adaptations in neurons and with the induction of LTP [63, 64]. With specific reference to the hippocampus, inhibition of calcium/calmodulin-dependent protein kinase II (CaMKII), a protein that is essential for learning and memory processes, was shown to attenuate morphine tolerance and withdrawal syndrome and blocked morphine-induced conditioned place preference (CPP) [25, 53]. Interestingly, Fan and coworkers [25] found these effects following injections of CaMKII inhibitors into the DG. Moreover, the morphine-induced CPP correlated with enhanced expression of the NR2B subunit of the NMDA receptor in the hippocampus and nucleus accumbens [54]. LTP induction, a presumptive cellular mechanism for learning and memory, is also implicated in the effects of addictive drugs. Incremented LTP has been found in the CA1 hippocampal subfield in rats receiving cocaine challenges [85] and in rats trained in cocaine self-administration, even long after withdrawal [86]. These data suggest that there is substantial commonality between the neural adaptations induced by drugs of abuse in the hippocampus and those implicated in hippocampal-dependent learning.
Evidence supporting a general role for the hippocampus in drug abuse has come from different sources. Fuchs and colleagues reported that functional inactivation of the dorsal hippocampus with tetrodotoxin abolished context-induced reinstatement of drug seeking in rats previously trained in cocaine self-administration [26]. This evidence suggests that activation of hippocampal networks is essential to reinstate drug seeking by a context previously paired with drug experiences. Previously, Vorel and collaborators had shown that stimulation of the hippocampus at theta frequency caused reinstatement of drug taking behaviour in rats that had learned to lever-press for cocaine reinforcement and had undergone extinction [88]. The authors indicated that hippocampal stimulation might have produced a “read-out” of an encoded association between the context and the cocaine experience. Other pieces of evidence point in the same direction. Conditioned reinstatement of alcohol seeking behaviour by a contextual stimulus correlated with the induction of c-Fos, a protein whose expression is thought to reflect neuronal activation, in the prefrontal cortex, nucleus accumbens and hippocampus [18]. Furthermore, inhibition of GABAA receptors in the hippocampus dose-dependently reduced alcohol-maintained responding in an operant task [39], suggesting that the hippocampus plays a role in active alcohol seeking behaviours. As well as having a potential role in the acquisition of drug taking and in relapse to drug seeking, the hippocampus is involved in the rewarding effects of abused drugs according to data obtained in place preference paradigms. For example, the acquisition and expression of cocaine-induced CPP was blocked by lesions [58] or by functional inactivation [59] of the dorsal hippocampus. In addition, inhibition of protein synthesis within the hippocampus disrupted the consolidation of an established morphine-induced-CPP [60]. These data suggest that recruitment of hippocampal activity is necessary for the acquisition, consolidation and expression of drug-related memories.
Data in humans is another source of compelling evidence implicating the hippocampus in addictive behaviour. Imaging evidence is consistent with animal data in suggesting that the hippocampus might mediate in part the rewarding effects of abused substances. Morphine exposure in opioid-naïve volunteers elicited mild euphoria and positive functional magnetic resonance imaging (fMRI) signals in reward-related brain regions including nucleus accumbens, extended amygdala, orbitofrontal cortex and hippocampus [8]. Rush ratings induced by cocaine infusions in cocaine-dependent individuals correlated with increased fMRI signals in the striatum, prefrontal cortex and hippocampus [10]. Furthermore, human data suggest that the hippocampus might be involved in drug craving. Drug craving evoked by autobiographical scripts in opiate-dependent abstinent subjects [17] or in crack cocaine dependent individuals [43] was associated with enhanced positron emission tomography (PET) signal in the hippocampus and frontocortical regions. In addition, alcohol-associated pictures presented to abstinent alcoholics produced bilateral activation of the hippocampus, measured by fMRI [34].
The evidence reviewed above strongly suggests a role for the hippocampus in the learning and memory processes underlying addiction, including the acquisition, consolidation and recollection of drug memories. Moreover, the hippocampus appears to be one of the neural substrates for drug-induced reward and, especially, for conditioned reinstatement, craving and relapse. If and how adult hippocampal neurogenesis contributes to these processes remains undetermined. Specific experiments have not been performed involving manipulations of adult hippocampal neurogenesis to examine systematically their effects on addictive behaviours. As a result, many critical questions are yet unresolved. Does impaired adult neurogenesis modify the sensitivity to drug-induced reward? Does it disturb inhibitory control over drug seeking? Does it increase the vulnerability to relapse? Researchers working in the field of adult neurogenesis are urged to address these critical questions.
