Abstract
Objective
The ADHEAR system (MED-EL, Innsbruck, Austria) is a new adhesive bone conduction hearing aid. This study evaluates the audiological benefit and subjective satisfaction as well as the manageability in everyday life in children with unilateral conductive hearing loss.
Methods
Ten children with unilateral hearing loss of different origin were included in the study. The audiological assessment included sound field audiometry and speech intelligibility in quiet and in noise, which was tested unaided and after 4 weeks of wearing the hearing system. Subjective benefit and satisfaction with the system was assessed using the SSQ for parents. With a second system-specific questionnaire, suitability for everyday use and quality of life were queried.
Results
With ADHEAR, speech perception in quiet improved by 44%. The word recognition score in noise improved from 11.7% in the unaided situation to 46.7% with the ADHEAR system. The SSQ for parents demonstrates a subjective benefit and satisfaction with the system.
Conclusion
ADHEAR is an effective treatment option for children with unilateral conductive hearing loss. Especially children who are not eligible for semi-implantable hearing systems or do not accept hearing devices on a softband can benefit from this device.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with single-sided conductive hearing loss have various technical treatment options to achieve a sufficient hearing sensation. These include percutaneous conduction devices (PBCD) such as BAHA® (Cochlear Corp., Sydney, Australia) [1] or semi-implantable hearing systems such as Bonebridge or Vibrant Soundbridge (MED-EL, Innsbruck, Austria). However, surgical intervention is required to use these systems. Furthermore, semi-implantable hearing systems are also subject to admission restrictions regarding the age of the children at implantation, so they cannot be used in all patients.
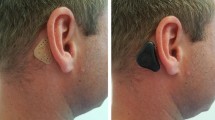
For patients who do not want to undergo surgery or do have a high anesthesia risk due to secondary illnesses, these systems cannot be applied. Various non-implantable bone-conduction hearing aids which are attached to a softband or spectacles are available for these patients [2, 3]. A disadvantage of these treatment options is their visibility, which can lead to perceived or experienced stigmatization in children, potentially leading to poor acceptance of the hearing aids. Headband systems also require pressure to transmit the sound energy to the skull which can be another reason for lower acceptance of these hearing systems. The ADHEAR-system (MED-EL, Innsbruck, Austria) is a new non-implantable bone conduction hearing system, consisting of two components, the audio processor (AP), which converts the acoustic signals into mechanical vibrations, and an adhesive adapter (AA), which is placed retroauricularly on the hairless skin. The AP is clicked onto the AA using a snap connector. In contrast to conventional systems, the sound is transmitted to the ipsilateral cochlea without the application of pressure. It has already been shown that this pressure-free bone conduction hearing aid have advantages in terms of acceptance and wearing time compared to conventional devices in adults [4].
There are numerous further publications on the performance of the ADHEAR system in adults, but very few on the performance of this device in children. All these examined a heterogeneous group of patients regarding the cause of hearing loss, while we focused our work on cases with unilateral conductive hearing loss (CHL). Thus, this study intends to evaluate the audiological benefit, the applicability and the subjective satisfaction with the ADHEAR hearing system in children with unilateral CHL. Compared to the existing studies in children, we used an extended audiological test battery including the adaptive speech test.
Materials and methods
Subjects and materials
Data was collected in the Department of Phoniatrics and Pediatric Audiology of the University Hospital Frankfurt between October 2017 and February 2019. Parents of all children declared informed consent on the day of enrollment. Children aged 4 to 18 years with unilateral conductive hearing loss were eligible for the study. Bone conduction thresholds (BC) had to be equal or better than 25 dB HL at the frequencies 0.5, 1, 2 and 4 kHz. Inclusion criteria included sufficient knowledge of the German language to be able to follow the test instructions and to complete the speech testing. Also ability to use the device had to be ensured. Combined or retrocochlear hearing loss and skin diseases that impaired the attachment of the adhesive adapter were exclusion criteria for participation in the study.
Ten children aged 4.0–16.7 years (average age 7 years, three female and seven male) were included in the study. In eight children the right ear was affected by the conductive hearing loss, in two children the left ear. After user training, all patients were given the device for daily use. The follow-up was scheduled after a 4 week period.
The study was approved by the ethics committee of the medical faculty (EC code 92/17).
Audiological Assessments
Audiometric tests were carried out in a soundproofed cabin with standardized sound signals. The audiometric instruments used were calibrated and certified as correct by the responsible technical supervisory authority. All participants were evaluated unaided at the day of study inclusion. The second testing was carried out in the aided situation after 4 weeks of wearing the device.
