Abstract
Both glucocorticoids and H1-antihistamines were widely used on patients with allergic rhinitis (AR) and obstructive airway diseases. However, their direct effects on airway smooth muscle were not fully explored. In this study, we tested the effectiveness of prednisolone (Kidsolone) and levocetirizine (Xyzal) on isolated rat trachea submersed in Kreb’s solution in a muscle bath. Changes in tracheal contractility in response to the application of parasympathetic mimetic agents were measured. The following assessments of the drug were performed: (1) effect on tracheal smooth muscle resting tension; (2) effect on contraction caused by 10−6 M methacholine; (3) effect of the drug on electrical field stimulation (EFS) induced tracheal smooth muscle contractions. The result revealed sole use of Kidsolone or Xyzal elicited no significant effect or only a little relaxation response on tracheal tension after methacholine treatment. The tension was 90.5 ± 7.5 and 99.5 ± 0.8 % at 10−4 M for Xyzal and 10−5 M for Kidsolone, respectively. However, a dramatically spasmolytic effect was observed after co-administration of Kidsolone and Xyzal and the tension dropped to 67.5 ± 13.6 %, with statistical significance (p < 0.05). As for EFS-induced contractions, Kidsolone had no direct effect but Xyzal could inhibit it, with increasing basal tension. In conclusion, using glucocorticoids alone had no spasmolytic effect but they can be synergized with antihistamines to dramatically relax the trachea smooth muscle within minutes. Therefore, for AR patients with acute asthma attack, combined use of those two drugs is recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive airway diseases (COAD), including asthma and chronic obstructive pulmonary diseases (COPD), are a developing pandemic expected to become the world’s third leading cause of death by 2020 [1]. Two major drug classes used in COAD encompassed β2-adrenergic receptor (β2AR) agonists and the glucocorticoids, both typically being administered by inhalation [2]. β2AR agonists worked primarily on airway smooth muscle cells, causing relaxation within seconds or minutes, whereas glucocorticoids primarily improved airway function via their anti-inflammatory action, the genomic pathway, within hours or days [3, 4]. In recent studies, researchers showed that glucocorticoids can also elicit much more rapid responses, probably through the non-genomic pathway [5], corresponding to the stimulation within seconds or minutes. However, most of those studies mainly emphasized and explained how glucocorticoids help β2AR in relaxing the constricting smooth muscle. Few studies discussed the direct effect of glucocorticoids on airway tone during an acute asthma attack.
Histamine is the salient mediator released after immunologic challenge, initiating multiple pathologic processes of the allergic reaction that result in bronchial smooth muscle contraction, vasodilation, mucus hypersecretion, and edema. It has been reported to play an important role in pathogenesis of bronchial asthma [6, 7]. However, antihistamines were not recommended as the first drug for asthma therapy, because they were not very effective at the doses recommended for allergic rhinitis (AR), while higher doses caused obvious side effects [6]. Recently, cumulative clinical evidence indicated that antihistamines may have a beneficial effect on asthma symptoms and improve quality of life, by attenuating the symptoms associated with early and late-phase allergic reactions [8]. Meanwhile, comorbidity of asthma and AR was very high and they shared similar allergic inflammation [6, 7]. Hence, antihistamines were alternatives for patients with both asthma and AR.
Smooth muscle, the main structure of the airway walls, plays a major role in the contraction of the trachea. Its excessive contraction may be one of the crucial factors that directly cause the asthmatic syndrome [9]. Therefore, the direct effect of glucocorticoids and antihistamines on the airway smooth muscle merited further exploration. Our previous report developed a simple and rapid test for screening parasympathetic mimetic agents and potential tracheal contraction agents [10]. We tried to use this technique to identify how glucocorticoids or antihistamines affect the isolated trachea smooth muscle directly in vitro. Understanding this information will further future pharmacotherapeutic strategies.
