Abstract
Background and objectives
The Pro51Ser (P51S) COCH mutation is characterized by a late-onset bilateral sensorineural hearing loss (SNHL) and progressive vestibular deterioration. The aim of this study was to carry out a systematic review of all reported hearing and vestibular function data in P51S COCH mutation carriers and its correlation with age.
Materials and methods
Scientific databases including Medline, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, ISI Web of Knowledge, and Web of Science were searched to accumulate information about hearing outcome and vestibular function. Eleven genotype–phenotype correlation studies of the P51S COCH variant were identified and analyzed.
Results
The SNHL starts at the age of 32.8 years. The Annual Threshold Deterioration is 3 decibel hearing loss (dB HL) per year (1–24 dB HL/year). Profound SNHL was observed at 76 years on average (60–84 years). 136 individual vestibular measurements were collected from 86 carriers. The onset of the vestibular dysfunction was estimated around 34 years (34–40 years), and vestibular deterioration rates were higher than those of the SNHL, with complete bilateral loss observed between 49 and 60 years.
Conclusion
Both audiometric and vestibular data were processed with much different methodologies and pre-symptomatic P51S carriers were systematically underrepresented. Further delineation of this correlation would benefit cross-sectional and longitudinal study involving all (pre-symptomatic and symptomatic) P51S carriers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gene mutations account for more than 60% of congenital sensorineural hearing loss (SNHL) in Western Countries [1, 2]. Hereditary SNHL does not necessarily start at birth, however, as many causative gene mutations only begin to express at much later ages, for example, DFNA9, also known as the ninth locus that was discovered for autosomal dominant SNHL [3]. It is characterized by a late-onset of rapidly progressive SNHL together with accompanying vestibular impairment [3]. The first reported DFNA9 patients were carrying the c.151C > T mutation in COCH, which is the result of a substitution of cytosine by thymine nucleotide of the 151th base pair in codon 51 (c.151C > T, P51S) [3, 4].
The SNHL in P51S carriers is estimated to start in the 4th decade, followed by a rapid progression to severe hearing and balance deficiencies in the 6th decade. The balance dysfunction is more discrete, but nevertheless, a progression to bilateral vestibulopathy (BV) with complete peripheral vestibular areflexia at later ages is observed in many DFNA9 patients. A considerable part of these patients, however, present Menière-like symptoms, which suggests that the vestibular signs are more heterogeneous than the auditory dysfunction [5,6,7]. For these reasons, we hypothesize that the age of onset of vestibular deterioration of P51S carriers is more difficult to assess than the SNHL. In the perspective of innovative future hearing and vestibular treatments, such as gene therapy, stem cell therapy, neural regeneration, in association with cochlear and/or vestibular implantation, a better understanding of the onset of the very first signs of any deterioration, including the balance system, is important.
The objective of this systematic review is to identify studies related to DFNA9, caused by the P51S COCH variant, with special attention to the subclinical period in this late-onset progressive disorder affecting cochleovestibular function.
Materials and methods
Data sources
The strategy and methodology used for the systematic review was based on the PRISMA Statement (preferred reporting items for systematic reviews and meta-analysis) [8].
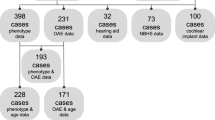
Medline, PubMed, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, ISI Web of Knowledge and Web of Science were searched. Information was retrieved about COCH mutations causing DFNA9, including phenotype, genotype, pathophysiology and imaging. Figure 1 shows the PRISMA 2009 Flow Diagram of publications found using the following search strategies: “P51S” and “cochleovestibular” and ‘deterioration”; “P51S” and “dizziness”; “P51S” and “DFNA9”; “P51S” and “hearing” and “impairment”; “COCH” and “mutation”; “phenotype” and “DFNA9”; “DFNA9” and “dizziness”; “DFNA9” and “COCH” and finally “COCH” and “mutation”.
Study selection
All studies were screened for eligibility in three phases based on study subject mentioning DFNA9 and/or COCH mutation. In the first phase, all English-written studies from the late 1980s until present were screened on title and abstract. In case of missing abstract, but with applicable title, the study was included to the second phase. In the second phase, the studies were screened in abstract and full-text based in study subject mentioning the P51S COCH mutation. A third screening on the presence of audiometric and/or vestibular function assessment was carried out.
