Abstract
Oral squamous cell carcinoma (OSCC) is the most common oral cancer. Hypoxia inducible factor (HIF) is involved in many malignant tumors’ growth and metastasis and upregulated by hypoxia, including oral cancer. Many studies have studied about the prognostic value of HIF expression in OSCC; however, they do not get the consistent results. Therefore, this study explored the correlation between the HIF expression and the prognosis of OSCC. It conducted a meta-analysis of relevant publications searched in the Web of Science, PubMed, and ISI Web of Knowledge databases. Totally, this study identified 12 relevant articles reporting a total of 1112 patients. This analysis revealed a significant association between increased risk of mortality (RR = 1.20; 95 % CI 0.74–1.95; I 2 85.4 %) and overexpression of HIFs. Furthermore, different HIF isoforms were associated with overall survival [HIF-1α (RR = 1.18; 95 % CI 0.66–2.11; I 2 87.2 %) and HIF-2α (RR = 1.40; 95 % CI 0.93–2.09; I2 0.0 %)]. These results show that overexpression of HIFs, regardless of whether the HIF-1α or HIF-2α isoforms are overexpressed is significantly associated with increased risk of mortality in OSCC patients. In this study, the funnel is symmetric, suggesting existed no publication bias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cancer is defined as any cancerous tissue growth located in the oral cavity and is now one of the most common malignancies [1]. OSCC constitutes about 90 % of oral malignancies [2]. Although the diagnostic techniques and treatment modalities have improved a lot, the prognosis of OSCC still remains poor, mainly because of the high rate of recurrence and the new malignant changes in the original field [2, 3]. Oral cancer patients are usually vulnerable since the visible nature of the disfigurement of the face because of the results from treatments [4], besides there are some other side-effects including swallowing problems and mucositis. Therefore, in this setting, it is of great importance to identify reliable prognostic indicators.
It is known that tumor hypoxia has a negative impact on the efficacy of radiotherapy and chemotherapy so it is a prognostic factor in solid tumors. Hypoxia inducible factors (HIFs), especially HIF-1 and HIF-2, are crucial characteristic markers which mediate cellular responses to hypoxic stress. HIF-1 is composed of a hypoxia inducible HIF-1α subunit and a constitutively expressed HIF-1β subunit which is a heterodimeric transcription factor [5]. HIF-1 is able to promote erythropoiesis, angiogenesis, anaerobic energy production, and some other adaptive processes to enable cells to survive under conditions of hypoxia [6]. Recently, some studies showed that HIF-2 plays a role in resistance to radiotherapy and chemotherapy [7].
Many studies have shown that high tumor expression of HIF-1 and HIF-2 suggested an adverse prognostic indicator in OSCC, but it is not a universal finding. Therefore, the present work tried to evaluate the prognostic significance of HIFs in OSCC.
Materials and methods
Identification and eligibility of relevant studies
Literature searches of the PubMed, Web of Science, and ISI Web of Knowledge databases were conducted using all possible combination of the following search terms: “HIF”, “hypoxia inducible factor”, “OSCC”, “Oral Squamous Cell Carcinoma”, “upper aerodigestive tract cancer”,“UADTC” “prognosis”. An upper date limit of Jan 12, 2016 was applied; no lower date limit was used. Furthermore, the analysis was focused on studies conducted in humans and only full-text articles were included. Conference abstracts, reviews, letters, case reports, or experiments conducted in animal models were excluded from the analysis. The reference lists of reviews and retrieved articles were searched manually.
Data extraction and management
Both investigators (Q. Jiang and WG. Xu) reviewed eligible studies and extracted data independently. Issues of controversy were settled by consensus between all authors. The following information was collected from each study: the first author’s name, year of publication, country, cancer type, patient sex and age, survival data, and HIF isoforms. We did not contact the author of the primary study to request the information.
Methodological assessment
The present work used the Newcastle–Ottawa quality assessment scale (NOS) [8] to access the methodological quality of the included studies. There were 0 to 9 stars which were designated as the lowest to highest quality. Studies awarded more than 6 stars including 6 stars, were regarded to be of high quality. Two researchers provided the scores independently and disagreements were settled by a consensus value for each item was achieved.