Adult neurogenesis and the regulation of mood, affect and relapse to drug-seeking
Another possible role for adult hippocampal neurogenesis, with similar potential to influence the expression of addictive behaviour, is the regulation of mood and affect. Drugs of abuse elicit intense internal affective states which might become associated with discrete external conditioned stimuli and, more generally, with configural contextual cues. Discontinuation and withdrawal from the drug is associated with mood dysregulation, anxiety, stress and self-reports of depression. Moreover, stress and depression are associated with increased vulnerability to addiction and enhanced craving and relapse in drug addicts [7, 29, 47, 55, 82, 90].
One of the most exciting recent developments in the neuroscience of affective disorders ties in mood regulation with adult neurogenesis in the hippocampus (see Czéh and Lucassen, Vollmayr et al. this issue). There appears to be a negative relationship between stress and depression and adult dentate neurogenesis. Stress-induced decreases in cell proliferation in the DG have been attributed to activation of the hypothalamic-pituitary-adrenal (HPA) axis [12, 31, 32]. It is noteworthy that most addictive drugs exert similar effects on the HPA system. By contrast, chronic treatment with antidepressants increases neurogenesis in the adult hippocampus. The serotonin uptake inhibitor fluoxetine increased BrdU incorporation and Ki-67 immunoreactivity in the DG [24, 37, 56]. Similarly, chronic exposure to imipramine incremented cell proliferation in the mouse DG [75].
Affective dysregulation and stress have an established role in maintaining ongoing drug use and inducing relapse to drug seeking [82]. Relapse is a process of associative learning, whereby stimuli or contexts previously paired with drug experiences acquire incentive-motivational properties, evoke memories of past drug-induced euphoria and signal drug availability. Such conditioned stimuli are potent triggers of relapse to drug seeking in animals that have extinguished responding. In addition to conditioned cues, non-contingent drug presentations and, especially, stress, can stimulate resumption of drug seeking. Activation of the HPA axis is involved in most aspects of drug self-administration. For example, increased levels of circulating corticosterone enhance sensitivity to cocaine reinforcement and, likewise, corticotropin-releasing hormone plays a part in the maintenance of cocaine self-administration [29]. In addition, both corticosterone and corticotropin-releasing hormone are involved in stress- and cue-induced reinstatement of extinguished cocaine seeking behaviour. There exists considerable overlap between the neural substrates evoking drug desire by conditioned cues and stress. Exposure to stressors or cocaine-associated cues and episodic memories could stimulate the stress circuits, thus producing craving and promoting relapse. It has been postulated that recurrent activation of such stress pathways could be one of the keys elements underlying the negative motivational state that drives addiction [47, 90]. In the light of the documented relationship between stress hormones, depression and hippocampal neurogenesis, and between drugs of abuse and neurogenesis, it is tempting to speculate that abnormal activation of hippocampal circuits subsequent to impaired neurogenesis could induce a psychopathological state characterized by affective dysregulation and increased vulnerability to relapse. Clearly, further experiments using animal models of addiction are needed to test this hypothesis.
Conclusions and future directions
The finding that most addictive drugs impair neurogenesis in the adult hippocampus has opened new avenues for exploring the neurobiological basis of drug addiction. However, major gaps remain in our understanding of the relationship between drug addiction and the effects that psychoactive drugs produce on adult-generated granule neurons. The research carried out so far has been for the most part descriptive. The studies reviewed in the foregoing sections have been critical in determining the influence of drug exposure on proliferation, survival, maturation and differentiation of newborn granule cells. However, this research has done little more than scratching the surface of the baffling problem of addiction. Neuroscientists have exquisitely delineated over the last two decades the neurocircuitry components and signalling mechanisms that mediate conditioned drug seeking, craving and relapse in experimental animals. Learning theorists have described with finesse a wealth of key mnemonic and motivational processes implicated in drug self-administration. Similarly, significant advances have been made in characterizing the cognitive abnormalities exhibited by drug-dependent individuals. Future research on adult neurogenesis would greatly benefit from this ferment produced by years of endeavours investigating the neuroscience of drug addiction. Therefore, toxicological experiments should give way to experiments examining the influence of adult-generated cells in processes such as drug discrimination, drug sensitivity, and reinstatement of drug seeking by, for example, contextual cues and stress.
The evidence implicating the hippocampus in addictive behaviours is substantial. The neuronal pathways and the intracellular events that mediate memory consolidation converge with those mediating drug-induced neuroplasticity. Hippocampal neurogenesis might be essential for efficient cognitive processing by the DG and by the hippocampal formation by extension. One way by which impaired neurogenesis could contribute to addiction would be by disrupting associative learning, by promoting the generalization of associations between drug experiences and stimuli, both internal and external, and by increasing susceptibility to relapse. Abused drugs also activate central stress circuits. The evidence reviewed supports a role for adult neurogenesis in stress, depression and affective disorder, which are major driving forces in addiction. Abnormal neurogenesis in the adult hippocampus could bring about affective and motivational instability and sensitize neural pathways to drug-induced reward. These hypotheses are amenable to experimental examination. Further investigations along these pathways could significantly increase our understanding of the processes involved in drug dependence and addiction.
