Abstract
The objective of the study was to examine the prognostic value of hypoxia-inducible factor-1α (HIF-1α), carbonic anhydrase-IX (CA-IX), cyclooxygenase-2 (COX-2), Ki-67, and erythropoietin receptor in patients with oral tongue squamous cell carcinoma. Immunohistochemical analysis of marker expression was performed on tissue samples from 25 patients with tongue squamous cell carcinoma. The Kaplan–Meier method, univariate and multivariate analyses, and the Cox proportional hazards model were used to examine associations between patient and tumor characteristics, and the immunohistochemical results and disease-specific survival. There was no association between the expression of the five markers and disease-specific survival, and there was no statistically significant difference in the hazards ratio according to postoperative radiotherapy. There was no correlation between marker expression and prognosis. There was no association between marker expression and radioresistance or disease-specific survival. Therefore, HIF-1α, CA-IX, COX-2, Ki-67, and erythropoietin receptor are not suitable prognostic markers for tongue squamous cell carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent progress in the field of targeted therapy has provided new insights into the treatment of head and neck squamous cell carcinoma (HNSCC). HNSCC cases with a similar histological grade and clinical stage often have a different prognosis, and recent studies suggest that these differences may be associated with the expression of specific immunohistochemical markers [1–3]. Therefore, the identification of immunohistochemical markers associated with the clinicopathological features of HNSCC is very important.
The clinical behavior of oral tongue squamous cell carcinoma (OTSCC) is highly variable; therefore, it is difficult to predict the prognosis of patients with the disease. In some cases, OTSCC can be cured by surgery; however, the disease is occasionally aggressive in nature. At present, there is a lack of reliable markers that predict the clinical course of OTSCC. Therefore, there is a need to identify markers associated with disease prognosis. Several immunohistochemical markers have been studied, but none are routinely used in clinical practice.
Here, we examined five immunohistochemical markers that are of potential prognostic significance for patients with OTSCC. These markers are hypoxia-inducible factor-1α (HIF-1α), carbonic anhydrase-9 (CA-IX), cyclooxygenase-2 (COX-2), Ki-67, and erythropoietin receptor (EPOR). The aim was to ascertain whether the expression of these markers influenced the outcome and survival of patients who underwent surgery as the primary treatment for OTSCC at a single center.
Materials and methods
Patients
Twenty-five patients with squamous cell carcinoma of the tongue, who were treated at the Department of Otolaryngology-Head and Neck Surgery at Gyeongsang University Hospital, Jinju, Korea between 1998 and 2009, were examined. Demographic and clinicopathologic data, including gender, age, and TNM category, were collected retrospectively from the patients’ charts.
Surgery was the primary treatment modality. Twenty-four patients (96 %) underwent partial glossectomy and one underwent total glossectomy. The neck was managed by supraomohyoid neck dissection in 12 (48 %) patients, modified radical neck dissection in eight (32 %), and radical neck dissection in one. Four patients (16 %) with early-stage tongue squamous cell carcinoma (the depth of invasion less than 4 mm) did not undergo neck dissection. Twenty-two (88 %) patients underwent ipsilateral neck dissection, whereas three (12 %) underwent bilateral neck dissection.
Eight patients (32 %) received postoperative radiotherapy. The indications for postoperative radiotherapy were a primary tumor larger than 4 cm (≥T3), a lymph node larger than 3 cm, and multiple metastatic lymph nodes (≥N2) (Table 1).
Tissue microarrays
Paraffin-embedded pretreatment biopsy specimens containing sufficient carcinoma cells for immunohistochemical staining were available. Core tissue biopsies (2 mm in diameter) were obtained from individual formalin-fixed and paraffin-embedded archival tissue (donor blocks) and arranged in a new recipient paraffin block using a trephine apparatus (Quick-RAY™, Unitma, Seoul, Korea). One tissue core from the most representative portion was analyzed in each case. The collection of specimens was approved by the Gyeongsang University Hospital Institution Review Board, Jinju, Korea.
Immunohistochemistry
Immunohistochemical staining was performed on 4 μm-thick tissue sections as follows. Sections were deparaffinized, rehydrated, and then incubated in 3 % H2O2 for 10 min to reduce nonspecific background staining caused by endogenous peroxidases. For epitope retrieval, specimens were placed in 10 mmol/L citrate buffer (pH 6.0) and heated in a microwave oven (700 W) for 20 min. The slides were then incubated with Ultra V Block (Lab Vision Corporation, Fremont, CA, USA) for 7 min at room temperature to block background staining, followed by incubation with rabbit polyclonal anti-COX-2 (Epitomics, Burlingame, CA, USA; dilution 1:100), mouse monoclonal anti-Ki-67 (DAKO, Carpinteria, CA, USA; dilution 1:1,000), rabbit polyclonal anti-CA-IX (Novus Biologicals, Littleton, CO, USA; dilution 1:1,000), rabbit polyclonal anti-HIF-1α (Epitomics, Burlingame, CA, USA; dilution 1:200), or rabbit polyclonal anti-EPOR (Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:100) antibodies at room temperature. Antibody binding was detected using the UltraVision LP detection system (Lab Vision Corporation) according to the manufacturer’s recommendations. Color development was performed with 3-3′-diaminobenzidine and the slides were counterstained with hematoxylin.
