Abstract
Objective
The objective of this prospective, single-subject, repeated measures study was to evaluate the audiological benefit and patient satisfaction with an adhesive, pressure-free bone conduction hearing system (ADHEAR; MED-EL, Innsbruck, Austria) in patients who underwent middle ear surgery with transient hearing loss due to auditory canal tamponade.
Methods
Eleven adult subjects suffering from transient conductive hearing loss were enrolled in the study and followed up to 3 weeks after middle ear surgery. Bone and air conduction thresholds were measured pre and postoperatively to evaluate eligibility for enrollment. Postoperative unaided and aided sound-field thresholds, as well as speech tests in quiet and noise were compared to confirm hearing improvement with the hearing system. To determine patient satisfaction, the SSQ12 and a system-specific quality of life questionnaire was administered to all subjects.
Results
Speech perception for monosyllables in quiet improved by 46%, with statistical significance for the ADHEAR system compared to the unaided condition after one week. The functional hearing gain improved by 19 dB. Speech perception in noise with the device was − 6.7 dB SNR on average, with a statistically significant improvement of 2.7 dB SNR. The results of the questionnaire showed a high level of patient satisfaction and subjective hearing improvement. No serious skin reactions or other severe complications occurred.
Conclusion
As long as the auditory canal is blocked due to tamponade, patients benefit from hearing rehabilitation. This adhesive hearing system is a safe and effective device to treat transient conductive hearing loss and may considerably improve treatment for patients even with short-term hearing loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transient conductive hearing loss can be observed in various clinical situations, e.g. otitis externa, acute or chronic otitis media with or without effusion or middle ear trauma. In addition, it can also occur following middle ear surgery due to a postoperatively placed tamponade blocking the external auditory canal (EAC).
The duration of such transient conductive hearing loss can vary substantially from days to even months, depending on the underlying aetiology. Currently, the effects of this hearing impairment tend to not be considered adequately with patients being left untreated, although transient CHL may affect a subject’s quality of life quite severely, e.g. hampering their ability to work, impairing speech communication and social interaction. Moreover, difficulties perceiving environmental sounds can be a safety issue.
Conventional hearing aids do present a therapy option, but their benefit is often limited in clinical practice. Thus, an alternative treatment is required.
Currently available non-surgical bone conduction devices such as the BAHA® Softband, BAHA® SoundArc (Cochlear Corp., Sydney, NSW, Australia) or hearing spectacles (e.g. Coselgi, BHM, Austria) have also been developed to treat this group of patients [1]. However, in these cases, the hearing aids are plainly visible, and require static force for their retention, which is not always tolerated by adults. The ADHEAR system (MED-EL, Innsbruck, Austria) is a novel, non-invasive bone conduction hearing aid, which is attached to the hairless skin behind the ear without the application of any pressure. It functions by the connection of two components, the audio processor (AP) and the adhesive adapter (AA) (Fig. 1). The single-use, water resistant AA is placed behind the auricle in the mastoid area and remains there for 3–7 days. The AP is then connected via the snap connector on the AA. The AP transfers vibrations to the inner ear without applying pressure to the skin as is required by other comparable non-implantable bone conduction devices (e.g. headbands, softbands, SoundArc). Recent studies have already shown that this pressure free adhesive system may have an advantage over other conventional bone conduction hearing aids regarding wearing time [2] and acceptance in adults and children [3, 4]. The audiometric outcomes also seem comparable to other non-surgical devices [4, 5].
To the best of our knowledge, this study is the first to evaluate applicability and hearing benefit with this novel hearing system in patients with transient conductive hearing loss due to tamponade of the auditory canal after ear surgery. Furthermore, safety and efficacy, as well as patient satisfaction with the device in everyday life situations will be analysed.
Material and methods
Subjects and materials
This prospective, single-subject, repeated-measure study collected data between August 2017 and October 2018 at the ENT department of the University Hospital Frankfurt, following approval by the ethics committee of the medical faculty (EC code 92/17). It was designed and conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants included in the study.
