Abstract
Oxymetazoline is often used as a decongestant in rhinitis patients who are suffering from nasal obstruction. It is used as a nasal drop or spray solution. The effect on nasal mucosa in vitro or in vivo is well known. However, the effect of the drug on tracheal smooth muscle has rarely been explored. During administration of the drug to the nose, it might affect the trachea via inhalation. We used our preparation to test the effectiveness of oxymetazoline on isolated rat’s tracheal smooth muscle. A 5 mm long portion of rat trachea was submersed in 30 ml Kreb’s solution in a muscle bath at 37°C. Changes in tracheal contractility in response to the application of parasympathetic mimetic agents were measured using a transducer connected to a Pentium III computer equipped with polygraphy software. The following assessments were performed: (1) effect on tracheal smooth muscle resting tension; (2) effect on contraction caused by 10−6 M methacholine as a parasympathetic mimetic; (3) effect of oxymetazoline on electrically induced tracheal smooth muscle contractions. Addition of parasympathetic mimetics to the incubation medium caused the trachea to contract in a dose-dependent manner. Addition of oxymetazoline induced a significant relaxation response when the preparation was up to 10−4 M. At the same concentration, the drug also could inhibit EFS induced spike contraction. Oxymetazoline had negligible effect on the basal tension of trachea as the concentration increased. The degree of drug-induced tracheal contraction or relaxation was dose-dependent. The study indicated that high concentrations of oxymetazoline might actually antagonize cholinergic receptors of the trachea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular or nasal mucosal strips have been used to study smooth muscle contractility in vitro [1, 2]. We used rat tracheas to develop a simple in vitro technique to test the effects of drugs that induce tracheal constriction or relaxation. Our technique was based on a previously described methods [3, 4] in which 10 mm strips of rabbit trachea are suspended in a tissue bath containing 20 ml of Kreb’s solution. One end of the strip is attached to a steel plate and the other to an isometric transducer and a steel plate. A passive tension of 8 g is applied to the strips.
A simple and rapid test is needed for screening parasympathetic mimetic agents and potential tracheal contraction agents and for identifying agents that affect tracheal smooth muscle directly. Development of such a test may help to resolve the inconsistencies in responses of the trachea to drugs in vivo. In addition, the aim of this study was to determine the effects of oxymetazoline on isolated tracheal smooth muscle in vitro.
Materials and methods
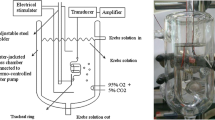
Chemicals used were of the highest purity available. All chemical reagents were obtained from Sigma (St Louis, MO, USA). We tested acetylcholine and methacholine as tracheal contraction drugs. Thirty rats were anesthetized by intraperitoneal administration of pentobarbital (45 mg/kg) and two pieces of trachea about 5 mm in length were removed from each rat. This study was approved by an animal experiment review board (LACUC-05-158). The tracheal specimen was mounted using two steel plates and submersed in a 30 ml muscle bath at 37°C. The bath (Fig. 1) was filled with 30 ml Kreb’s solution consisting of (mmol/l) NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgSO4·7H2O, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; and glucose, 10.0. The upper side of the tracheal strip was attached to a Grass FT-03 force displacement transducer (AstroMed, West Warwick, RI, USA) using a steel plate and a 3-0 silk ligature. The other side of the strip was fixed to a steel plate attached to the bath. A passive tension of 0.3 g was applied to the strips and subsequent changes in tension were recorded continuously using Chart V4.2 software (PowerLab, ADInstruments, Colorado Springs, CO, USA). Preliminary tests showed that a tracheal strip immersed in the bath solution used for subsequent experiments did not contract when basal tension was applied. Before drug assays were conducted, isolated tracheas were equilibrated in the bath solution for 15–30 min, during which continuous aeration with a mixture of 95% O2 and 5% CO2 was applied. Stepwise increases in the amount of drugs used were employed to study contraction or relaxation responses of tracheal strips. All drugs were administered by adding a defined volume of stock solution to the tissue bath solution.
Electrical field stimulation (EFS) (5 Hz, 5 ms pulse duration, at a voltage of 50 V, trains of stimulation for 5 s) was applied to the trachea strip with two wire electrodes placed parallel to the trachea strip and connected to a direct-current stimulator (Grass S44, Quincy, MA, USA). An interval of 2 min was imposed between each stimulation period to allow recovery from the response. Stimulation was applied contiguously to the trachea at 37°C.
The following assessments for oxymetazoline were performed: (1) effect on tracheal smooth muscle resting tension; (2) effect on contraction caused by 10−6 M methacholine; (3) effect of oxymetazoline on electrically induced tracheal smooth muscle contractions. In each experiment, one untreated strip served as a control.
Concentrations of drugs are expressed as concentrations present in the 30 ml bath solution. Data are presented as mean values and standard deviations (SDs). Differences between mean values were compared using Student’s t test. Differences were assumed to be significant at P < 0.01.
Results
The degree of contraction or relaxation of tracheal strips was estimated from the tension applied to the transducer. Tracheal contraction induced by a small dose of acetylcholine was easily detected (Fig. 2) and the tissue remained in a contracted state until the drug was rinsed from the tissue.