References
Aberg E, Hofstetter CP, Olson L, Brene S (2005) Moderate ethanol consumption increases hippocampal cell proliferation and neurogenesis in the adult mouse. Int J Neuropsychopharmacol 8:557–567
Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le MM, Piazza PV (2002) Nicotine self-administration impairs hippocampal plasticity. J Neurosci 22:3656–3662
Abrous DN, Koehl M, Le MM (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569
Altman J, Everitt BJ, Glautier S, Markou A, Nutt D, Oretti R, Phillips GD, Robbins TW (1996) The biological, social and clinical bases of drug addiction: commentary and debate. Psychopharmacology (Berl) 125:285–345
Alvarez-Buylla A, Lim DA (2004) For the long run: maintaining germinal niches in the adult brain. Neuron 41:683–686
Alvarez-Buylla A, Lois C (1995) Neuronal stem cells in the brain of adult vertebrates. Stem Cells 13:263–272
Balfour DJ, Ridley DL (2000) The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav 66:79–85
Becerra L, Harter K, Gonzalez RG, Borsook D (2006) Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg 103:208–16, table
Becker S (2005) A computational principle for hippocampal learning and neurogenesis. Hippocampus 15:722–738
Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE (1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611
Burgess N, Maguire EA, O’Keefe J (2002) The human hippocampus and spatial and episodic memory. Neuron 35:625–641
Cameron HA, McKay RD (1999) Restoring production of hippocampal neurons in old age. Nat Neurosci 2:894–897
Canales JJ (2005) Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem 83:93–103
Christie BR, Cameron HA (2006) Neurogenesis in the adult hippocampus. Hippocampus 16:199–207
Crews FT, Nixon K (2003) Alcohol, neural stem cells, and adult neurogenesis. Alcohol Res Health 27:197–204
Crews FT, Nixon K, Wilkie ME (2004) Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol 33:63–71
Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ (2003) Functional connectivity analysis of the neural circuits of opiate craving: “more” rather than “different”? Neuroimage 20:1964–1970
Dayas CV, Liu X, Simms JA, Weiss F (2006) Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry
Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, Canales JJ (2006) Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci 24:586–594
Duman RS, Malberg J, Nakagawa S (2001) Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther 299:401–407
Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ (2000) Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA 97:7579–7584
Eisch AJ, Harburg GC (2006) Opiates, psychostimulants, and adult hippocampal neurogenesis: insights for addiction and stem cell biology. Hippocampus 16:271–286
Eisch AJ, Mandyam CD (2004) Drug dependence and addiction, II: adult neurogenesis and drug abuse. Am J Psychiatry 161:426
Encinas JM, Vaahtokari A, Enikolopov G (2006) Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA 103:8233–8238
Fan GH, Wang LZ, Qiu HC, Ma L, Pei G (1999) Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol 56:39–45
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309
Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J (1998) Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol 36:249–266
Gilbert PE, Kesner RP, Lee I (2001) Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus 11:626–636
Goeders NE (2002) Stress and cocaine addiction. J Pharmacol Exp Ther 301:785–789
Goldman D, Oroszi G, Ducci F (2005) The genetics of addictions: uncovering the genes. Nat Rev Genet 6:521–532
Gould E, Tanapat P (1999) Stress and hippocampal neurogenesis. Biol Psychiatry 46:1472–1479
Gould E, Woolley CS, McEwen BS (1991) Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. effects of glucocorticoids on cell death. J Comp Neurol 313:479–485
Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA (1996) Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 383:713–716
Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A (2006) Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res 30:1349–1354
Hernandez-Rabaza V, Dominguez-Escriba L, Barcia JA, Rosel JF, Romero FJ, Garcia-Verdugo JM, Canales JJ (2006) Binge administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) impairs the survival of neural precursors in adult rat dentate gyrus. Neuropharmacology 51:967–973
Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM (2003) Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA 100:7919–7924
Huang GJ, Herbert J (2006) Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry 59:619–624
Jiang W, Zhang Y, Xiao L, Van CJ, Ji SP, Bai G, Zhang X (2005) Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest 115:3104–3116
June HL, Harvey SC, Foster KL, McKay PF, Cummings R, Garcia M, Mason D, Grey C, McCane S, Williams LS, Johnson TB, He X, Rock S, Cook JM (2001) GABA(A) receptors containing (alpha)5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: an extended ethanol reward circuitry. J Neurosci 21:2166–2177
Kelley AE (2004) Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44:161–179
Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452
Kempermann G, Wiskott L, Gage FH (2004) Functional significance of adult neurogenesis. Curr Opin Neurobiol 14:186–191
Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP (2001) Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58:334–341
Kippin TE, Kapur S, van der KD (2005) Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci 25:5815–5823
Kochman LJ, dos Santos AA, Fornal CA, Jacobs BL (2006) Despite strong behavioral disruption, Delta9-tetrahydrocannabinol does not affect cell proliferation in the adult mouse dentate gyrus. Brain Res 1113:86–93