A total of 153 records were listed, and another 18 records were added by manual searching to get a better understanding of the gain-of-function effect, pathophysiology of COCH mutation and cochlin function [7, 9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. A hundred and two were removed, of which 82 duplicates, 18 irrelevant subject and 2 without full text, leaving 69 papers for further analysis. Only 20 records were specifically dealing with the P51S COCH variant, as 31 were dealing with other COCH mutations, whereas 18 were non-audiological reports [6, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45].
The 20 selected records were further analyzed with regard to phenotypical aspects of the P51S COCH variant. Eleven records met all selection criteria and contained presumed useful audiometric and/or vestibular data for the evaluation of the deterioration in relation to age [5, 26,27,28, 30,31,32,33,34, 36, 37].
Data extraction
In case of different audiometric data presentation (e.g., audiograms or different pure tone average (PTA) plots against age) a comprehensive assessment and inventory of all individual measurements was conducted. All available measurements at both ears per frequency were collected and a binaural mean value for each frequency per age was calculated and plotted as cumulative age-related typical audiogram (ARTA). Since the individual 95th percentile threshold values of presbyacusis in relation to the patient’s sex and age were derived for each frequency by the ISO 7029 method in almost all selected records, an identical procedure was carried out for the data retrieved from the two papers in which this methodology was not reported [46]. If longitudinal measurements of the same individual were shown, all the available data were included in the assessment (see discussion below). For the vestibular function, we first analyzed all different methods and parameters that were used to represent the vestibular function and we looked for the reference method for the respective scores. Normative values, if mentioned, were used to evaluate the measurements. An overall inventory of all individual vestibular measurements as function to age was carried out. The flow of included articles can be found in Fig. 1.
Results
Assessment of the auditory function in P51S carriers
Belgian and Dutch researchers carried out a series of phenotype studies in large COCH P51S families originating from the Low Countries [3, 5, 26, 28, 30,31,32,33,34, 37, 38].
The P51S COCH variant is by far the most prevalent COCH variant in this region (Belgium and The Netherlands) [5, 6, 26,27,28, 30,31,32,33,34, 37, 38, 40, 43]. Other variants include G88E, G87W and I109T in Dutch families and P98* in a Belgian family with Moroccan roots [41, 44, 47,48,49]. The P51S COCH variant was also found in one North-American family [36].
Of the 153 records selected for DFNA9 cochleovestibular deficiency, only 11 phenotype studies of P51S carriers could be held back for detailed analysis of age-related SNHL [26,27,28, 30,31,32,33,34, 36, 37]. The methodology and the audiometric data of these 11 selected records are summarized in Table 1 [5, 26,27,28, 30,31,32,33,34, 36, 37].
Individual hearing thresholds were available in six papers, whereas the audiometric data was limited to a variety of pure tone average (PTA) or descriptive statistics without available raw data in two records [26, 28, 30,31,32,33,34, 36]. In the three remaining records, audiometric data were missing and were, therefore, unavailable for the evaluation of the SNHL in relation to age [5, 27, 37]. The majority of the records were cross-sectional studies. However, additional longitudinal data assessment was found in 7 out of 11 records, which were available in plots as function to age per frequency for those affected subjects of whom at least three different audiometric measurements of the same affected subject were available over a period of at least 3 years, as defined by the majority of the authors [26, 28, 30, 31, 33, 34, 36].
Regression analysis of auditory data
Until 2003, many authors used linear regression analysis to evaluate the progression of the hearing deterioration in DFNA9 as function to age [26, 28, 31, 32]. However, since the onset of the SNHL is late and because maximal hearing threshold values are fixed, due to scale limitations of audiometers (120 dB HL), a more realistic trajectory of the auditory deterioration would rather be non-linear instead of a straight line [33]. The logarithmic dose–response equation results in a sigmoidal curve that plots the hearing thresholds in decibel hearing level (dB HL) as function to age in years [32,33,34, 41, 48, 49].