Subjects enrolled had to be 18 years of age or older with sufficient German language skills to perform all tests and be able to use the hearing system. Subjects who had undergone middle ear surgery with an endaural surgical approach and presented with pre and postoperative bone conduction (BC) thresholds better or equal to 25 dB HL at the frequencies 0.5, 1, 2, 4 kHz were eligible for the study. Due to tamponade in the EAC as part of the wound care, transient CHL was expected. Exclusion criteria were retrocochlear or central auditory disorders, skin or scalp conditions that may preclude attachment of the adhesive adaptor and any other physio and/or psychological issues interfering with the conduct of the study.
Eleven patients were enrolled in the study. Patient number seven was lost to follow-up before device fitting. Ten patients (four female and six male, average age 38 years, ranging from 21 to 62 years) received the device and were followed up to 3 weeks post-endaural tympanoplasty or auditory canal mold. Six subjects were attended with a disintegrating tamponade (Gelita®) after tympanoplasty and four subjects with a non-disintegrating tamponade (Merocel), due to external auditory canal reconstruction. The integrity of the tamponade was assessed visually by the medical investigator after 1 and 3 weeks. Eight subjects were treated with the adhesive hearing system on the right and two on the left side. Demographic details and etiologies are shown in Table 1.
Audiological assessments
Audiometric testing was conducted in an audiometric sound-attenuated room, using calibrated signals and equipment according to accepted ISO standards. All included subjects were evaluated at regular intervals to include one preoperative (PRE) and three postoperative test sessions. The first postoperative session (1st POST) was scheduled for the first or second day after surgery, the second postoperative session (2nd POST) approximately one week and the third postoperative session (3rd POST) approximately three weeks (earliest point before tamponade removal) after surgery.
Device fitting
The participants were instructed how to use the device and the best hairless position of the skin behind the ear on the mastoid bone was identified. Before attaching the adhesive adapter, the skin was cleaned with alcohol. Patients were asked to use the device in the default program and in their preferred volume setting. The volume could be adjusted via a wheel on the AP. Measurements were then taken using these device settings.
Pure tone audiometry
Air conduction (AC, 0.5–8 kHz) and bone conduction (BC, 0.5–4 kHz) pure-tone thresholds were tested for both ears using insert earphones, headphones or a calibrated BC vibrator, as appropriate on each ear individually at the preoperative session and at the 1st postoperative session (Table1).
Sound-field audiometry
Sound-field tests were conducted under the S0 or S0N0 configuration with the contralateral ear plugged and covered. The loudspeakers were placed 1 m away from the patient’s head. Sound-field thresholds (SF) were measured using warble tones in the unaided and aided conditions at the 1st, 2nd and 3rd postoperative sessions. Speech intelligibility was tested using word recognition scores (WRS), speech recognition thresholds (SRT) in quiet and SRT in noise (signal to noise ratio—SNR) [6]. The WRS was measured with monosyllables at 65 dB SPL signal level (Freiburger Einsilber) [6]. The SRT50 was defined as the level needed to understand 50% of the words in a list. The SRT50 in quiet was measured with polysyllabic numbers (Freiburger Zahlen) [6]. The SRT50 in noise was measured using the Oldenburger sentence test (OLSA) with a fixed background noise level at 65 dB SPL and adaptive speech levels [7]. Unaided and aided postoperative outcomes of the WRS, the SRT50 in quiet and noise were measured at the 2nd and 3rd postoperative sessions.
Subjective device satisfaction
The "Speech, Spatial, and Qualities of Hearing" (SSQ) test measures self-reported auditory disability across a wide variety of domains. The short form of the original version, SSQ12, was validated in 2013 by Noble et al. [8] and brings consistent results and better usability compared to the long version. The SSQ12 asks the patient to rate each answer on a scale from 0 to 10. The SSQ12 was administered at the preoperative session and at the 1st, 2nd and 3rd postoperative sessions. To be able to evaluate the patient’s unaided experience with transient CHL, the subjects were asked to refrain from using the hearing aid for at least 1 day after discharge from the hospital, report their outcomes on the SSQ12 (1st postoperative SSQ12) and then start to use the system. At the 3rd postoperative session, an additional questionnaire consisting of 21 questions regarding the usability of the device in daily use was administered.