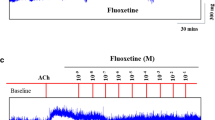
Contractile responses to another parasympathomimetic agent, methacholine, are shown in Fig. 3. Tracheal mucosa was slightly less sensitive to methacholine than acetylcholine when present in low concentrations. The degree and rate of tissue contraction induced by both agents was dose dependent (Fig. 3). Addition of the nasal decongestant, oxymetazoline, on its own elicited negligible response (not shown), but resulted in relaxation of the trachea when introduced after the addition of a constricting agent such as methacholine (Fig. 4). Low doses of oxymetazoline resulted in a slight decrease in contraction and higher doses relaxed the trachea much more quickly. Higher doses of oxymetazoline also decreased the spike contraction induced by EFS (Fig. 5).
Discussion
Although in vitro assays for monitoring tracheal responses to drugs have been developed by other groups [3–6], they all have disadvantages. In such assays, a tracheal mucosa strip measuring 8 mm × 20 mm is attached to an isometric transducer and suspended in a tissue bath containing 30 ml of Kreb’s solution. This is a relatively invasive procedure as it requires several centimeters of trachea; sufficient quantities of trachea are difficult to obtain from rats. Our test only required a few millimeters of trachea, which was excised as an intact ring of trachea without injury to the smooth muscle or mucosa. An intact tracheal ring was an important component of our technique. Previous authors have used tracheal smooth muscle strips to conduct drug tests [3–6]. Our test was simpler and more robust than the tests in which tracheal rings were destroyed. An intact tracheal ring was much more representative of a physiological situation than smooth muscle strips.
The results of our experiments should be interpreted within the context of the test materials used. Although it was difficult to determine which tissue component of the trachea was responsible for drug-induced contraction, the nature of specific tissues and their responses to specific drugs provide some indication. Firstly, the tracheal strips used in our study were crude preparations that contained cartilage and tracheal smooth muscle (Fig. 6). The smooth muscle of the trachea appeared to be the main tissue component responsible for contraction as the other components (epithelium, glands, connective tissue, nerves, and cartilage) did not contract to a significant extent.
Because the method involved cross contraction, changes in tension were caused by radial contraction of the tracheal ring. Responses to drugs and electrical stimulation have been verified for similar preparations [4–6]. However, the contractile response observed was probably an aggregate of the responses of various types of muscle tissue. Secondly, the isolated tracheal preparations used in our experiments were excised from rats without damaging the endothelium or smooth muscle. It was therefore reasonable to assume that tracheal responses to test agents in our study were comparable to those observed after application of a spray to the trachea during an asthma attack.
Both contracting agents tested were commonly used. It was noteworthy that drug-induced relaxation of tissue was dependent on prior partial contraction of smooth muscle using acetylcholine or methacholine. It should thus be possible to assay the effects of common drugs and agents supposedly responsible for relieving asthma. Oxymetazoline, a nasal decongestant, could reduce methacholine-induced contraction. It is known as a directing-acting α-adrenergic agonist [7]. How the α-adrenergic agonist affected the trachea smooth muscle requires further study to elucidate.
EFS-induced contraction of the canine nasal mucosa disappeared after ipsilateral cervical sympathetic ganglionectomy [8]. The EFS-induced spike contraction of nasal mucosa was proved from the stimulation of sympathetic innervation [8]. In this study, EFS-induced spike contraction of the tracheal smooth muscle was believed from the stimulation of parasympathetic innervation. Therefore, EFS-induced contraction of the trachea was decreased during the oxymetazoline concentration was increased. This suggested that an adrenergic agonist could antagonize the parasympathetic innervation in trachea smooth muscle contraction. What we observed in this study was very interesting and additional research is needed to clarify these phenomena.
Conclusion
The degree of drug-induced tracheal contraction or relaxation was dose-dependent. The study indicated that high concentrations of oxymetazoline might actually antagonize cholinergic receptors of the trachea.
References
Beny J, Pacicca C (1994) Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol 266:H1465–H1472
Ichimura K, Jackson RT (1983) Calcium, calcium blockers, and nasal smooth muscle. Arch Otolaryngol 109:593–597
Bratton DL, Tanaka DT, Grunstein MM (1987) Effects of temperature on cholinergic contractility of rabbit airway smooth muscle. J Appl Physiol 63:1933–1941
Gonzalez O, Santacana GE (2001) Effect of low temperature on tracheal smooth muscle contractile and relaxing responses evoked by electrical field stimulation. Phys Res 20:237–243
Yau KI, Ko FN, Chien CH (1999) Effects of prokinetic agents on contractile responses to electrical field stimulation of isolated guinea pig trachea. J Formos Med Assoc 98:567–572
Yau KI, Hwang TL (2002) The nonadrenergic noncholinergic system can modulate the effect of prokinetic agents on contractile response of isolated guinea pig trachea segments to electrical field stimulation. J Formos Med Assoc 101:695–699
Hoffman BB (2001) Adrenergic-activating and other sympathomimetic drugs. In: Katzung BG (ed) Basic and clinical pharmacology, 8th edn. McGraw-Hill, San Francisco, pp 120–154
Wang H-W, Jackson RT (1988) Do cholinergic neurons directly innervate nasal blood vessels? Rhinology 26:139–146
Acknowledgments
This work was supported in part by the Tri-Service General Hospital (TSGH C96-27).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, HW., Wu, CC. Effects of oxymetazoline on isolated rat’s tracheal smooth muscle. Eur Arch Otorhinolaryngol 265, 695–698 (2008). https://doi.org/10.1007/s00405-007-0509-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-007-0509-4