Abstract
Objectives
To present the outcomes of our case series of head and neck necrotizing fasciitis (HNNF) in which vacuum-assisted closure (VAC) is used in most of the cases in the treatment.
Methods
Case series in a tertiary referral center.
Results
Eleven patients were treated for HNNF between January 2008 and January 2017. Patients were two females and nine males, the mean age was 57.1. Oral cavity and tracheotomy/tracheostomy sites were the main aetiological foci of the infection. Three patients were treated with aggressive debridements and conventional dressing, whereas eight patients were treated with incision and exploration followed by limited skin excisions and VAC dressing. The mean number of surgical debridements was 2.3. The mean length of hospital stay was 41.8 days. Complications were observed in all patients except one. The mortality rate of HNNF in our series was 18%. The cause of death was severe sepsis and multi-organ failure in one case and mediastinitis followed by respiratory distress syndrome in the other case.
Conclusion
HNNF is still a mortal disease and surgical debridements are crucial. The current study is the only case series in the literature in which VAC treatment was used in consecutive cases of HNNF. VAC treatment can play a major role in the post-operative care of HNNF patients. It reduces the amount of excised skin during debridements and stimulates wound healing. VAC treatment may be included in the treatment protocol of HNNF alongside surgical debridements and medical therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After the first encounter with necrotizing fasciitis (NF), the clinician will probably have a lifetime memory of the disease. This catastrophic and mostly polymicrobial bacterial [1] infection is characterized by a rapid and destructive spread within the subcutaneous tissue and along the superficial fascial planes. Even an insect bite can be the cause of NF [2]. Bacterial toxins and enzymes facilitate the spread of the pathogen and cause necrosis of subcutaneous tissue and fascia [3]. It is reported that necrosis of soft tissues can progress as fast as 1 in. an hour [4]. After the invasion and blockage of blood vessels by pathogens, vasoconstriction and thrombosis occur, which cause skin necrosis as well as diminished delivery of antibiotics and systemic treatments to the affected area [5]. Crepitation of the soft tissue as a result of subcutaneous bacterial gas production is an important and specific finding which unluckily occurs in the late stages of the disease.
NF usually appears in the abdominal wall and limbs. The incidence of Head and Neck NF (HNNF) is extremely low (approximately 4 of 1000 cases of necrotizing fasciitis per year) [6]. Mostly, the oral cavity is the origin of HNNF. The clinical course is characterized by a drastic hyperacute sepsis and a mortality rate of up to 70% [7]. As a consequence of this chain of pathologic processes and high mortality rates, prompt and repeated aggressive surgical debridements are the main choice of treatment. Debridements are followed by systemic antibiotherapy. The benefits of hyperbaric oxygen (HBO) therapy and intravenous immunoglobulin (IVIG) therapy are controversial [8]. Recent case reports have pointed out the positive effect of vacuum-assisted closure (VAC) on HNNF, which facilitates wound healing and stimulates the formation of new well-vascularized granulation tissue [9].
The aim of this study is to present our clinical experience in the diagnosis and management of HNNF and to emphasize the role of VAC, which has been routinely used in the post-operative treatment of HNNF in our department for the last 10 years.
Materials and methods
The medical records of 11 consecutive patients, who were treated for HNNF between January 2008 and January 2017 in our institution, were retrospectively reviewed. Informed consents were obtained from all individual participants included in the study. The diagnosis of necrotizing fasciitis was made according to clinical and computed tomography (CT) scan findings and the diagnosis was histologically confirmed by necrosis of the fascia and subcutaneous tissue. Complete blood count, biochemical studies (including C-reactive protein) and CT scans of neck and chest were performed preoperatively and post-operatively in all cases. Samples of the necrotic tissue were also sent for histopathology and microbiological cultures.
Demographic data of the patients, etiological focus, bacteriology, co-morbidities, Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scores [10], time period between the outset of symptoms and surgical intervention, number of surgical procedures, presence of VAC therapy, length of intensive care unit (ICU) and hospital stay, duration of antibiotic treatment, accompanying complications, presence of tracheotomy, prognosis and mortality were evaluated.
Results
Demographic data and pre-operative findings of patients are summarized in Table 1. The clinical courses are summarized in Table 2.