Assessment of protein expression
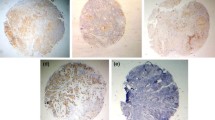
Antigen expression was examined by an investigator who was blinded to the clinical data. For COX-2, Ki-67, and HIF-1α, the samples were scored according to the percentage of positively stained cells as follows: 1+, 1–10 %; 2+, 11–50 %; 3+, 51–80 %; and 4+, >80 % of cancer cells stained. For CA-IX, the samples were scored as follows: 1+, 1–10 %; 2+, 11–30 %; 3+, 31–50 %; and 4+, >50 % of cancer cells stained. A cutoff point was used to categorize samples into low-expression and high-expression groups: negative, 1+, or 2+ staining = low expression; +3 and above = high expression. For EPOR, the samples were considered positive if 50 % or more cancer cells were stained. Less than 50 % stained cells were classed as negative (Fig. 1).
Statistical analysis
Locoregional control was analyzed using the Kaplan–Meier method, and prognostic factors were assessed using the log-rank test. Univariate analysis and the Cox proportional hazards model were used to analyze the association between patient and tumor characteristics, and immunohistochemical results and locoregional control.
Differences were considered statistically significant at P < 0.05. All statistical analyses were performed using the Statistical Package for Social Sciences, version 14.0.0 (SPSS, Chicago, IL, USA).
Results
Follow-up data
The clinicopathologic characteristics of the patients included in the study are shown in Table 1. The mean follow-up period was 56 months (range 11–116 months). Of the 25 patients enrolled, 5 died from the disease, 1 died of other causes, and 19 were alive at the last follow-up. The overall survival rate was 76 %. Five patients (20 %) presented with recurrent locoregional disease after primary treatment. There was nodal involvement in seven patients (28 %). Sixty-four percent of tumors were larger than 2 cm. Tumor depth was >0.5 cm in 21 patients (84 %) and >1 cm in 11 patients (44 %). Immunohistochemical staining of tissue microarrays showed that most tumors were negative or weakly positive for all five immunohistochemical markers.
None of the immunohistochemical markers predicts prognosis
Univariate Cox regression analysis showed that none of the immunohistochemical markers were predictive of disease-specific survival (Table 2). Kaplan–Meier survival curves showed no significant association between the expression of the five immunohistochemical markers and disease-specific survival (Fig. 2). There was no statistically significant association between disease-specific survival and other clinicopathological variables (age, gender, nodal involvement, tumor size and depth, and tumor differentiation) (Table 2). None of the immunohistochemical markers showed a significant association with disease-specific survival, regardless of whether a patient received postoperative radiotherapy (Table 3).
Discussion
Approximately, 275,000 new patients are diagnosed with cancer of the oral cavity every year, and the disease is estimated to cause 1.7 % of all cancer-related deaths worldwide [4, 5]. OTSCC is the most common cancer of the oral cavity (comprising 25–40 % of oral carcinomas) and is generally a disease of the elderly, with a peak incidence in the sixth and seventh decades of life [6]. The prognosis for OTSCC is significantly worse than that for similar lesions at other oral sites [7]. The tongue has a rich lymphatic network and a highly muscular structure, both of which make it the site most frequently associated with cervical metastasis of oral cancers [6]. Despite considerable advances in diagnostic and therapeutic techniques, the estimated 5 year overall survival rate for OTSCC patients is only 56 % [8].
The management of OTSCC is dependent upon the TNM staging system, which is based on clinical evaluation. The TNM stage, however, is not always sufficient to predict prognosis. For example, some small T1 tumors behave in an aggressive manner, leading to a surprisingly poor prognosis. Prognostic factors for OTSCC are important for clinicians because they can help to identify aggressive cancers and determine the most appropriate postoperative therapy. Thus, it would be of great benefit to identify more aggressive tumors at the time of diagnosis.
Several immunohistochemical markers are associated with poor clinical outcome in cancer patients. Of these, we examined HIF-1α, CA-IX, COX-2, Ki-67, and EPOR. These immunohistochemical markers have been suggested as possible prognostic markers for other HNSCCs [1–3].