Statistical analysis
Relative risk (RR) with 95 % confidence intervals (CIs) was used to determine the strength of associations between the HIFs and the prognosis of OSCC. As hazard ratio (HR) was broadly equivalent to RR [9], HRs were directly considered as RRs. Odds ratios (ORs) were transformed into RRs using the formula RR = OR/[(1-P’)i + (P’ × OR)] where P stands for the incidence in control group [10].
Publication bias was evaluated by the linear regression asymmetry test described by Egger et al. [11]. All data were analyzed using STATA12.0 (Stata-Corp, College Station, TX, USA).
Results
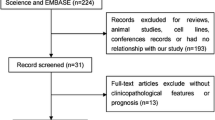
Figure 1 summarizes the study selection procedure. From an initial 392 potentially relevant articles, 12 were included in the final analysis (Fig. 1). Of these, all studies [12–19] assessed the relationships between HIF-1α expression and OSCC prognosis, while two studies [14, 17] evaluated the association of HIF-2α expression and prognosis.
Table 1 shows the methodological quality of the included studies, according to the Newcastle–Ottawa quality assessment scale. Table 2 show the characteristics of populations and cancer types of the studies included in the study.
A forest plot of the data revealed a significant association between overexpression of HIFs and increased risk of mortality (RR = 1.20; 95 % CI 0.74–1.95; I 2 85.4 %). Furthermore, the expression of different HIF isoforms was associated with overall survival (OS) [HIF-1α (RR = 1.18; 95 % CI 0.66–2.11; I 2 87.2 %) and HIF-2α (RR = 1.40; 95 % CI 0.93–2.09; I2 0.0 %)] (Fig. 2).
Figure 3 shows the funnel plot used to evaluate the publication bias. In the study, the funnel is symmetric, suggesting there exist no publication bias.
Discussion
Hypoxia is a basic characteristic of locally advanced solid tumors. It has emerged as a significant factor influencing the tumor (patho-)physiome because of its ability to promote tumor progression and resistance to radiotherapy and chemotherapy. Hypoxia, independent of standard prognostic factors such as tumor stage and nodal status, has been implicated as an adverse prognostic factor for cancer patients. Persistent hypoxia leads to the selection of genotypes promoting survival and accelerating tumor angiogenesis, metastasis and invasiveness, epithelial-to-mesenchymal transition, and suppressing immune reactivity [20]. Therefore, hypoxia is a prognostic factor for a variety of cancers including OSCC. HIF-1α and HIF-2α are two of the most significant transcription factors mediating the cellular response to hypoxia [21]. HIF-1α and HIF-2α are hydroxylated by specific prolyl hydroxylases in aerobic conditions, which promote the von Hippel–Lindau protein bind to the HIF-1α and HIF-2α. Von Hippel–Lindau protein becomes the substrate recognition module for the E3 ubiquitin ligase complex. After that, HIF-1α and HIF-2α protein is quickly degraded by ubiquitination. While in hypoxic conditions, prolyl hydroxylases becomes inactivated, so HIF-1α and HIF-2α become activated and steady. Then, it could activate the downstream target genes which regulate numbers of biological processes including cell proliferation and survival, angiogenesis, glucose metabolism, migration, and pH regulation [22]. The evidence about the fundamental role of HIFs in tumor progression is becoming more and more.
The present work found that in OSCC patients, HIF overexpression was significantly associated with increased risk of mortality, regardless of the HIF-1α or HIF-2α isoform expressed. Therefore, HIF expression is shown to be a prognostic marker in evaluating the severity of OSCC and guiding the decision of clinical treatment for patients.
However, some limitations of the present work should be noted. The relatively small size of the sample may increase the risk of bias in the study. Only two studies were included for the analysis of HIF-2α. More researches are needed to validate the relationship between HIF-2α and OSCC. Besides, oropharyngeal squamous cell cancer might also be associated with HIF [23].
Despite these limitations, the results of the present work suggest that HIF expression is a prognostic marker for OSCC patients. Although HIF-2α has been reported to suppress or promote different types of cancer, it is widely accepted HIF-2α leads to tumor angiogenesis [24–28]. Some studies showed that a hypoxic microenvironment could stabilize HIF-1α inside the cancer [29]. HIF-1α could lead to a rapid expression of VEGF, therefore promoting angiogenesis [29] and increasing tumor oxygenation and vascularization [18].