The resulting sigmoidal curve still contains a linear section, however, which starts at X10 and ends at X90, both representing the age at which the threshold attains 10% of the whole trajectory and the age at which the threshold attains 90% of the whole trajectory, respectively [32]. The variable slope of the linear segment represents the annual threshold deterioration (ATD), in decibel per year (dB/year) and can be calculated per frequency or for different PTA indices (Table 2) [33, 50]. Moreover, it allows the estimation of the age of onset of the hearing loss, which is derived from the scores at X10 [33, 34].
In five studies from this same centre, similar regression analyses was used to calculate ATD’s, which we have summarized in Table 2 [26, 31,32,33,34]. The ATD is on average 3 dB HL per year, ranging from 1 to 24 dB HL per year. The evaluation of the decline of speech perception as function to age, using consonant–vowel–consonant (CVC) word scores was conducted in a similar fashion [32]. An average annual deterioration of speech perception around 2.9% per year was observed [32].
Age-related typical audiogram (ARTA)
Audiometric data were presented in a variety of different manners, evolving from simple superimposed audiograms in early studies to plots of hearing thresholds or PTA’s as function to frequency by means of averaged thresholds per age group or decade in the more recent papers [26, 28, 33].
Verstreken et al., from their side, presented Box and Whisker boxes per frequency by means of three plots representing hearing thresholds (from the patient’s best ear) of three subgroups of P51S carriers of different age categories (< 35 years, 36–55 years, > 55 years) [30]. Bom et al., first developed “age-related typical audiogram” (ARTA) as a way of depicting binaural median averaged hearing thresholds of all assessed subjects in just one audiogram, avoiding superimposition of data and unnecessary subdivision into different audiograms [33, 50]. The ARTA for the P51S COCH variant was first derived from 32 P51S carriers originating from the same Dutch family (w98-011), as shown in Table 3, which was only available in one record [33]. It is unclear whether additional data of other affected subjects of the same and/or other DFNA9 families may have been supplemented to the data of these first 32 P51S mutation carriers for calculation of the ARTA shown in two more recent papers [33, 34, 49]. Bom’s ARTA provides a clear overview of typical mean hearing thresholds per frequency per age group (decade). However, it is based on a limited number of affected subjects (n = 32) when compared to the large amount of available individual hearing data in the 11 selected records. For this reason, it was worth the effort to calculate a cumulative ARTA, based on a much larger number of affected subjects assembled with all the available raw data in the 11 papers (Fig. 2). For this purpose, we collected all individual hearing thresholds which could be derived from available cross-sectional raw data in audiograms (six records) as well as frequency-specific longitudinal data against age (seven records). In case ISO 7029 method was not used or not addressed (2 records; 24 subjects), individuals were considered affected if the best hearing ear showed thresholds beyond the 95th percentile threshold value for presbyacusis. This way, we were able to analyze a total of 243 cumulative individual hearing threshold measurements, representing audiometric data of at least 100 P51S carriers collected from all studies in Table 2 [26, 28, 31,32,33,34]. The frequency-specific median values are plotted against age in decades in Fig. 2 (cumulative ARTA). The age distribution of the assessed measurements (in decades) are depicted in Fig. 3. Thirty-eight hearing measurements were collected from younger subjects (10–40 years) compared with 207 individual measurements of subjects aged 41 and older (Fig. 3). The age of onset of SNHL is estimated at a median age of 40, ranging from 35 to 56 years (Table 2). Besides one exceptionally early onset at the age of 18 years in one Belgian sibling with a homozygous carriership of the P51S, high-frequency SNHL starts at 32.8 years, and the lower frequencies at 40.7 years. Profound SNHL is achieved at 76 years.
Cumulative ARTA (based on bilateral averaged hearing levels) based on 243 measurements in P51S carriers. Note that the averaged hearing levels of the P51S carriers aged 80 years and above are comparable to the preceding age-group. This is probably the result of the missing values corresponding to all out-of-scale hearing measurements which were excluded by several authors, who feared biased binaural averaged values using 130 dB versus 120 dB. Open dots: P51S carriers aged < 20 years. c-shaped dots: P51S carriers aged 30 years. Open triangles: P51S carriers aged 40 years. Open boxes: P51S carriers aged 50 years. x-dots: P51S carriers aged 60 years. full dots: P51S carriers aged 70 years. Full boxes: P51S carriers aged 80 years
The assessment of the vestibular function in COCH mutations
Only six studies of DFNA9 patients caused by the P51S COCH variant contain detailed individual data of the vestibular function which are summarized in Table 3 [26, 28, 30, 31, 34, 37]. The Dutch investigators used the velocity-step test (VST), with the time constant ‘T’, in seconds (s), as cardinal parameter for the vestibular function, whereas the Belgian researchers, on the contrary, preferred calculating the gain (°/s) of the eye nystagmus slow phase velocity obtained from the caloric stimulation by successive irrigation of both ears with water at 30° and 44 °C.