Materials and methods
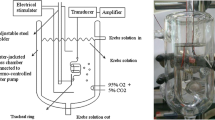
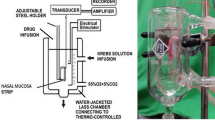
Rat trachea tissue preparation
This study was approved by the Animal Experiment Review Board of Taipei Medical University (LAC-101-0062). Thirty rats were anesthetized by intraperitoneal administration of pentobarbital (60 mg/kg), and two pieces of trachea about 5 mm in length were removed from each rat. The equipment and process were designed based on our previous study [10, 11]. The tracheal specimen was mounted using two steel plates and submersed in a 30-mL muscle bath at 37 °C. The bath was filled with 30 mL of Krebs solution consisting of (mmol/L): NaCl (118), KCl (4.7), CaCl2 (2.5), MgSO4·7H2O (1.2), KH2PO4 (1.2), NaHCO3 (25.0), and glucose (10.0). The upper side of the tracheal strip was attached to a Grass FT-03 force displacement transducer (AstroMed, West Warwick, RI) using a steel plate and a 3-0 silk ligature. The other side of the strip was fixed to a steel plate attached to a bath. A passive tension of 0.5 g was applied to the strips and subsequent changes in tension were recorded continuously using Chart V4.2 software (PowerLab; AD Instruments, CO Springs, CO). The chemicals used were of the highest purity available. All chemical reagents were obtained from Sigma (St. Louis, MO, USA).
Methacholine test
We used methacholine, a parasympathetic mimetic, as a tracheal contraction drug. This contracting agent is a synthetic choline ester that acts as a non-selective cholinergic agonist. Before drug assays were conducted, isolated tracheas were equilibrated in the bath solution for 30–45 min, during which continuous aeration with a mixture of 95 % O2 and 5 % CO2 was applied. It is worthy of note that the drug-induced relaxation of tissue was dependent on prior partial contraction of the smooth muscle in response to methacholine. Preliminary tests showed the tracheal strip immersed in the bath solution used for subsequent experiments did not contract when basal tension was applied.
Electrical field stimulation test
Electrical field stimulation (EFS; 5 Hz, 5-ms pulse duration, at a voltage range of 30–90 V, trains of stimulation for 5 s) was applied to the trachea strip with two wire electrodes placed parallel to the trachea strip and connected to a direct-current stimulator (Grass S44; Grass Instrument Co., Quincy, MA). An interval of 2 min was imposed between each stimulation period to allow recovery from the response. Stimulation was applied contiguously to the trachea at 37 °C.
Kidsolone and Xyzal assessments
We selected the levocetirizine dihydrochloride (Xyzal, UCB Farchim SA), a second-generation antihistamine, and prednisolone (Kidsolone oral solution, Center Laboratories, INC., Taiwan) as our testing agents. The following assessments for Kidsolone and Xyzal were performed: (1) effect on tracheal resting tension—this test examined the effect of the drug on the simulating condition of the resting trachea condition; (2) effect on contraction caused by 10−6 M of methacholine—this procedure was concerned with examining postsynaptic events such as muscle receptor blockade, enhancement, and second messengers; and (3) effect of Kidsolone and Xyzal on electrically induced contractions—electrical stimulation of this tissue causes parasympathetic nerve remnants in the trachea to release the transmitter acetylcholine. If there is interference with transmitter release, electrical stimulation does not cause contraction. Thus, presynaptic events were seen more easily with this procedure. All drugs were administered by adding a defined volume of stock solution to the tissue bath solution. Concentrations of drugs are expressed as concentrations present in the 30-mL bath solution. Stepwise increases in the amount of testing agents were used to study the contraction or relaxation responses of tracheal strips. At the end of the study, we added xylocaine, which is classified as an anticholinergic drug, to thoroughly assay the drug effect on 10−6 M methacholine-induced contraction. In each experiment, one untreated strip served as a control and repetitions were performed at least six times.
Statistical analysis
Data were presented as mean values and SD. The differences between mean values were compared using Student’s t test, and were assumed to be significant at p < 0.05.
Ethical considerations
The research protocol has been reviewed and approved by an animal experiment review board (LAC-101-0062).