Indeed, several studies have examined their role in OTSCC. For example, studies show that HIF-1α [9, 10], Ki-67, CA-IX, and EPOR [7, 11, 12] may predict the prognosis of patients with OTSCC. A study suggested that COX-2 expression was associated with prognosis in patients with tongue cancer [13], whereas another suggested that Ki-67 was not a prognostic factor for OTSCC [14]. Thus, it is possible that these markers are prognostic for OTSCC. However, the results of the present study suggest that none are prognostic for OTSCC.
Tumor hypoxia is a prognostic factor for locoregional control after radiotherapy. A large body of clinical evidence suggests that tumor hypoxia has a negative impact on the outcome of radiotherapy [2]. Several proteins are related to the transcriptional response to hypoxia, and these proteins are expressed in tumor tissues. These include HIF-1α and CA-IX, both of which are currently being discussed as ‘endogenous hypoxia-related markers’ [15]. Several studies show that HIF-1α and CA-IX are predictive of a poor outcome after radiotherapy for various types of cancer, including HNSCC [3, 16]. However, we found that none of the immunohistochemical markers examined, including HIF-1α and CA-IX, had a significant effect on disease-specific survival after postoperative radiotherapy.
Conclusion
HIF-1α, CA-IX, COX-2, Ki-67, and EPOR do not appear to have prognostic value in OTSCC, suggesting that OTSCC has biological characteristics that are distinct from those of other HNSCCs.
References
Schrijvers ML, van der Laan BF, de Bock GH, Pattje WJ, Mastik MF, Menkema L, Langendijk JA, Kluin PM, Schuuring E, van der Wal JE (2008) Overexpression of intrinsic hypoxia markers HIF1alpha and CA-IX predict for local recurrence in stage T1–T2 glottic laryngeal carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys 72:161–169
Moeller BJ, Richardson RA, Dewhirst MW (2007) Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev 26:241–248
Vordermark D, Brown JM (2003) Endogenous markers of tumor hypoxia predictors of clinical radiation resistance? Strahlenther Onkol 179:801–811
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2008) Estimates of worldwide burden of cancer in: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Suslu N, Hosal AS, Aslan T, Sozeri B, Dolgun A (2013) Carcinoma of the oral tongue: a case series analysis of prognostic factors and surgical outcomes. J Oral Maxillofac Surg 71:1283–1290
Gueiros LA, Coletta RD, Kowalski LP, Lopes MA (2011) Clinicopathological features and proliferation markers in tongue squamous cell carcinomas. Int J Oral Maxillofac Surg 40:510–515
Bettendorf O, Piffko J, Bankfalvi A (2004) Prognostic and predictive factors in oral squamous cell cancer: important tools for planning individual therapy? Oral Oncol 40:110–119
Liang X, Zheng M, Jiang J, Zhu G, Yang J, Tang Y (2011) Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncol 47:92–97
Dunkel J, Vaittinen S, Grenman R, Kinnunen I, Irjala H (2013) Prognostic markers in stage I oral cavity squamous cell carcinoma. Laryngoscope 123:2435–2441
Kim SJ, Shin HJ, Jung KY, Baek SK, Shin BK, Choi J, Kim BS, Shin SW, Kim YH, Kim JS, Oosterwijk E (2007) Prognostic value of carbonic anhydrase IX and Ki-67 expression in squamous cell carcinoma of the tongue. Jpn J Clin Oncol 37:812–819
Li HG, Li JS, Chen WL, Wang L, Wu DH, Lin ZY (2009) Prognostic significance of erythropoietin and erythropoietin receptor in tongue squamous cell carcinoma. Br J Oral Maxillofac Surg 47:470–475
Wang YH, Wu MW, Yang AK, Zhang WD, Sun J, Liu TR, Chen YF (2011) COX-2 Gene increases tongue cancer cell proliferation and invasion through VEGF-C pathway. Med Oncol 28(Suppl 1):S360–S366
Sittel C, Ruiz S, Volling P, Kvasnicka HM, Jungehulsing M, Eckel HE (1999) Prognostic significance of Ki-67 (MIB1), PCNA and p53 in cancer of the oropharynx and oral cavity. Oral Oncol 35:583–589
Jonathan RA, Wijffels KI, Peeters W, de Wilde PC, Marres HA, Merkx MA, Oosterwijk E, van der Kogel AJ, Kaanders JH (2006) The prognostic value of endogenous hypoxia-related markers for head and neck squamous cell carcinomas treated with ARCON. Radiother Oncol 79:288–297
Vaupel P, Mayer A (2007) Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 26:225–239
Conflict of interest
The authors declare no conflicts of interest, financial or otherwise.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. S. Hwa and O. J. Kwon made an equal contribution to the manuscript.
Rights and permissions
About this article
Cite this article
Hwa, J.S., Kwon, O.J., Park, J.J. et al. The prognostic value of immunohistochemical markers for oral tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol 272, 2953–2959 (2015). https://doi.org/10.1007/s00405-014-3254-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-3254-5