The methodology for the time constant ‘T’ measurement was well defined and comparable in all selected (Dutch) studies, in which T was derived from a VST by determining the computer-based analysis of the time of speed decay of the elicited post-rotational nystagmus till 37% of its initial value [26, 28, 34, 37]. They established a classification of the vestibular–ocular reflex (VOR) according to the value of the time constant ‘T’, considering T scores from 13 to 23 s as normal [34, 51]. A T score of 0 s was allocated to areflexia, a score of less than 5 was assigned to severe hyporeflexia close to areflexia, whereas scores from 5 to 12 were considered hyporeflexia. Hyperreflexia, on the other hand, was diagnosed when T score was above 23 s [34]. Verstreken et al. used summation of the slow phase’s gain derived from of all 4 caloric response as a measure of the vestibular function, using the normative data of the gain scores according to Vanderstappen et al. [30, 52]. Lemaire et al. also used the caloric response as a parameter, however, without reporting a reference method [31]. The raw data of Verstreken et al. unfortunately, were unavailable [30]. Moreover, the study outcome measures served almost exclusively to determine a possible correlation between the unilaterality of the vestibular dysfunction and the asymmetry of the SNHL [30].
Many older papers presented rudimentary and anecdotal results of vestibular function of a limited number of patients, whereas other authors attempted the calculation of the annual vestibular deterioration (AVD) rate, based on the time Constant ‘T’ plotted as function to age [30, 31, 34]. According to some, at least 25% of affected subjects presented Menière-like symptoms, especially in early stages of the disease, whereas others reported similar fluctuating hearing function, but not in such proportions [26, 28, 30, 31, 34]. Menière’s disease and DFNA9 are unrelated, however [53].
The subjective age of onset of the vestibular signs ranged from 5 to 53 years, with a median of 39 years (Table 3). The vestibular symptoms were first considered appearing at the same time as the onset of auditory signs [5, 26, 28, 30, 31]. Other studies with a bit larger groups of affected individuals, however, claim vestibular signs preceding the SNHL by 9 years [34].
The time constant ‘T’ was used as the main vestibular parameter in the majority of (Dutch) studies, contributing to 128 cumulative vestibular measurements performed on more or less 76 affected subjects [26, 28, 34, 37]. Caloric responses of another eight affected individuals were added to the previous data [31].
This way, a total of 136 cumulative individual vestibular measurements of approximately 84 P51S carriers were analyzed (Fig. 4). There were no measurements of subjects under 21 years. Bilateral vestibular areflexia (BV) was observed in all affected individuals aged 61 years or older, while this condition was already achieved in the 82.75% of measurements in affected subjects aged between 51 and 60 years (24 out of 29). However, individuals aged between 31 and 50 years showed a marked heterogeneity due to a wide variety in the degree of the vestibular impairment, ranging from normal to total bilateral areflexia. In this critical age period, bilateral vestibular hyporeflexia accounted for 10 out of 41 measurements (24.4%) and the same percentage showed BV. In contrast, no areflexia was found in patients aged under 31 years. Vestibular hyperreflexia, however, was observed in 5 out 16 measurements in subjects aged between 21 and 30 years (31%) and it is definitely an exclusive feature of the early phase in the peripheral vestibular dysfunction.
The wide variety of different vestibular conditions in the age period between 31 and 50 years suggests the highest deterioration rate that occurs in this period. When these data are divided into smaller age group of 5-year intervals, as depicted in Fig. 5, hyperactive vestibular function are absent at ages above 41 years and all showed some degree of impairment above the age of 45. The strongest decline starts at 36 years and ends around 60 years. BV was registered in 46, 68.8 and 77% of all measurements in affected subjects of the next three age groups (46–50, 51–55 and 56–60 years, respectively). This suggests that the vestibular deterioration starts around the age of 36 years, whereas 100% completion is achieved about 60 years. For comparison, Bischoff’s annual vestibular deterioration (AVD) rate of time constant ‘T’ was 1.5 s per year, with age of onset at 34 years and BV achieved at about 49 years [34]. Here also, a proportionally underrepresentation of presymptomatic subjects is evident, since none of them were investigated under the age of 21 years, while only 5 and 11 measurements were inventoried in the 3rd and 4th decade, respectively.