Results
The degree of contraction or relaxation of tracheal strips was estimated from the tension applied to the transducer. Tracheal contraction induced by a small dose of methacholine was easily detected (not shown), and the tissue remained in a contracted state until the drug was rinsed from the tissue.
The pharmacologic effects on tracheal contraction caused by methacholine
Adding the Kidsolone to the basal tension had a negligible effect (Fig. 1a). It resulted in no relaxation of the trachea when introduced after adding a constricting agent such as 10−6 M of methacholine. As the concentration of Kidsolone increased from 10−8 to 10−5 M, it had no effect on contraction (Fig. 1b). At 10−8 M of Kidsolone, the tension was 99.2 ± 0.8 % of the control values. At 10−6 and 10−5 M of Kidsolone, the tensions were 99.2 ± 0.5 and 99.5 ± 0.8 %, respectively (Fig. 2a). The difference in tension among the specimens treated with 10−8 M of Kidsolone and 10−6 or 10−5 M of Kidsolone was not statistically significant. The total relaxation of the 10−6 M methacholine-induced contracted tracheal strip was observed when adding 10−4 M of xylocaine among the specimens treated with Kidsolone (Fig. 1b).
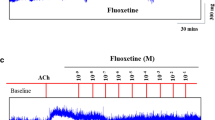
Tension changes in rat’s trachea after applying Kidsolone or Xyzal at various concentrations. Kidsolone alone had a minimal effect on the basal tension of the trachea (a). On 10−6 M of methacholine-induced contraction of rat’s trachea, the effects of Kidsolone was minimal (b). Xyzal exhibits little spasmolytic effect only at high doses (c). A dramatic relaxation was observed when adding those two drugs simultaneously (d)
Effects of drugs on 10−6 M methacholine-induced contraction (contraction area was calculated at 100 % with no addition of Kidsolone or Xyzal) of rat’s trachea (a). Combined use of those two drugs showed statistically significant relaxation as compared to the single use of each of them (p < 0.05) (b)
Addition of the Xyzal, on the basal tension also elicited a negligible response (not shown). A small relaxation of the trachea was revealed when introduced after the addition of a constricting agent such as 10−6 M methacholine (Fig. 1c). Low doses of Xyzal resulted in a mild effect on contraction and higher doses slightly relaxed the trachea smooth muscle (Fig. 2a). At 10−8 M Xyzal, the tension was 98.3 ± 1.6 % of control values. While at 10−5 and 10−4 M Xyzal, the tensions were 93.8 ± 4.3 and 90.5 ± 7.5 %, respectively (Fig. 2a).
Surprisingly, a synergistic effect of Kidsolone with Xyzal was demonstrated on the contracted trachea (Fig. 1d). At 10−4 M Xyzal, the tension was 90.5 ± 7.5 %. The tension dropped dramatically to 67.5 ± 13.6 % when we added 10−5 M of Kidsolone among the specimens simultaneously (Fig. 2b). This spasmolytic effect when the 10−4 M Xyzal and 10−5 M of Kidsolone was co-administrated was statistically significant (p < 0.05) as compared to the single use of each of them.
The pharmacologic effects on electrically induced tracheal contractions
EFS-induced contraction of the trachea did not decrease as the Kidsolone concentration was increased (Fig. 3a). The peak tension of the tracheal strip evoked by EFS upon the addition of 10−8 M Kidsolone was 101 ± 1.9 %, whereas at 10−6 and 10−5 M Kidsolone the peaks were 102 ± 2.4 and 103 ± 1.4 %, respectively. The difference of tension among 10−8 M Kidsolone and 10−6 or 10−5 M Kidsolone was not statistically significant. These findings suggest that Kidsolone could not antagonize the parasympathetic innervation responsible for trachea smooth muscle contraction.
On the other hand, Xyzal inhibited the spike contraction induced by EFS, and the basal tension was increased at the same time (Fig. 3b). The peak tension of the tracheal strip evoked by EFS upon the addition of 10−8 M Xyzal was 84.1 ± 12.5 %, while at 10−5 and 10−4 M Xyzal they were 4.3 ± 0.3 and 0 %. The peak tension values of the tracheal strip evoked by EFS at 10−5 and 10−4 M Xyzal addition were significantly lower than that at 10−8 M Xyzal.