Device fitting
On the day of enrolment, the children and their parents received training in the use of the ADHEAR system by the medical investigator. This included the correct attachment of the adhesive adapter and the speech processor, as well as the correct usage of the device. The participants and their parents were advised to operate the device in the default mode. The volume could be individually adjusted to the most comfortable level by each child. This setting was then used for the audiological testing after 4 weeks.
Pure tone audiometry
On the day of enrollment, the hearing thresholds for air and bone conduction were tested separately for each ear. Insert earphones or headphones were used to examine the air conduction threshold, and a calibrated BC vibrator was used to determine the bone conduction threshold.
Sound Field audiometry
A Mainzer Kindertisch was used to perform sound-field tests. The sound and speech signals were presented in S0 or S0N0 configuration. The participants were placed at a distance of one meter from the loudspeaker. Warble tones were presented to determine hearing thresholds in the sound-field. Word recognition scores (WRS) were used to assess speech intelligibility by presenting monosyllables at 65 dB SPL signal level (Freiburger Einsilber or Mainzer II, dependent on the age of the children) [5]. Speech recognition thresholds (SRT) in quiet and SRT in noise (signal to noise ratio—SNR) were also measured. Therefore, the sound level was determined at which 50% of the presented test words in a list were correctly understood (SRT50). Polysyllabic numbers or words (Freiburger Zahlen/Mainzer II) were used to determine SRT50 in quiet. The SRT50 in noise was measured using Mainzer II. Older children of the collective performed the Oldenburger sentence test for children (OLKISA) with a fixed background noise level at 65 dB SPL and adaptive speech levels [6]. All tests described were taken unaided on the day of enrollment and aided after 4 weeks of wearing the device.
Patient satisfaction
Subjective restrictions on hearing were recorded with the “Speech, Spatial, and Qualities of Hearing” (SSQ) test for parents, in which parents assess children's hearing in different aspects of daily life [7]. Each answer is rated on a scale from 0 to 10. The SSQ for parents was administered at enrolment in the study to evaluate the hearing impairment in the unaided situation. After wearing the hearing system for 4 weeks, parents were asked to complete the SSQ again to assess the aided situation. At this point in time, the parents were also given a system-specific questionnaire to evaluate the manageability of the system in everyday life.
Skin safety
Parents were instructed to examine the skin every time they changed the adhesive adapter and to report any abnormalities immediately. After a period of 4 weeks, an examination of the skin in the area of the mastoid bone was carried out by the medical investigator to detect and document skin irritation caused by the adhesive adapter.
Statistics
To compare groups, the values were first checked for normal distribution using the Kolmogorov–Smirnov normality test and the D’Agostino-Pearson normality test. If these tests showed a positive result, the student’s t test or the Wilcoxon test was applied for further analysis. The level of statistical significance was determined to a p value < 0.05. Statistical calculations were carried out with Graphpad Prism 6.0. This program was also used to create the graphs. Tables were created with Microsoft Excel.
Results
Audiometric results
Mean sound-field hearing thresholds in the unaided situation and the aided condition after 4 weeks of wearing the device are shown in Fig. 1. The functional hearing gain was calculated by comparing the difference between the average hearing thresholds in the frequencies 0.5, 1, 2 and 4 kHz in the unaided situation and with the hearing system. It showed a significant improvement by 19.6 HL (n = 10, p < 0.0039) (Fig. 2).
The WRS results in quiet were 34.0 ± 23.5% unaided and 84.5 ± 17.9% (n = 10) aided. It shows a significant improvement of ± 50.5% (n = 10, p < 0.0039) using the system compared to the unaided condition (Fig. 3). WRS in noise (Mainzer II, noise at 60 dB, 5 dB SNR) was 11.7 ± 9.8% in the unaided situation and 46.7 ± 18.6% with the ADHEAR system, which means an improvement of 35% (n = 6, p < 0.0313) compared to the unaided situation (Fig. 4).
Results of SRT50 in quiet are shown in Fig. 5. The average threshold was 69.4 ± 8.0 dB SPL unaided and significantly improved by 16.6 dB SPL to 52.8 ± 7.1 dB SPL using the hearing device (n = 9, p < 0.0195).
The mean SRT50 in noise was assessed with the Oldenburg sentence test for children (OLKISA) (Fig. 6). SRTs showed an improvement from an SNR of 0.2 ± 5.5 dB SNR in the unaided condition to an SRT of − 1.7 ± 3.9 dB SNR with the device (n = 4, no p value calculated because of small n).