Vestibular dysfunction as function age (5-year interval). The vast majority of vestibular function was measured on symptomatic carriers aged 55 years or more. The younger carriers (< 30 years) are largely underrepresented. The maximal decline rate is noted between 36 and 55 years of age, whereas carriers aged 56 and older have reached advanced stages of bilateral vestibular dysfunction
Discussion
DFNA9 is caused by no less than 24 different mutations in COCH and it has been found to originate in four different continents, except for Africa. This suggests the prevalence of COCH mutations may still be underestimated. It also confirms that COCH plays an important role in human inner ear.
The three main limitations for the comparison of the audiological data were the following: the tendency of successively reusing identical study populations of the same family pedigrees in consecutive papers over a period of time (7 out of 11), absence of audiometric data of any kind (3 out of 11), the use of different PTA indices without displaying the raw data (2 out of 11) and different data assessments (linear regression (n = 3), dose–response curve (n = 3), box plots (n = 1). The problems to overcome when assessing of the vestibular data were the differences in test method, choice of parameter and data processing.
The reuse of audiometric data of the same affected subjects in successive studies holds the risk of double or even triple registration of identical measurements in a single individual, resulting in the distortion of figures representing one or more age-related subgroups of patients compared to other subgroups, due to absence of unequivocal data in many records. Besides raw data restricted to those measured at the best ear of the subjects by some, the superimposition of a series of numerous audiograms by others were also potentially confusing. Other limitations, such as the absence of the correction of the SNHL for individual 95th percentile threshold values of presbyacusis in relation to age and sex (ISO 7029) or the omission of mentioning the total number of included individuals in a few records, further added to the complexity of the review [5, 26, 30, 31, 36].
Nonetheless, the abundancy of all the available audiometric data of patients suffering from DFNA9 when compared to other hereditary SNHL must be exploited. For these reasons all available audiometric data from all 11 selected studies were collected to compare the cumulative figures with those already available in the literature (Table 1; Fig. 2).
As a final note on the audiometric assessment, a clear underrepresentation of young subjects (38 versus 207) suggests proportionally scarce enrollment of presymptomatic P51S COCH carriers in the available studies.
Figures 4 and 5, which represent data of 136 measurements, also illustrates, however, that BV may only be observed at 60 years instead of 49 years, even though the decline of vestibular function remains more severe than the SNHL [34]. Because the high-frequency SNHL already starts at 32.8 years, vestibular dysfunction may not precede the SNHL by 9 years, but rather start simultaneously. This discrepancy may be due the fact that the statistical estimates were based on one single parameter (time constant ‘T’), which may not have adequate sensitivity to detect early (unilateral) vestibular dysfunction, even though it is considered very suitable in bilateral vestibulopathy [54]. The proportionally small sample size of presymptomatic P51S carriers may limit our insight into the vestibular deterioration rate in the group.
Rotatory chair and caloric response test protocols involve the stimulation of the horizontal (lateral) SCC, however, at completely different frequencies (0.002–0.004 Hz in caloric tests) [55,56,57]. The sinusoidal harmonic acceleration test (SHAT) uses only low frequency sensitivity (0.005–0.64 Hz), whereas the VST involve more high-frequency components closer to those of (video) head impulse test (vHIT) [55]. In case of unilateral or bilateral vestibular dysfunction, SHAT has higher sensitivity, however, both VST and SHAT show abnormal response in 53% of BV [58]. Caloric response tests, in contrast, are less sensitive for BV mainly due to missing initial physiological values as a reference [58]. Important factors for the interpretation of vestibular function are the inherent limitations of caloric and both VST and SHAT tests as well as the necessity of carrying out normative studies for each vestibular laboratory. With the exception of one record, all phenotype studies of P51S COCH carriers were conducted at least 10 years ago. Vestibular evoked myogenic potentials (VEMP) and (video) head impulse test (vHIT) were not implemented at that time, whereas they are now incorporated as part of the vestibular testing battery worldwide [59, 60]. Except for VEMP tests, which assess the otolith organs, all other tests use a variety of different sensitivity components of one of more SCC (from 0 to 6 Hz), which are covering the optimal frequency sensitivity range of the SCC [55, 59].