Discussion
Airway remodeling is a classic feature of COAD [12]. It is characterized by variable airflow obstruction that is secondary to an allergic pattern of inflammation in the airways, which involves the activation of resident mast cells and dendritic cells by allergens [13]. Major drug classes used in COAD encompass bronchodilators, which act mainly by reversing airway smooth muscle contraction, and anti-inflammatory drugs, which suppress inflammation in the airways. β2-agonists are by far the most effective bronchodilators for COAD [1]. They can relax airway smooth muscle cells by activating the adenylyl cyclase via the stimulatory G protein (Gs) [9]. Certain calcium-mobilizing contractile agonists, particularly muscarinic cholinergic agonists [e.g., acetylcholine (Ach)], directly inhibit adenylyl cyclase activity. Therefore, anticholinergics also have some additive bronchodilator effect.
Glucocorticoids are the most effective therapy for controlling COAD [14]. Glucocorticoids can modulate the transcription of many inflammatory mediators, via mechanisms known as trans-activation and trans-repression [15]. The major action was to switch off multiple activated inflammatory genes that code for cytokines, chemokines, adhesion molecules, inflammatory enzymes, and receptors. In addition, in vitro studies indicated that glucocorticoids inhibited proliferation of airway smooth muscle cells, indicating that they can also alter airway smooth muscle contractile properties [16]. Glucocorticoids reduced the production of extracellular matrix proteins and, therefore, inhibited airway smooth muscle remodeling.
Histamine is released by mast cells and basophils and is the predominant mediator after immunologic challenge [17]. Histamine receptors belong to the G protein-coupled receptor family [17]. The inflammatory responses resulting from the release of histamine is primarily mediated by the H1-receptor. The classic H1-mediated responses include bronchial smooth muscle contraction, edema, and mucus hypersecretion [18]. Therefore, H1-antihistamines were widely used in clinics and were recommended as first-line drugs to treat AR in the guidelines [7, 19]. Rather than “true” antagonists, H1-antihistamines are actually inverse agonists at the histamine H1-receptor. Meanwhile, they are typically moderately to highly potent muscarinic acetylcholine receptor antagonists (anticholinergic) as well [18, 20]. This lack of receptor selectivity and its anticholinergic features might explain the additive bronchodilator effect demonstrated by our previous studies [21]. The prior partial contracted trachea smooth muscle, which was induced by methacholine, relaxed by a small extent after the application of a H1-antihistamine at high doses. Accordingly, we believed H1-antihistamines still have a role in relieving symptoms of chronic asthma, although some authors held their concern that antihistamines had drying effects on the airways, which could contribute to mucous retention and increase airway obstruction [6].
The surprising synergistic effects of glucocorticoids with H1-antihistamines in relaxing the trachea smooth muscle were demonstrated by this study. Using Kidsolone alone had no significant effect on the basal tension of the trachea or on those which were pre-sensitized by methacholine. However, the obvious relaxation of the contracted tracheal strip was observed within minutes when we added 10−4 M of Xyzal simultaneously. We believe this synergism might not be merely based on anti-inflammatory properties, the genomic pathway, via the intracellular glucocorticoids receptor. The genomic effect of glucocorticoids is known to occur with a time lag of hours or even days. The non-genomic pathway, probably through enhancing the anticholinergic properties of H1-antihistamines, might explain this rapidly spasmolytic phenomenon. The non-genomic effects of the glucocorticoids have been shown to modulate hormone secretion, neuronal excitability, carbohydrate metabolism, cell morphology, cell behavior, and other processes [22]. Sun et al. [23] observed that a high concentration of glucocorticoids exerted rapid spasmolytic effects on the contraction in guinea pig tracheal smooth muscle induced by histamine. The authors concluded that glucocorticoids had non-genomic effects in airway smooth muscle cells because the application of RU486, the selective glucocorticoid receptors antagonist, and actidione, the blocker of protein synthesis, did not affect the spasmolytic effect of glucocorticoids. Rapid glucocorticoid actions were triggered by or at least dependent on, membrane associated G protein-coupled receptors and activation of downstream signaling cascades. Glucocorticoids might affect the muscarinic receptor–Gq coupling or decrease the intracellular calcium level through affect the ion channel directly. Downstream from the putative membrane receptors, various signaling pathways have been implicated in the rapid actions of glucocorticoids, such as altering the activity of the adenylyl cyclase, the protein kinase C (PKC) and the Rho kinase. However, those changes in these signal molecules probably had clinical therapeutic effect only while glucocorticoids and H1-antihistamines were co-administrated. Therefore, sole use of glucocorticoids or H1-antihistamines in treating acute asthma or COPD attacks may be suboptimal, since our findings confirmed that glucocorticoids had no direct anticholinergic effects in vitro, and H1-antihistamines had little spasmolytic effect even at high doses.