Skin safety
Skin safety while using the adhesive adapter was evaluated by the medical investigator after week 1 and week 3. Relevant results included redness or other skin reactions.
Statistics
Results were summarised by calculating the mean and standard deviation (mean ± SD).
The D'Agostino–Pearson normality test and Kolmogorov–Smirnov normality test were used to test for normal distribution of datasets. If both tests were positive, the student's t test or the Wilcoxon test was used to compare between groups. A p value below 0.05 (p < 0.05) indicated statistical significance. GraphPad Prism 6.0 was used for statistical analysis and creating graphs and Microsoft Excel was used for the tables.
Results
Tamponade integrity
After 1 week, the disintegrating tamponade (Gelita®) was still intact, as was the non-disintegrating (Merocel) after 1 week and 3 weeks. However, the non-disintegrating tamponade appeared dried out and shrunk after 3 weeks in patient 8. In all patients fitted with the disintegrating tamponade, this tamponade had mostly dissolved after 3 weeks. After 3 weeks, patient 9 still had ear blockage, as a coagulum occluded the EAC during audiometric testing. A similar component also remained in patient 10 after tamponade removal.
Audiometric results
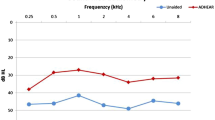
Mean sound-field hearing thresholds in the unaided condition and with the hearing system on the day of fitting and after 1 and 3 weeks are shown in Fig. 2. The overall average functional hearing gain, calculated as the mean difference between the unaided and aided sound-field audiometry (with the contralateral ear blocked) for the frequencies 0.5, 1, 2 and 4 kHz (PTA 4) improved significantly by 15 ± 2.9 dB HL (n = 9, p < 0.0001) on the day of fitting and by 19 ± 7.4 dB HL after one week (n = 10, p < 0.0001). At the first follow-up one week postoperatively, the tamponade was intact regardless of the type.
After 3 weeks, the functional hearing gain was 7 ± 7.0 dB HL for the disintegrating tamponade (n = 5) and 15 ± 11.4 dB HL for the non-disintegrating tamponade (n = 4). Due to early tamponade removal, the audiometric evaluation of patient 1 was not possible at the third postoperative session.
WRS results are shown in Fig. 3. A significant improvement of 46 ± 19.4% (n = 10, p < 0.0001) was achieved using the system compared to the unaided condition as long as the occlusion due to tamponade was present (2nd POST). At this time point, the WRS results were 29 ± 20.0% unaided and 75 ± 16.2% (n = 10) aided. After 3 weeks, the WRS was 56 ± 37.5% unaided and 75 ± 14.1% aided for patients with a disintegrating tamponade (n = 5). For patients with a non-disintegrating tamponade (n = 4), the unaided and aided results were 61 ± 34.7% and 85 ± 8.2%, respectively.
The average SRT50 in quiet (Fig. 4) was 57 ± 7.6 dB SPL unaided and significantly improved by 17 dB SPL to 40 ± 9.9 dB SPL with the adhesive hearing aid after one week (n = 10, p = 0.0020). After 3 weeks, the SRT50 in quiet improvement was 6 ± 8.4 dB SPL for subjects with a disintegrating tamponade (n = 5) and 11 ± 5.1 dB SPL for those with a non-disintegrating tamponade (n = 4).
Mean SRT50 in noise on the Oldenburg sentence test (OLSA) values are shown in Fig. 5. SRTs improved significantly from an SRT of – 4.0 ± 3.5 dB SNR to an SRT of – 6.7 ± 2.7 dB SNR with the device (after 1 week, n = 10, p = 0.0039). After 3 weeks, the SRT50 in noise was − 5.3 ± 1.6 dB SNR unaided and − 5.4 ± 1.8 dB SNR with the disintegrating tamponade (n = 5) and − 6.6 ± 2.9 dB SNR unaided and − 7.5 ± 1.6 dB SNR with the non-disintegrating tamponade (n = 4).