For those reasons, and in light of the limitations that are inherent to the rotatory chair and caloric vestibular tests, rotatory chair or caloric test are to be considered complementary with other vestibular test and are not to be seen as the only test in the diagnosis of BV or unilateral vestibulopathy.
A multicentric prospective cross-sectional study is needed, involving symptomatic as well as presymptomatic P51S carriers and using comprehensive audiometric and vestibular test battery, including VNG, VEMP and vHIT tests, to gain new insights and more accurate figures on cochleovestibular deterioration meanwhile avoiding the limitations described in this section.
Conclusion
The present review of all available phenotype studies of the most prevalent COCH mutation in the Low Countries (the P51S variant) confirms the late onset of the SNHL (43 years), characterized with an annual threshold deterioration of 3 dB HL per year and with profound SNHL at 76 years on average, whereas vestibular dysfunction was first observed around 34 years and BV was achieved from about 41 to 60 years. Hence, high-frequency SNHL already starts at 32.8 years, which is earlier than the estimated onset of the vestibular signs.
The main limitations are the fact that presymptomatic P51S carriers are clearly underrepresented in the literature, both in the assessment of SNHL as well as the peripheral vestibular function. Moreover, the deterioration rates of the vestibular function were only calculated by means of the time constant ‘T’ derived from the velocity-step test in the overwhelming majority of the data.
Further work is needed to highlight the subclinical period in this late onset progressive trait affecting cochleovestibular function, involving all (presymptomatic and symptomatic) P51S carriers while using state-of-the-art vestibular testing (incl. VEMP and vHIT).
References
Sommen M, Wuyts W, Van Camp G (2017) Molecular diagnostics for hereditary hearing loss in children. Expert Rev Mol Diagn 17(8):751–760
Shearer AE, Hildebrand MS, Smith RJH (1993–2019) Hereditary hearing loss and deafness overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) Genereviews (R), University of Washington, Seattle
Verhagen WI, Huygen PL, Joosten EM (1988) Familial progressive vestibulocochlear dysfunction. Arch Neurol 45(7):766–768
Manolis EN et al (1996) A gene for non-syndromic autosomal dominant progressive postlingual sensorineural hearing loss maps to chromosome 14q12–13. Hum Mol Genet 5(7):1047–1050
Fransen E et al (1999) High prevalence of symptoms of Meniere’s disease in three families with a mutation in the COCH gene. Hum Mol Genet 8(8):1425–1429
Verhagen WI et al (2000) Familial progressive vestibulocochlear dysfunction caused by a COCH mutation (DFNA9). Arch Neurol 57(7):1045–1047
De Belder J et al (2017) Does otovestibular loss in the autosomal dominant disorder DFNA9 have an impact of on cognition? A systematic review. Front Neurosci 11:735
Moher D et al (2009) Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 89(9):873–880
Robertson NG et al (1998) Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat Genet 20(3):299–303
Robertson NG et al (2001) Inner ear localization of mRNA and protein products of COCH, mutated in the sensorineural deafness and vestibular disorder, DFNA9. Hum Mol Genet 10(22):2493–2500
Robertson NG et al (2014) Cochlin in normal middle ear and abnormal middle ear deposits in DFNA9 and Coch (G88E/G88E) mice. J Assoc Res Otolaryngol 15(6):961–974
Robertson NG et al (2006) Cochlin immunostaining of inner ear pathologic deposits and proteomic analysis in DFNA9 deafness and vestibular dysfunction. Hum Mol Genet 15(7):1071–1085
Robertson NG et al (2003) Subcellular localisation, secretion, and post-translational processing of normal cochlin, and of mutants causing the sensorineural deafness and vestibular disorder, DFNA9. J Med Genet 40(7):479–486