The coexistence of AR with asthma was widely recognized by clinicians. The upper and lower airways are connected anatomically, physiologically, and immunologically [24]. New evidence supports previous Allergic Rhinitis and its Impact on Asthma (ARIA) statements, such as: (1) AR is a risk factor for asthma; (2) most patients with asthma have AR and (3) a combined strategy should be used to treat both upper and lower airways [7]. Both intranasal H1-antihistamines and glucocorticoids were widely used in treating AR patients. Although there was an underlying concern regarding antihistamines that their anticholinergic effects may be harmful in patients with asthma, the ARIA still concluded that antihistamines should not be withheld from patients with asthma who require treatment for concomitant disorders such as AR and urticaria. Our study offers another support for co-administration of antihistamines and glucocorticoids, especially for AR patients with acute asthma attacks. Pahl et al. [25] also confirmed the synergistic effects of the anticholinergic agent with anti-inflammatory drugs in inhibiting inflammatory mediators, the genomic pathway. Another study performed by Nabishah et al. [26] showed that glucocorticoids, but not mineralocorticoids, could decrease the muscarinic receptor on normal rats’ bronchial smooth muscle after 7 days of administration, which might relieve bronchospasm by reducing the cholinergic hypersensitivity. Our data showed another advantage of this combination in its direct effect on airway smooth muscle, the non-genomic pathway. Therefore, we recommended the combined use of those two class drugs, especially in AR patients with asthma or COPD attack.
The isolated tracheal preparations used in our experiments were excised from rats without damaging the endothelium or smooth muscle. Therefore, it was reasonable to assume the tracheal responses to test agents in our study were comparable with those observed after applying an inhaler to the trachea during the asthma attack. However, since this was an in vitro study, there were reservations as to its comparability with an in vivo situation in humans. In the in vivo situation, the response might be much more complicated than that in the in vitro situation. Therefore, the results of our experiments should still be interpreted within the context of the test materials used. Further investigation was needed to elucidate the precise molecular mechanism of the glucocorticoids action and define the downstream targets of its pathway.
Conclusion
In conclusion, this study indicated glucocorticoids alone had no anticholinergic effect but they can relax the trachea smooth muscle with the aid of antihistamines within minutes. Therefore, combined use of inhalation glucocorticoids and antihistamines in AR patients with acute asthma attack may be an alternative.