The mean age of the patients was 57.1 ± 9.9 years. The patients consisted of two females and nine males. Most of the patients had co-morbidities including diabetes mellitus (DM) and malignancies. Only one patient had no underlying predisposition to infection. Oral cavity and tracheotomy/tracheostomy sites were the main etiological foci. Two patients had tracheostomies as they had been operated for laryngeal cancer previously, and one of these patients had a recurrent tumor at the stoma. Two patients had tracheotomies due to severe radionecrosis and larynx edema which occurred after radiotherapy treatment. One of these patients was operated for submandibular gland malignancy, whereas the other was operated for laryngeal cancer.
Wound bacteriology showed both streptococcal species and polybacterial colonization. LRINEC scores were greater than six in nine cases and equal or greater than eight in four cases. The time period between patients’ admission to the hospital and surgical intervention was shorter than 48 h except for one patient, whose differential diagnosis was challenging. This patient did not show typical signs of HNNF and was treated for a deep neck infection for 2 days. The mean number of surgical debridements was 2.3. In three cases without VAC dressing, the surgical intervention consisted of the exploration of deep neck spaces and debridement of necrotic tissue and skin until the healthy bleeding tissue was reached. Conventional gauze dressings were changed twice a day. In eight cases with VAC dressing, a limited debridement was applied: single or multiple incisions were made to explore subcutaneous tissue and fascia. Necrotic subcutaneous tissues and secretions were washed out and cleaned, but only totally necrotic skin portions were removed and any discolored or edematous skin areas were kept in place. Afterwards, VAC dressing was applied to the subcutaneous space and was changed every 2 days. Subsequent debridements were performed if necessary.
In our treatment protocol, a portable VAC therapy device was used (Vac Veraflo™, KCl International, San Antonio, TX, USA). The foam of the dressing was cut and placed according to the shape of the wound and wound edges were covered with the drape to block air inflow. In cases with a tracheotomy, it can be challenging to create a closed system without air inflow. In this occasion, a biocompatible foam was used to seal the edges of the dressing and the tracheostomy cannula. The VAC device was set to intermittent therapy at − 100 mmHg, cycling at 5 min on and 2 min off. The dressing was changed every 2 days after initial placement. The decision to end VAC treatment was based mainly on the clinical examination of the wound. If the amount of granulation tissue was considered to be sufficient, the wound was primarily closed or patients were referred to the department of plastic and reconstructive surgery for reconstruction.
The mean length of hospital stay was 41.8 days. Four patients required ICU care because of poor general conditions. Other patients were treated in the ENT department under close follow-up.
The initial choice of antibiotics was varied among the patients as they had diverse etiological focuses and accompanying complications. Initial regime consisted of piperacillin–tazobactam and metronidazole in non-complicated cases. The management strategies were adjusted by the infectious disease specialists according to clinical response and culture results. The spectrum of antibiotherapy included ceftriaxone, vancomycin, imipenem, teicoplanin, and linezolid. The mean duration of antibiotic treatment was 36.1 days. None of the patients received intravenous immunoglobulin combined with antibiotherapy.
Complications occurred in all patients except two. The most dangerous complication of HNNF: necrotizing mediastinitis (NM), was diagnosed in four patients. NM was classified based on the study of Endo et al. [11]. One case had Type I (localized NM above the carina and the innominate artery) mediastinitis, whereas other three cases had Type IIa (diffuse NM with extension to the lower anterior mediastinum) mediastinitis. These cases were treated with mediastinotomy and pleural drainage, performed by thoracic surgeons.
Liver enzyme elevation and leukopenia were detected in two patients and were considered adverse effects of antibiotic therapy. They showed spontaneous regression after the alteration of the antibiotic regimen.
After the diagnosis of HNNF, a tracheotomy was performed in three patients because of airway obstruction. Two patients already had tracheotomies and two patients had tracheostomies. Other patients did not require an airway intervention. Decannulation was achieved before discharge in both patients.
Only one patient received HBO treatment as an adjunct therapy. HBO was applied in a multiple chamber 2.8 atmosphere pressure for 60 min for 10 days after the first surgery.
The mortality rate of HNNF in our series was 18%. The cause of death was severe sepsis and multi-organ failure in one patient. NM with massive pleural effusion followed by respiratory distress syndrome was the cause of death in the other patient.