Patient satisfaction
Two questionnaires were used to survey the patients’ acceptance and handling of the hearing device in daily life. The Speech, Spatial and Qualities of Hearing scale (SSQ) revealed improved subjective satisfaction with use of the hearing aid postoperatively in the three subdomains and there was a significant improvement in the total score (n = 7, p = 0.0313, Fig. 6). The initial SSQ total score before ear surgery, unaided and without a tamponade occluding the ear was 7.2 ± 1.9 points; this score dropped to 5.0 ± 2.4 points after surgery when the transient CHL was present but untreated. The total score improved to 7.1 ± 1.6 points when the transient CHL was aided with the system. Three weeks postop., three of these seven patients still had the ear occluded with the tamponade and their score remained elevated at 7.2 ± 1.8 points.
The system-specific questionnaire was used to assess the usability of this device.
It was rated a “valuable” or “very valuable” aid by eight out of ten patients (Fig. 7). The remaining two patients described the system as “partially valuable”. The interval at which they changed the adhesive adapter was 1 day for one patient, 3–4 days for the majority (n = 7) and once a week for two patients. The sound quality of the system was rated “acceptable” (n = 5), “good” (n = 4) or “very good” (n = 1). The average daily wearing time of the audio processor was 11.7 ± 6.3 h. Wearing the audio processor behind the ear was reported as not disturbing in any situation, while five patients sometimes forgot they were wearing the device and two patients did not notice it most of the time. Six out of ten patients found that other people rarely noticed the device. Five out of ten patients tried wearing spectacles and the hearing aid at the same time and found this to be both possible and comfortable. Six patients tried wearing headwear (e.g. helmets, hats) and the audio processor at the same time; half of them found this to be uncomfortable. By the end of 3 weeks, the adhesive adapter fell off only one time in three patients.
Skin safety
Nine out of ten patients reported no complications or skin reactions. Only one patient reported the skin to be “a little” irritated, but did not require medical treatment. The patient’s skin was reddish underneath the adhesive adapter; the broken skin at this site had previously been removed. The patient continued to wear the device all day despite this condition, replacing the adhesive adapter twice a week.
Discussion
There are numerous treatments available for mixed and conductive hearing loss.
In cases of transient hearing loss, e.g. due to temporary postoperative tamponade of the auditory canal or latency time for reconstructive middle ear surgery, non-invasive options can ensure the most achievable compensation for disability. Likewise, if surgical treatments may cause increased anaesthetic risk due to multimorbidity or if the patient simply does not wish to undergo surgery, alternative treatments are necessary.
Hearing disorders—even short term—have the potential to diminish a patient’s quality of life and could negatively influence daily life, especially when binaural hearing is necessary (e.g. road traffic, work, social situations, etc.).
The bone conduction hearing aids that are currently available such as the BAHA® Softband, BAHA® SoundArc (Cochlear Corp., Sydney, NSW, Australia) or hearing spectacles (e.g. Coselgi, BHM, Austria) are plainly visible devices and therefore not always tolerated by adults and children [9]. Furthermore, the hearing sensation is generated using pressure to overcome the damping effect of the skin. This may cause discomfort or pain to the users. Verhagen et al. showed that 50% of children using a steel spring headband or softband suffer from pressure points [10].
The pressure-free nature of this adhesive bone conduction device could be an advantage over the recently available hearing aids.
This study aimed to analyse the audiological performance, applicability and subjective satisfaction of the adhesive hearing system in patients with transient hearing loss following ear surgery.
During the study, we realised that subjects were separated into two groups: patients who received a disintegrating tamponade (Gelita, n = 6) and those who received a non-disintegrating tamponade (Merocel®, n = 4), depending on the surgical procedure involved. We decided to analyse the measurements of each group individually to show the effect of the different auditory canal occlusion.