As a regulator of lymphangiogenesis and angiogenesis in OSCC, HIF-1α may play an important role in regional lymph node metastasis by a possible novel pathway involving vascular endothelial growth factor (VEGF). Studies have shown that microvessel density (MVD) is directly related with VEGF expression and vascularization [30]. According to previous reports,the high HIF-1α expression is related to higher MVD, probably as a result of VEGF pathway activation [30].
Lymph node metastasis and angiogenesis may have some negative impact on human, leading a worse prognosis [31]. As a consequence, HIF-1α could be a particularly promising target in OSCC for controlling regional lymph node metastases through antiangiogenic and anti-lymphangiogenic effects [31].
In conclusion, this study suggests that the overexpression of HIFs is significantly associated with increased risk of mortality in OSCC patients. Further subgroup analysis showed that HIF overexpression, regardless of the isoform (HIF-1α or HIF-2α) is significantly associated with a poorer prognosis. Although more well-designed studies with larger sample sizes are required to confirm the associations between HIF-2α and the OSCC. The relationship between the OSCC and HIF-1α is clear.
References
Rajwar YC, Jain N, Bhatia G, Sikka N, Garg B, Walia E. Expression and significance of cadherins and its subtypes in development and progression of oral cancers: a review. J Clin Diag Res JCDR. 2015;9(5):ZE05–7. doi:10.7860/jcdr/2015/11964.5907.
Feller LL, Khammissa RR, Kramer BB, Lemmer JJ. Oral squamous cell carcinoma in relation to field precancerisation: pathobiology. Cancer Cell Int. 2013;13:31. doi:10.1186/1475-2867-13-31.
Kimple AJ, Welch CM, Zevallos JP, Patel SN. Oral cavity squamous cell carcinoma—an overview. Oral health Dent Manage. 2014;13(3):877–82.
Mangalath U, Aslam SA, Abdul Khadar AH, Francis PG, Mikacha MS, Kalathingal JH. Recent trends in prevention of oral cancer. J Int Soc Prev Commun Dent. 2014;4 Suppl 3:S131–8. doi:10.4103/2231-0762.149018.
Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268(29):21513–8.
Chun YS, Kim MS, Park JW. Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci. 2002;17(5):581–8.
Zhao J, Du F, Luo Y, Shen G, Zheng F, Xu B. The emerging role of hypoxia-inducible factor-2 involved in chemo/radioresistance in solid tumors. Cancer Treat Rev. 2015;41(7):623–33. doi:10.1016/j.ctrv.2015.05.004.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi:10.1007/s10654-010-9491-z.
Spruance SL, Reid JE, Grace M, Samore M. Hazard ratio in clinical trials. Antimicrob Agents Chemother. 2004;48(8):2787–92. doi:10.1128/aac.48.8.2787-2792.2004.
Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. 1998;280(19):1690–1.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Educ). 1997;315(7109):629–34.
Fillies T, Werkmeister R, van Diest PJ, Brandt B, Joos U, Buerger H. HIF1-alpha overexpression indicates a good prognosis in early stage squamous cell carcinomas of the oral floor. BMC Cancer. 2005;5:84. doi:10.1186/1471-2407-5-84.
Lin PY, Yu CH, Wang JT, Chen HH, Cheng SJ, Kuo MY, et al. Expression of hypoxia-inducible factor-1 alpha is significantly associated with the progression and prognosis of oral squamous cell carcinomas in Taiwan. J Oral Pathol Med Off Pub Int Assoc Oral Pathol Am Acad Oral Pathol. 2008;37(1):18–25. doi:10.1111/j.1600-0714.2007.00571.x.
Zhu GQ, Tang YL, Li L, Zheng M, Jiang J, Li XY, et al. Hypoxia inducible factor 1 alpha and hypoxia inducible factor 2 alpha play distinct and functionally overlapping roles in oral squamous cell carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(19):4732–41. doi:10.1158/1078-0432.ccr-10-1408.