Patient satisfaction
The acceptance and manageability of the system in everyday life was queried with two questionnaires. Regarding the Speech, Spatial and Qualities of Hearing scale for parents (SSQ for parents) there was an improvement of subjective satisfaction with use of the ADHEAR system in each subdomain. The total score showed a significant improvement (n = 7, p = 0.0313) (Fig. 7) with an initial score (in the unaided situation) of 6.5 ± 1.8 points. It improved to 7.8 ± 1.0 points when the unilateral hearing loss was aided with the system.
The usability of the system was inquired using a system-specific questionnaire (Table 1).
Five out of nine patients found the system to be “valuable” or “very valuable”. Three patients described the system as “partially valuable”. One patient did not answer the question. The adhesive adapter was changed each day by three patients, every second day by one patient. Three patients changed the adapter every 3–4 days. One patient stated to change the adhesive adapter once a week or less than once a week. The sound quality of the system was rated “acceptable” (n = 3), “good” (n = 3), or “very good” (n = 1). Two Patients did not answer the question. The audio processor was worn for an average of 6.9 h a day. Three out of ten patients wore the hearing system at the same time with glasses and did not perceive this as uncomfortable. Six patients wore the device simultaneously with headgear (e.g., hats, helmets). Four patients reported disturbing feedback noises, which did not allow a combination of hearing system and headgear. Two patients stated that the combination of hearing system and headgear was possible without any problems. One patient had problems with the fixation of the adhesive adapter due to a mastoidal retraction after mastoidectomy.
Skin safety
Nine out of ten patients denied skin reactions due to the adhesive adapter. Only one patient showed a slight reddening under the adhesive adapter during the visit after 4 weeks. Medical treatment was not required. The adapter was nevertheless worn daily by the patient.
Discussion
Conventional bone conduction hearing aids such as the BAHA® Softband or BAHA® SoundArc (Cochlear Corp., Sydney, NSW, Australia) are very visible and can, therefore, lead to reduced patient acceptance [1]. Since sound transmission to the skull requires pressure, the use of these systems can lead to pressure points on the skin [8]. Semi-implantable systems need surgical intervention and are currently only licensed from the age of 5, so that application in younger children is not approved. For this group of children who are too young to use semi-implantable hearing systems or do not tolerate a softband-related device, be it for cosmetic reasons or the development of pressure points, there had so far been no meaningful therapy option.
In this study, we were able to show that children with unilateral conductive hearing loss achieved a significant improvement in functional hearing gain by using the pressure-free bone conduction device ADHEAR compared to the unaided situation. The overall average functional hearing gain, calculated as the mean difference between the unaided and aided sound-field audiometry (PTA 4) improved significantly by 19.6 dB (n = 10, p < 0.0039) to a mean threshold of 29.8 dB HL. These results are comparable with other studies in children. Neumann et al. showed a very similar functional hearing gain in children between 33.3 and 35.8 dB, resulting in an aided threshold of 29.7 dB HL [9]. Osborne showed an aided threshold of 26 dB HL in a collective of 21 children [10]. The WRS improved in our collective by 50–84.5% in the aided situation. This increase in WRS is somewhat less than in previous studies in children, in which the improvement was between 55.2% and 77.5% and a WRS between 91.3% and 94.6% in the aided situation was achieved [9, 11, 12]. This difference may be explained by the inhomogeneous patient groups of the individual studies, in which children with different degrees of conductive hearing loss were included.
So far no adaptive speech test has been performed in children using the ADHEAR system. Existing studies in adults used the Oldenburg sentence (OLSA) test as adaptive speech tests for evaluation of the ADHEAR system. We used the Oldenburg sentence test for children (OLKISA) to assess the SRT50. The SRT50 improvement in our population was − 1.9 dB. This improvement is comparable to the data in adults, which showed an improvement between −0.8 and − 3.6 dB SNR [4, 13,14,15].
One limitation of our study is the lack of comparison to other bone conduction hearing aids. Existing studies suggest that the audiometric benefit of bone conduction hearing aids on a softband are comparable to that of the ADHEAR system [4, 9]. The ADHEAR system can be an alternative for patients who suffer from pressure points on the skin with a bone conduction hearing aid on a softband or stigmatization due to the higher visibility of the system.
By analyzing the system-specific questionnaire, we could see high patient acceptance of the applied hearing device. Five out of eight patients described the device as a valuable or very valuable aid. The average daily wearing time for the system was 6.9 h. Dahm et al. published similar results and a significantly longer wearing time with the ADHEAR (8.1 h/day) compared to a conventional BCD on softband or headband (4.5 h/day) [15].