Robertson NG et al (2008) A targeted Coch missense mutation: a knock-in mouse model for DFNA9 late-onset hearing loss and vestibular dysfunction. Hum Mol Genet 17(21):3426–3434
Grabski R et al (2003) Mutations in COCH that result in non-syndromic autosomal dominant deafness (DFNA9) affect matrix deposition of cochlin. Hum Genet 113(5):406–416
Liepinsh E et al (2001) NMR structure of the LCCL domain and implications for DFNA9 deafness disorder. Embo j 20(19):5347–5353
Trexler M, Banyai L, Patthy L (2000) The LCCL module. Eur J Biochem 267(18):5751–5757
Bae SH et al (2014) Identification of pathogenic mechanisms of COCH mutations, abolished cochlin secretion, and intracellular aggregate formation: genotype–phenotype correlations in DFNA9 deafness and vestibular disorder. Hum Mutat 35(12):1506–1513
Yao J et al (2010) Role of protein misfolding in DFNA9 hearing loss. J Biol Chem 285(20):14909–14919
Py BF et al (2013) Cochlin produced by follicular dendritic cells promotes antibacterial innate immunity. Immunity 38(5):1063–1072
Eavey RD et al (2000) Mutations in COCH (formerly Coch5b2) cause DFNA9. Adv Otorhinolaryngol 56:101–102
Ikezono T et al (2001) Identification of the protein product of the Coch gene (hereditary deafness gene) as the major component of bovine inner ear protein. Biochim Biophys Acta 1535(3):258–265
Khetarpal U (2000) DFNA9 is a progressive audiovestibular dysfunction with a microfibrillar deposit in the inner ear. Laryngoscope 110(8):1379–1384
Merchant SN, Linthicum FH, Nadol JB Jr (2000) Histopathology of the inner ear in DFNA9. Adv Otorhinolaryngol 56:212–217
Nagy I, Trexler M, Patthy L (2008) The second von Willebrand type A domain of cochlin has high affinity for type I, type II and type IV collagens. FEBS Lett 582(29):4003–4007
Verhagen WI et al (2001) Hereditary cochleovestibular dysfunction due to a COCH gene mutation (DFNA9): a follow-up study of a family. Clin Otolaryngol Allied Sci 26(6):477–483
de Kok YJ et al (1999) A Pro51Ser mutation in the COCH gene is associated with late onset autosomal dominant progressive sensorineural hearing loss with vestibular defects. Hum Mol Genet 8(2):361–366
Bom SJ et al (1999) Progressive cochleovestibular impairment caused by a point mutation in the COCH gene at DFNA9. Laryngoscope 109(9):1525–1530
Fransen E, Van Camp G (1999) The COCH gene: a frequent cause of hearing impairment and vestibular dysfunction? Br J Audiol 33(5):297–302
Verstreken M et al (2001) Hereditary otovestibular dysfunction and Meniere’s disease in a large Belgian family is caused by a missense mutation in the COCH gene. Otol Neurotol 22(6):874–881
Lemaire FX et al (2003) Progressive late-onset sensorineural hearing loss and vestibular impairment with vertigo (DFNA9/COCH): longitudinal analyses in a belgian family. Otol Neurotol 24(5):743–748
Bom SJ et al (2001) Speech recognition scores related to age and degree of hearing impairment in DFNA2/KCNQ4 and DFNA9/COCH. Arch Otolaryngol Head Neck Surg 127(9):1045–1048
Bom SJ et al (2003) Cross-sectional analysis of hearing threshold in relation to age in a large family with cochleovestibular impairment thoroughly genotyped for DFNA9/COCH. Ann Otol Rhinol Laryngol 112(3):280–286
Bischoff AM et al (2005) Vestibular deterioration precedes hearing deterioration in the P51S COCH mutation (DFNA9): an analysis in 74 mutation carriers. Otol Neurotol 26(5):918–925
Bischoff AM et al (2007) Vertical corneal striae in families with autosomal dominant hearing loss: DFNA9/COCH. Am J Ophthalmol 143(5):847–852
Hildebrand MS et al (2009) Mutation in the COCH gene is associated with superior semicircular canal dehiscence. Am J Med Genet A 149A(2):280–285
Alberts B et al (2018) Bayesian quantification of sensory reweighting in a familial bilateral vestibular disorder (DFNA9). J Neurophysiol 119(3):1209–1221