Koob GF (1992) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13:177–184
Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP (2004) Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27:739–749
Kreek MJ, Nielsen DA, LaForge KS (2004) Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular Med 5:85–108
Lee I, Hunsaker MR, Kesner RP (2005) The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci 119:145–153
Lee I, Kesner RP (2003) Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behav Neurosci 117:1044–1053
Lee I, Kesner RP (2004) Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus 14:66–76
Leuner B, Gould E, Shors TJ (2006) Is there a link between adult neurogenesis and learning? Hippocampus 16:216–224
Lu L, Zeng S, Liu D, Ceng X (2000) Inhibition of the amygdala and hippocampal calcium/calmodulin-dependent protein kinase II attenuates the dependence and relapse to morphine differently in rats. Neurosci Lett 291:191–195
Ma YY, Guo CY, Yu P, Lee DY, Han JS, Cui CL (2006) The role of NR2B containing NMDA receptor in place preference conditioned with morphine and natural reinforcers in rats. Exp Neurol 200:343–355
Majewska MD (1996) Cocaine addiction as a neurological disorder: implications for treatment. NIDA Res Monogr 163:1–26
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110
McDonald RJ, White NM (1993) A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci 107:3–22
Meyers RA, Zavala AR, Neisewander JL (2003) Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport 14:2127–2131
Meyers RA, Zavala AR, Speer CM, Neisewander JL (2006) Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120:401–412
Milekic MH, Brown SD, Castellini C, Alberini CM (2006) Persistent disruption of an established morphine conditioned place preference. J Neurosci 26:3010–3020
Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250
Nacher J, Crespo C, McEwen BS (2001) Doublecortin expression in the adult rat telencephalon. Eur J Neurosci 14:629–644
Nestler EJ (2002) Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem 78:637–647
Nestler EJ (2001) Neurobiology. Total recall-the memory of addiction. Science 292:2266–2267
Nixon K (2006) Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus 16:287–295
Nixon K, Crews FT (2002) Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem 83:1087–1093
Nixon K, Crews FT (2004) Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci 24:9714–9722
Ohtani N, Goto T, Waeber C, Bhide PG (2003) Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci 23:2840–2850
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216
Powrozek TA, Sari Y, Singh RP, Zhou FC (2004) Neurotransmitters and substances of abuse: effects on adult neurogenesis. Curr Neurovasc Res 1:251–260
Rao MS, Shetty AK (2004) Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19:234–246
Robbins TW, Everitt BJ (2002) Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem 78:625–636
Robinson TE, Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95(Suppl 2):S91–S117
Rolls ET, Kesner RP (2006) A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol 79:1–48
Sairanen M, Lucas G, Ernfors P, Castren M, Castren E (2005) Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25:1089–1094
Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia VJ, Berger MS, varez-Buylla A (2004) Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427:740–744
Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187
Shingo AS, Kito S (2005) Effects of nicotine on neurogenesis and plasticity of hippocampal neurons. J Neural Transm 112:1475–1478
Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, Sershen H, Lajtha A (2004) Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res 29:1779–1792
Snyder JS, Kee N, Wojtowicz JM (2001) Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol 85:2423–2431
Squire LR, Stark CE, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27:279–306
Stewart J (2003) Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am J Addict 12:1–17
Stolerman I (1992) Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci 13:170–176
Teuchert-Noodt G, Dawirs RR, Hildebrandt K (2000) Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm 107:133–143
Thompson AM, Gosnell BA, Wagner JJ (2002) Enhancement of long-term potentiation in the rat hippocampus following cocaine exposure. Neuropharmacology 42:1039–1042
Thompson AM, Swant J, Gosnell BA, Wagner JJ (2004) Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience 127:177–185
van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034
Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL (2001) Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292:1175–1178
Wang S, Scott BW, Wojtowicz JM (2000) Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol 42:248–257
Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR (2001) Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci 937:1–26
White NM (1996) Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction 91:921–949
Wise RA (2005) Forebrain substrates of reward and motivation. J Comp Neurol 493:115–121
Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T (2004) Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann NY Acad Sci 1025:351–362
Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26:3–11
Acknowledgements
This work was supported by grants from the Generalitat Valenciana (Conselleria de Sanidad and Conselleria de Empresa, Universidad y Ciencia), Fundación Bancaja and Ministerio de Educacion y Ciencia (Plan Nacional de Biomedicina).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canales, J.J. Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosc 257, 261–270 (2007). https://doi.org/10.1007/s00406-007-0730-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-007-0730-6