References
Schmidt M, Michel MC (2011) How can 1 + 1 = 3? beta2-adrenergic and glucocorticoid receptor agonist synergism in obstructive airway diseases. Mol Pharmacol 80:955–958
Dekkers BG, Pehlic A, Mariani R, Bos IS, Meurs H, Zaagsma J (2012) Glucocorticosteroids and beta(2)-adrenoceptor agonists synergize to inhibit airway smooth muscle remodeling. J Pharmacol Exp Ther 342:780–787
Yick CY, Zwinderman AH, Kunst PW et al (2013) Glucocorticoid-induced changes in gene expression of airway smooth muscle in patients with asthma. Am J Respir Crit Care Med 187:1076–1084
Mendes ES, Rebolledo P, Wanner A (2012) Acute effects of salmeterol and fluticasone propionate alone and in combination on airway blood flow in patients with asthma. Chest 141:1184–1189
Wang C, Li YJ, Zheng YQ, Feng B, Liu Y, Cao JM (2012) Glucocorticoid decreases airway tone via a nongenomic pathway. Respir Physiol Neurobiol 183:10–14
Larsen JS (2001) Do antihistamines have a role in asthma therapy? Pharmacotherapy 21:28S–33S
Cruz AA, Popov T, Pawankar R et al (2007) Common characteristics of upper and lower airways in rhinitis and asthma: ARIA update, in collaboration with GA(2)LEN. Allergy 62(Suppl 84):1–41
Walsh GM (2002) Second-generation antihistamines in asthma therapy: is there a protective effect? Am J Respir Med 1:27–34
Barnes PJ (2011) Biochemical basis of asthma therapy. J Biol Chem 286:32899–32905
Wang HW, Chou YL, Chu YH (2010) Azelastine nasal spray inhibiting parasympathetic function of tracheal smooth muscle. Rhinology 48:211–215
Wang HW, Wu CC (2008) Effects of oxymetazoline on isolated rat’s tracheal smooth muscle. Eur Arch Otorhinolaryngol 265:695–698
Black JL, Roth M, Lee J, Carlin S, Johnson PR (2001) Mechanisms of airway remodeling. Airway smooth muscle. Am J Respir Crit Care Med 164:S63–S66
Halayko AJ, Amrani Y (2003) Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Respir Physiol Neurobiol 137:209–222
Sin DD, Park HY (2013) Steroids for treatment of COPD exacerbations: less is clearly more. JAMA 309:2272–2273
Chang PJ, Bhavsar PK, Michaeloudes C, Khorasani N, Chung KF (2012) Corticosteroid insensitivity of chemokine expression in airway smooth muscle of patients with severe asthma. J Allergy Clin Immunol 130:877 e875–885 e875
Chiappori A, Folli C, Riccio AM et al (2012) Salbutamol: how does it enter smooth muscle cells? Int J Immunopathol Pharmacol 25:541–546
Bachert C, Maspero J (2011) Efficacy of second-generation antihistamines in patients with allergic rhinitis and comorbid asthma. J Asthma 48:965–973
Liu H, Farley JM (2005) Effects of first and second generation antihistamines on muscarinic induced mucus gland cell ion transport. BMC Pharmacol 5:8
Bousquet J, Van Cauwenberge P, Khaltaev N (2001) Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 108:S147–S334
Gosens R, Zaagsma J, Meurs H, Halayko AJ (2006) Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7:73
Kao CH, Chu YH, Wang HW (2009) Effects of cetirizine on isolated rat’s tracheal smooth muscle. Eur Arch Otorhinolaryngol 266:753–757
Urbach V, Verriere V, Grumbach Y, Bousquet J, Harvey BJ (2006) Rapid anti-secretory effects of glucocorticoids in human airway epithelium. Steroids 71:323–328
Sun HW, Miao CY, Liu L et al (2006) Rapid inhibitory effect of glucocorticoids on airway smooth muscle contractions in guinea pigs. Steroids 71:154–159
Bachert C, Claeys SE, Tomassen P, van Zele T, Zhang N (2010) Rhinosinusitis and asthma: a link for asthma severity. Curr Allergy Asthma Rep 10:194–201
Pahl A, Bauhofer A, Petzold U et al (2006) Synergistic effects of the anti-cholinergic R, R-glycopyrrolate with anti-inflammatory drugs. Biochem Pharmacol 72:1690–1696
Nabishah BM, Morat PB, Kadir BA, Khalid BA (1991) Effect of steroid hormones on muscarinic receptors of bronchial smooth muscle. Gen Pharmacol 22:389–392
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, SC., Chu, YH., Kao, CH. et al. Steroids and antihistamines synergize to inhibit rat’s airway smooth muscle contractility. Eur Arch Otorhinolaryngol 272, 1443–1449 (2015). https://doi.org/10.1007/s00405-014-3240-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-3240-y