Fransen E et al (2001) A common ancestor for COCH related cochleovestibular (DFNA9) patients in Belgium and The Netherlands bearing the P51S mutation. J Med Genet 38(1):61–65
de Varebeke SP et al (2014) Focal sclerosis of semicircular canals with severe DFNA9 hearing impairment caused by a P51S COCH-mutation: is there a link? Otol Neurotol 35(6):1077–1086
Kemperman MH et al (2002) DFNA9/COCH and its phenotype. Adv Otorhinolaryngol 61:66–72
Kemperman MH et al (2005) Audiometric, vestibular, and genetic aspects of a DFNA9 family with a G88E COCH mutation. Otol Neurotol 26(5):926–933
Vermeire K et al (2006) Good speech recognition and quality-of-life scores after cochlear implantation in patients with DFNA9. Otol Neurotol 27(1):44–49
Cremers CW et al (2005) From gene to disease; a progressive cochlear-vestibular dysfunction with onset in middle-age (DFNA9). Ned Tijdschr Geneeskd 149(47):2619–2621
JanssensdeVarebeke SPF et al (2018) Bi-allelic inactivating variants in the COCH gene cause autosomal recessive prelingual hearing impairment. Eur J Hum Genet 26(4):587–591
Parzefall T et al (2018) Identification of a rare COCH mutation by whole-exome sequencing: implications for personalized therapeutic rehabilitation in an Austrian family with non-syndromic autosomal dominant late-onset hearing loss. Wien Klin Wochenschr 130(9–10):299–306
ISO 7029:2017 (2017) Statistical distribution of hearing thresholds related to age and gender. Acoustics. https://www.iso.org/standard/42916.html
Collin RW et al (2006) Identification of a novel COCH mutation, G87W, causing autosomal dominant hearing impairment (DFNA9). Am J Med Genet A 140(16):1791–1794
Pauw RJ et al (2007) Clinical characteristics of a Dutch DFNA9 family with a novel COCH mutation, G87W. Audiol Neurootol 12(2):77–84
Pauw RJ et al (2007) Phenotype description of a novel DFNA9/COCH mutation, I109T. Ann Otol Rhinol Laryngol 116(5):349–357
Huygen PLM, Cremers PR (2003) CWRJ, Characterizing and distinguishing progressive phenotypes in nonsyndromic autosomal dominant hearing impairment. Audiol Med 1:37–46
Fernandez C, Goldberg JM (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34(4):661–675
Van Der Stappen A, Wuyts FL, Van De Heyning PH (2000) Computerized electronystagmography: normative data revisited. Acta Otolaryngol 120(6):724–730
Usami S et al (2003) Mutations in the COCH gene are a frequent cause of autosomal dominant progressive cochleo-vestibular dysfunction, but not of Meniere’s disease. Eur J Hum Genet 11(10):744–748
Huygen PL, Verhagen WI, Nicolasen MG (1989) Correlation between velocity step and caloric response parameters. Acta Otolaryngol 108(5–6):368–371
Maes L et al (2008) Normative data and test-retest reliability of the sinusoidal harmonic acceleration test, pseudorandom rotation test and velocity step test. J Vestib Res 18(4):197–208
Jongkees LB (1973) Vestibular tests for the clinician. Arch Otolaryngol 97(1):77–80
Wuyts FL et al (2007) Vestibular function testing. Curr Opin Neurol 20(1):19–24
Hain TC, Cherchi M, Yacovino DA (2013) Bilateral vestibular loss. Semin Neurol 33(3):195–203
Maes L et al (2017) Comparison of the motor performance and vestibular function in infants with a congenital cytomegalovirus infection or a connexin 26 mutation: a preliminary study. Ear Hear 38(1):e49–e56
Curthoys IS (2010) A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol 121(2):132–144
Funding
There are no funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
The study is a systematic review, and therefore, with a retrospective design. Therefore, informant consent was not applicable for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
JanssensdeVarebeke, S., Topsakal, V., Van Camp, G. et al. A systematic review of hearing and vestibular function in carriers of the Pro51Ser mutation in the COCH gene. Eur Arch Otorhinolaryngol 276, 1251–1262 (2019). https://doi.org/10.1007/s00405-019-05322-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-019-05322-x