All tamponade, regardless of the type, appeared intact at the first follow-up 1 week postoperatively. The PTA4 measurements at this time point showed a significant improvement by 19 dB averaged over all patients (mean aided threshold 27 dB HL, n = 10). These results are comparable with the mean aided threshold achieved with the BAHA® Softband in other studies [10]. Recent studies analyzing the ADHEAR system in other patient groups showed similar results with an average functional hearing gain of 14.3–24.6 dB [2, 3, 5].
Speech perception in quiet also showed significant improvement after 1 week, by 46% in monosyllabic words. Likewise, speech perception in noise, using the Oldenburger sentence test, improved significantly by 2.7 dB SNR and was − 6.7 dB SNR on average. These results are comparable to the outcomes using a conventional headband BCD or other non-implantable wearing options like the BAHA® SoundArc (Cochlear Corp., Sydney, NSW, Australia) [10,11,12,13], although the indication criteria of this new adhesive device are more restrictive (bone conduction thresholds better than or equal to 25 dB HL at the frequencies 0.5, 1, 2, 4 kHz).
None of our patients had any serious skin reactions, although one patient suffered from reddish skin under the adhesive adapter due to prior removal of split skin in this area. No medical treatment was needed.
Measurements at the second follow-up (3 weeks postop.) still showed an improvement in the aided condition in patients with remaining conductive hearing loss due to tamponade or pathology (Pat. 3, 4, 5, 6, 9, 10). However, the SSQ12 questionnaire results for the aided condition at 3 weeks remained elevated regardless of whether or not hearing was restored by the device or by the restoration of an open auditory canal. By analysing the system-specific questionnaire, we could see high patient acceptance of the hearing solution (Fig. 7). Eight out of ten patients described the device as a valuable or very valuable aid. None of the patients had any problems with using the system in combination with their regular spectacles. Wearing time of the system was high, with an average of 12 h per day. Dahm et al. published similar results and a significantly longer wearing time with the ADHEAR (8.1 h/day) compared to a conventional BCD on softband or headband (4.5 h/day) [3]. Discomfort and pain were stated as the reasons for the shorter wearing time of the softband/headband devices. The subjects in our study found that wearing the audio processor behind the ear was not disturbing in any situation and five patients sometimes forgot they were wearing the device, while two patients did not notice it most of the time. Similar experiences were reported in children, where seven out of nine children did not accept a BCD on softband due to stigmatisation, inconvenience or pressure on the head, while the adhesive hearing device was well accepted and chosen for further treatment by eight of those nine children [4].
One limitation of our study is the missing comparison to other available bone conduction hearing aids. Due to audiometric results, we expect similar benefits using these devices, as seen in several studies [2, 4, 5, 11]. While this was not the focus of our present study, it will be an interesting topic for further studies.
Another limitation is the small number of subjects; the conclusions were therefore mainly drawn from the results 1 week postoperatively, which were consistent between subjects and highly comparable to already published data on this device. Further studies could be undertaken to evaluate the benefit in terms of the patients’ safety in daily life, ability to work and quality of life during the rehabilitation period after ear surgery with and without treatment of associated transient CHL. Such data would be valuable for usage in health technology assessment reports.
This adhesive bone conduction hearing system performed as a feasible treatment for patients who face a limited period of time with conductive hearing loss. At present, the potential costs of the system will be in the range of established bone conduction devices. In Germany, it is actually available as a loan unit and could be of further use as a temporary solution before hearing implant surgery. However, the costs have to be seen in comparison to the benefit in each individual case. In addition, the device could probably optimise hearing after one way bilateral tympanoplasty, helping save further resources.
Furthermore, Neumann et al. showed that this adhesive hearing solution effectively treats children with CHL and one can assume that the device could also be a good treatment option for children with episodes of otitis media that result in transient conductive hearing loss [4].
Conclusion
This study was the first to evaluate the novel adhesive bone conduction system ADHEAR as a treatment for transient CHL after ear surgery. The results of ten patients revealed good usability of the hearing device; there were no serious skin reactions and the device provided sufficient audiological gain for hearing rehabilitation.
In summary, this study showed that the adhesive system is a safe and effective device to treat transient conductive hearing loss. Treatment with this hearing aid would improve the current therapy options for patients suffering from surgery associated, short-term hearing loss.