Discussion
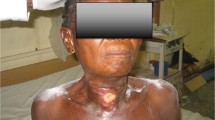
Prompt diagnosis and surgical debridements are crucial for the management of HNNF. Recurrent debridements are often necessary to clean necrotic tissues and secretions. They also help to evaluate the clinical course of the wound and the infection. Mao et al. defined debridement as a wide incision, followed by an adequate exploration of deep neck spaces and removal of necrotic tissue until healthy, bleeding tissue is encountered [12]. We performed this procedure in three patients in which VAC treatment was not used. Large skin and soft tissues were removed during these procedures. Conventional dressings were applied and changed twice daily until healthy granulation tissue covered the wound (Fig. 1).
Images of a head and neck necrotizing fasciitis (HNNF) patient at the time of diagnosis (a, b) (large portions of skin and subcutaneous tissue were already necrotic before the patient was referred to our hospital), intra-operative image during the first debridement (c), the wound and granulation tissue formation after 25 days of conventional dressing (d)
Argenta et al. described VAC therapy as a method which combines the benefits of both closed and open wound treatment, protects the wound against contamination and provides an optimal physiologic environment for tissue repair [13]. VAC therapy also stimulates tissue growth and plays a major role in cleaning accumulated secretions from the wound, which is essential in the treatment of HNNF. Although our study did not include the sufficient number of cases to compare the outcomes of VAC treatment statistically, we observed an apparent difference in wound healing between cases with and without VAC. Formation of healthy granulation tissue may be achieved faster with VAC. Pre-operative, intra-operative and post-operative 10th day images of an HNNF patient with VAC treatment are shown in Fig. 2.
Images of a head and neck necrotizing fasciitis (HNNF) patient at the time of diagnosis (a), the wound at initial debridement (the “dishwasher pus” is evident) (b), the wound after 2 days of vacuum-assisted closure (VAC) treatment (c), the wound and granulation tissue formation after 10 days of VAC treatment (d)
The second benefit of VAC treatment is the amount of excised skin. It is clear that all necrotic skin and soft tissues should be removed in the treatment of HNNF. In contrast to aggressive surgical debridements, less invasive debridement procedures such as incision drainage can be performed with VAC treatment. In discolored and edematous skin portions, the VAC dressing can be placed subcutaneously after the exploration and cleaning of the wound. This procedure reduces the amount of excised skin drastically and helps cutaneous and subcutaneous tissues sustain their vitality, especially if the patient is diagnosed in the early stages of the disease. The clinical course of a patient who was treated with wide incisions and extensive exploration followed by VAC dressing is shown in Fig. 3. Limited skin excisions also help to avoid long-term complications of skin reconstruction, such as contractures, which are likely to be more often in patients with large defects. The contracted wound of the patient in Fig. 1 1 year after surgery is shown in Fig. 4.
Images of a tracheotomy-originated head and neck necrotizing fasciitis (HNNF) patient at initial diagnosis (a), vacuum-assisted closure (VAC) dressing in use (b), healed wound before closure (c), primarily closed wound (d). With the help of VAC treatment, limited skin excisions were performed and the wound was suitable for primary closure, skin grafts were not necessary
VAC in the treatment of HNNF was presented in the case reports of Novelli et al. and Oczenski et al. [9, 14]. Both authors evaluated VAC as a valuable tool for post-operative wound management. To our knowledge, our study presents the only case series in which VAC treatment was routinely used in 72% (8/11) of cases. Our findings also concur that this technique could be effective in the management of HNNF.
Sumi et al. described an alternative procedure for the treatment of HNNF in which the integrity of the skin is preserved: percutaneous catheter drainage under real-time sonographic guidance [15]. They reported that this non-operative management may be used as an effective treatment for HNNF with and without mediastinitis. However, it is obvious that this procedure is safe only in experienced hands and it is harder to handle intra-operative complications compared to open surgery.
The most common nidus of HNNF is the oral cavity, especially the dental infections [16, 17]. Alongside these regions, we observed that tracheotomies and tracheostomies, which were not mentioned as the possible foci in previous reports, can also be the nidus of HNNF. Especially in cases with a history of head and neck malignancy or radiation therapy, the infections at these regions may lead to HNNF.