Eckert AW, Lautner MH, Schutze A, Bolte K, Bache M, Kappler M, et al. Co-expression of Hif1alpha and CAIX is associated with poor prognosis in oral squamous cell carcinoma patients. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2010;39(4):313–7. doi:10.1111/j.1600-0714.2009.00829.x.
Eckert AW, Lautner MH, Schutze A, Taubert H, Schubert J, Bilkenroth U. Coexpression of hypoxia-inducible factor-1alpha and glucose transporter-1 is associated with poor prognosis in oral squamous cell carcinoma patients. Histopathology. 2011;58(7):1136–47. doi:10.1111/j.1365-2559.2011.03806.x.
Liang X, Zheng M, Jiang J, Zhu G, Yang J, Tang Y. Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncol. 2011;47(2):92–7. doi:10.1016/j.oraloncology.2010.11.014.
dos Santos M, Mercante AM, Louro ID, Goncalves AJ, de Carvalho MB, da Silva EH, et al. HIF1-alpha expression predicts survival of patients with squamous cell carcinoma of the oral cavity. PLoS One. 2012;7(9):e45228. doi:10.1371/journal.pone.0045228.
Mendes SO, dos Santos M, Peterle GT, Maia Lde L, Stur E, Agostini LP, et al. HIF-1alpha expression profile in intratumoral and peritumoral inflammatory cells as a prognostic marker for squamous cell carcinoma of the oral cavity. PLoS One. 2014;9(1):e84923. doi:10.1371/journal.pone.0084923.
Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi:10.1038/nrc3064.
Beasley NJ, Leek R, Alam M, Turley H, Cox GJ, Gatter K, et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62(9):2493–7.
Gong L, Zhang W, Zhou J, Lu J, Xiong H, Shi X, et al. Prognostic value of HIFs expression in head and neck cancer: a systematic review. PLoS One. 2013;8(9):e75094. doi:10.1371/journal.pone.0075094.
Hong A, Zhang M, Veillard AS, Jahanbani J, Lee CS, Jones D, et al. The prognostic significance of hypoxia inducing factor 1-alpha in oropharyngeal cancer in relation to human papillomavirus status. Oral Oncol. 2013;49(4):354–9. doi:10.1016/j.oraloncology.2012.11.006.
Imamura T, Kikuchi H, Herraiz MT, Park DY, Mizukami Y, Mino-Kenduson M, et al. HIF-1alpha and HIF-2alpha have divergent roles in colon cancer. Int J Cancer J Int Cancer. 2009;124(4):763–71. doi:10.1002/ijc.24032.
Acker T, Diez-Juan A, Aragones J, Tjwa M, Brusselmans K, Moons L, et al. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell. 2005;8(2):131–41. doi:10.1016/j.ccr.2005.07.003.
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25(13):5675–86. doi:10.1128/mcb.25.13.5675-5686.2005.
Covello KL, Simon MC, Keith B. Targeted replacement of hypoxia-inducible factor-1alpha by a hypoxia-inducible factor-2alpha knock-in allele promotes tumor growth. Cancer Res. 2005;65(6):2277–86. doi:10.1158/0008-5472.can-04-3246.
Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10(5):413–23. doi:10.1016/j.ccr.2006.08.026.
Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–14. doi:10.1016/j.tips.2012.01.005.
Astekar M, Joshi A, Ramesh G, Metgud R. Expression of vascular endothelial growth factor and microvessel density in oral tumorigenesis. J Oral Maxillofac Pathol JOMFP. 2012;16(1):22–6. doi:10.4103/0973-029x.92968.
Liang X, Yang D, Hu J, Hao X, Gao J, Mao Z. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res. 2008;28(3A):1659–66.
Acknowledgments
This study was financially supported by National Natural Science Foundation of China (81302351), Jiangsu Provincial Natural Science Foundation (BK 20131080), Distinguished young investigator project of Nanjing (JQX14010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Qian, J., Wenguang, X., Zhiyong, W. et al. Hypoxia inducible factor: a potential prognostic biomarker in oral squamous cell carcinoma. Tumor Biol. 37, 10815–10820 (2016). https://doi.org/10.1007/s13277-016-4976-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4976-3