Only one in nine patients showed a skin reaction in the sense of reddening in the area of the adhesive adapter after wearing it for 4 weeks, which, however, did not require medical treatment. In three patients, the adhesive adapter had to be changed daily. In the remaining patients, this was necessary between every 2 days and once a week. One of the children, who required daily change of the adhesive adapter had to be changed daily, had a retroauricular retraction after mastoidectomy, which made it more difficult for the adhesive adapter to fit securely due to the changed skull contour. In cases of altered anatomy, e.g., after retroauricular surgery, secure fitting of the adhesive adapter can be challenging; therefore, additional adhesive material could be useful for better fixation. The wide spread of the adhesion duration of the adapter seems to be due to different skin properties of the study participants (e.g., sweat and sebum production).
Conclusion
The aim of the study was to examine the audiological benefit and subjective satisfaction with the ADHEAR system in children with unilateral conductive hearing loss. The results showed an audiological benefit that is comparable to bone conduction hearing aids on the softband—the current non-surgical standard care. The system proved to be an effective and safe option for children with single-sided hearing loss who do not tolerate a softband bone conduction hearing aid and who are too young or are not accessible for surgical care for other reasons.
References
Hakansson B et al (1985) The bone-anchored hearing aid. Principal design and a psychoacoustical evaluation. Acta Otolaryngol 100(3):229–239
Zarowski AJ et al (2011) Headbands, testbands and softbands in preoperative testing and application of bone-anchored devices in adults and children. AdvOtorhinolaryngol 71:124–131
Reinfeldt S et al (2015) New developments in bone-conduction hearing implants: a review. Med Devices (Auckl) 8:79–93
Dahm V et al (2019) A Randomized cross-over trial comparing a pressure-free, adhesive to a conventional bone conduction hearing device. OtolNeurotol 40(5):571–577
Hahlbrock K-H (1953) ÜberSprachaudiometrie und neueWörterteste. ArchivfürOhrenNasenKehlkopfheilkunde 162:394–431
Weissgerber T et al (2012) German Oldenburg Sentence Test for Children: a useful speech audiometry tool for hearing-impaired children at kindergarten and school age. Folia PhoniatrLogop 64(5):227–233
Noble W et al (2013) A short form of the speech, spatial and qualities of hearing scale suitable for clinical use: the SSQ12. Int J Audiol 52(6):409–412
Verhagen CV et al (2008) The Baha Softband. A new treatment for young children with bilateral congenital aural atresia. Int J PediatrOtorhinolaryngol 72(10):1455–1459
Neumann K et al (2019) A new adhesive bone conduction hearing system effectively treats conductive hearing loss in children. Int J PediatrOtorhinolaryngol 122:117–125
Osborne MS et al (2019) First pediatric experience with a novel, adhesive adapter retained, bone conduction hearing aid system. OtolNeurotol 40(9):1199–1207
Zernotti M (2019) The adhesive bone conduction hearing system in children with Atresia and Microtia, in ESPCI 2019. Bucharest, Romania
Gavilan J (2019) Comparison of the non-invasive adhesive bone conduction system with passive transcutaneous bone conduction implants in children, in EPSCI2019. Bucharest, Romania
Weiss R et al (2020) A new adhesive bone conduction hearing system as a treatment option for transient hearing loss after middle ear surgery. Eur Arch Otorhinolaryngol 277(3):751–759
Gawliczek T et al (2018) Unilateral and bilateral audiological benefit with an adhesively attached, noninvasive bone conduction hearing system. OtolNeurotol 39(8):1025–1030
Dahm V et al (2018) First results with a new, pressure-free, adhesive bone conduction hearing aid. OtolNeurotol 39(6):748–754
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author Daniel Hirth and Roxanne Weiss received a financial support for attending the symposium “Deutscher HNO-Kongress” 2019 in Berlin. The other authors declare that they have no conflict of interest.
Human and/or animals rights
All procedures performed in this study involving human participants were in accordance with the ethical standards by the ethics committee of the medical faculty Frankfurt (EC code 92/17) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirth, D., Weiss, R., Stöver, T. et al. Audiological benefit and subjective satisfaction with the ADHEAR hearing system in children with unilateral conductive hearing loss. Eur Arch Otorhinolaryngol 278, 2781–2788 (2021). https://doi.org/10.1007/s00405-020-06364-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-06364-2