The LRINEC score was described by Wong et al. in 2004 as a robust index that is capable of detecting early cases of necrotizing fasciitis [10]. This scoring system uses C-reactive protein, total white cell count, hemoglobin, sodium, creatinine, and glucose levels. The maximum score is 13; a score of ≥ 6 raises the suspicion of necrotizing fasciitis and a score of ≥ 8 is strongly predictive of this disease. In our series, 8 cases out of 11 had LRINEC scores ≥ 6 and 4 of these cases had LRINEC score ≥ 4. Although this scoring system was first introduced as a practical tool that helps to diagnose HNNF, the meta-analysis of Fernando et al. confirmed that the LRINEC score should not be used to rule out necrotizing fasciitis because of the poor sensitivity [18]. In our series, this scoring system was not used to diagnose or to rule out HNNF. The scores were calculated in each case as preoperative blood works already include the parameters, and these parameters may also be used as an indicator of the severity of sepsis.
CT scan was the main imaging modality in our series and it has superior sensitivity and specificity to plain radiography in diagnosing HNNF. Fernando et al. performed a sensitivity analysis for CT and the authors concluded that CT scan had a relatively strong accuracy in diagnosis of HNNF [18]. We used CT scans in all our cases not only to diagnose HNNF but also to evaluate the presence of complications, especially necrotizing mediastinitis. Although Martinez et al. [19] stated that a negative intravenous contrast-enhanced CT scan could reliably rule out the need for surgical intervention in patients with initial suspicion of necrotizing soft tissue infections, still clinical evaluation is the mainstay of assessing HNNF.
In cases with negative CT scan findings, point-of-care (POC) ultrasound (US) was used by Kehrl et al. as a diagnostic tool to help the clinician make the early diagnosis [20]. Yen et al. evaluated the diagnostic characteristics of POC US in patients with suspected NF and reported a sensitivity of 88.2% and a specificity of 93.3% [21]. In contrast, Malghem et al. do not recommend US in adults with possible NF, as the infiltration of the hypodermis blocks ultrasound transmission [22]. In our series, CT scan was used as the main diagnostic tool in all cases. Therefore, we did not use the US routinely. We believe, imaging techniques may help to confirm soft tissue involvement but it should never delay prompt surgical intervention in these patients.
The knowledge of the diagnosis and treatment of HNNF has increased in the last decades. Consequently, the mortality rates showed a tendency to decrease. Lanisnik et al. reported a mortality rate of 2% (1/34) in their series of 34 HNNF cases [17]. Mao et al. reported the mortality rate of cranio-cervical NF patients with thoracic extension as 40% (4/10), whereas mortality of CCNF patients without thoracic extension was 0% (0/10) [12]. Both authors concluded that patients with thoracic extension had poorer outcomes. Sepsis followed by multiple organ failure was the main cause of mortality in all reports. The mortality rate in our series was 18% and the causes of mortality were similar to other reports.
The main limitation of our study was the lack of a statistical analysis between two groups of cases. This could help to compare the effect of VAC treatment. But considering the rarity of the disease and the presence of various co-morbidities and/or complications in these patients, it is difficult to create two homogenous groups and to perform a meaningful analysis. We believe this goal may be achieved with a multicenter collaboration.
Conclusions
Necrotizing fasciitis of the head and neck is still a mortal disease. Early diagnosis and surgical interventions are crucial for the treatment. The benefits of adjunctive therapies such as HBO and IVIG are not yet clear. The current study presents the only case series in which VAC treatment was routinely used as an adjunctive therapy in eight consecutive cases. Considering the pathophysiology of the disease, we observed that VAC treatment may play a major role in the post-operative care of these patients. It is easy to place and it may even be used in the ICU. It reduces the amount of excised skin during debridements and stimulates wound healing. With regard to these findings and our clinical experience, we came to the conclusion that VAC may be considered in the treatment protocol of HNNF in addition to surgical debridements and medical therapy.