References
Reinfeldt S, Håkansson B, Taghavi H, Eeg-Olofsson M (2015) New developments in bone-conduction hearing implants: a review. Med Devices (Auckl). 16(8):79–93. https://doi.org/10.2147/MDER.S39691
Dahm V, Auinger AB, Liepins R, Baumgartner WD, Riss D, Arnoldner C (2019) A randomized cross-over trial comparing a pressure-free, adhesive to a conventional bone conduction hearing device. Otol Neurotol. 40(5):571–577. https://doi.org/10.1097/MAO.0000000000002184
Dahm V, Baumgartner WD, Liepins R, Arnoldner C, Riss D (2018) First results with a new, pressure-free, adhesive bone conduction hearing aid. Otol Neurotol. 39(6):748–754. https://doi.org/10.1097/MAO.0000000000001829
Neumann K, Thomas JP, Voelter C, Dazert S (2019) A new adhesive bone conduction hearing system effectively treats conductive hearing loss in children. Int J Pediatr Otorhinolaryngol. 122:117–125. https://doi.org/10.1016/j.ijporl.2019.03.014
Gawliczek T, Munzinger F, Anschuetz L, Caversaccio M, Kompis M, Wimmer W (2018) Unilateral and bilateral audiological benefit with an adhesively attached, noninvasive bone conduction hearing system. Otol Neurotol. 39(8):1025–1030. https://doi.org/10.1097/MAO.0000000000001924
Hahlbrock K-H (1953) Über Sprachaudiometrie und neue Wörterteste. Archiv für Ohren-, Nasen- und Kehlkopfheilkunde 162:394–431
Wagener K, Brand T, Kühnel V, Kollmeier B (1999) Entwicklung und Evaluation eines Satztests für die deutsche Sprache I–III: design, Optimierung und Evaluation des Oldenburger Satztests. s.l. ZfA 38(1–3): 4–15, 44–56, 86–95
Noble W, Jensen NS, Naylor G, Bhullar N, Akeroyd MA (2013) A short form of the speech, spatial and qualities of hearing scale suitable for clinical use: the SSQ12. Int J Audiol. 52(6):409–412. https://doi.org/10.3109/14992027.2013.781278
Håkansson B, Tjellström A, Rosenhall U, Carlsson P (1985) The bone-anchored hearing aid. Principal design and a psychoacoustical evaluation. Acta Otolaryngol. 100(3–4):229–239
Verhagen CV, Hol MK, Coppens-Schellekens W, Snik AF, Cremers CW (2008) The Baha Softband. A new treatment for young children with bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol. 72:1455–1459
Gawliczek T, Wimmer W, Munzinger F, Caversaccio M, Kompis M (2018) Speech understanding and sound localization with a new nonimplantable wearing option for Baha. Biomed Res Int. 2018:5264124. https://doi.org/10.1155/2018/5264124
Ihler F, Blum J, Berger MU, Weiss BG, Welz C, Canis M (2016) The prediction of speech recognition in noise with a semi-implantable bone conduction hearing system by external bone conduction stimulation with headband: a prospective study. Trends Hear. 3:20 [pii: 2331216516669330]
Verstraeten N, Zarowski AJ, Somers T, Riff D, Offeciers EF (2009) Comparison of the audiologic results obtained with the bone-anchored hearing aid attached to the headband, the testband, and to the "snap" abutment. Otol Neurotol. 30(1):70–75
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author Roxanne Weiss received a financial support for attending the symposium “Deutscher HNO-Kongress” 2019 in Berlin. The other authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in this study involving human participants were in accordance with the ethical standards by the ethics committee of the medical faculty (EC code 92/17) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weiss, R., Loth, A., Leinung, M. et al. A new adhesive bone conduction hearing system as a treatment option for transient hearing loss after middle ear surgery. Eur Arch Otorhinolaryngol 277, 751–759 (2020). https://doi.org/10.1007/s00405-019-05769-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-019-05769-y