References
Wilson B (1952) Necrotizing fasciitis. Am Surg 18:416–431
Fernando DM, Kaluarachchi CI, Ratnatunga CN (2013) Necrotizing fasciitis and death following an insect bite. Am J Forensic Med Pathol 34:234–236
Wilkerson R, Pauli W, Coville FV (1987) Necrotizing fasciitis: review of the literature and case report. Clin Orthop Relat Res 216:187–192
Fink A, DeLuca G (2002) Necrotizing fasciitis: pathophysiology and treatment. Dermatol Nurs 14:324–327
Kessenich CR, Bahl A (2004) Necrotizing fasciitis: understanding the deadly results of the uncommon ‘flesh-eating bacteria’. Am J Nurs 104:51–55
Stone HH, Martin J Jr (1972) Synergistic necrotizing cellulitis. Ann Surg 175:702–711
Lin C, Yeh FL, Lin JT, Ma H, Hwang CH, Shen BH, Fang RH (2001) Necrotizing fasciitis of the head and neck: an analysis of 47 cases. Plast Reconstr Surg 107:1684–1693
Jallali N, Whitney S, Butler PE (2005) Hyperbaric oxygen as adjuvant therapy in the management of necrotizing fasciitis. Am J Surg 189:462–466
Novelli G, Catanzaro S, Canzi G, Sozzi D, Bozzetti A (2014) Vacuum assisted closure therapy in the management of cervico-facial necrotizing fasciitis: a case report and review of the literature. Minerva Stomatol 63:135–144
Wong CH, Khin LW, Heng KS, Tan KC, Low CO (2004) The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 32:1535–1541
Endo S, Murayama F, Hasegawa T, Yamamoto S, Yamaguchi T, Sohara Y, Fuse K, Miyata M, Nishino H (1999) Guideline of surgical management based on diffusion of descending mediastinitis. Jpn J Thorac Cardiovasc Surg 47:14–19
Mao JC, Carron MA, Fountain KR, Stachler RJ, Yoo GH, Mathog RH, Coticchia JM (2009) Craniocervical necrotizing fasciitis with and without thoracic extension: management strategies and outcome. Am J Otolaryngol 30:17–23
Argenta L, Morykwas M (1997) Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plastic Surg 38:563–577
Oczenski W, Waldenberger F, Nehrer G, Kneifel W, Swoboda H, Schwarz S, Fitzgerald RD (2004) Vacuum-assisted closure for the treatment of cervical and mediastinal necrotizing fasciitis. J Cardiothorac Vasc Anesth 18:336–338
Sumi Y, Ogura H, Nakamori Y, Ukai I, Tasaki O, Kuwagata Y, Shimazu T, Tanaka H, Sugimoto H (2008) Nonoperative catheter management for cervical necrotizing fasciitis with and without descending necrotizing mediastinitis. Arch Otolaryngol Head Neck Surg 134:750–756
Krenk L, Nielsen HU, Christensen ME (2007) Necrotizing fasciitis in the head and neck region: an analysis of standard treatment effectiveness. Eur Arch Otorhinolaryngol 264:917–922
Lanisnik B, Cizmarevic B (2010) Necrotizing fasciitis of the head and neck: 34 cases of a single institution experience. Eur Arch Otorhinolaryngol 267:415–421
Fernando SM, Tran A, Cheng W, Rochwerg B, Kyeremanteng K, Seely AJE, Inaba K, Perry JJ (2018) Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC score: a systematic review and meta-analysis. Ann Surg. https://doi.org/10.1097/SLA.0000000000002774
Martinez M, Peponis T, Hage A et al (2018) The role of computed tomography in the diagnosis of necrotizing soft tissue infections. World J Surg 42:82–87
Kehrl T (2014) Point-of-care ultrasound diagnosis of necrotizing fasciitis missed by computed tomography and magnetic resonance imaging. J Emerg Med 47:172–175
Yen ZS, Wang HP, Ma HM, Chen SC, Chen WJ (2002) Ultrasonographic screening of clinically-suspected necrotizing fasciitis. Acad Emerg Med 9:1448–1451
Malghem J, Lecouvet FE, Omoumi P, Maldague BE, Vande Berg BC (2013) Necrotizing fasciitis: contribution and limitations of diagnostic imaging. Joint Bone Spine 80:146–154
Funding
The research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Balcı, M.K., Ciğer, E., Arslanoğlu, S. et al. Necrotizing fasciitis of the head and neck: our experience with vacuum-assisted closure therapy. Eur Arch Otorhinolaryngol 275, 2555–2562 (2018). https://doi.org/10.1007/s00405-018-5096-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5096-z