Abstract
The objective of this retrospective study was to investigate the horizontal vestibulo-ocular reflex (hVOR) pathway with caloric test (low-frequency hVOR) and video head impulse test (vHIT) (high-frequency hVOR) in patients with sporadic vestibular schwannoma (69 patients, 27–86 years, mean age 58.1 years) and to compare both test methods in terms of their sensitivity and specificity to detect a retrocochlear lesion. Test results with a unilateral weakness (UWCaloric) >25 % (caloric test) or a Mean-GainvHIT <0.79/asymmetry ratio of Gain (AR-GainvHIT) >8.5 % and accompanied refixation saccades (vHIT) were considered abnormal. The overall sensitivity of the caloric test was 72 %. The evaluation of AR-GainvHIT detected more abnormal cases than did Mean-GainvHIT (44 vs. 36 %). In up to 4 %, a normal caloric test result was related to an abnormal vHIT. There was only a moderate correlation of UWCaloric and AR-GainvHIT (r = 0.54, p < 0.05) with a linear regression line intercept/slope of 32.2/0.9 (p < 0.05). Receiver operating characteristics curve analysis exhibited at a UWCaloric of 50 % a vHIT sensitivity/specificity/positive predictive value/negative predictive value of 0.45/0.9/0.94/0.42. Vestibular testing at varying frequencies provides deeper insights into hVOR function and is helpful in detecting a cerebello-pontine lesion. Whereas caloric test yields a high sensitivity for nerve dysfunction, vHIT test reveals a remaining function of hVOR in the high-frequency range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
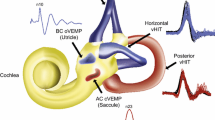
Caloric testing and the recently introduced video head impulse test (vHIT) are reliable test methods to evaluate the function of the lateral semicircular canal and superior vestibular nerve, i.e., horizontal vestibulo-ocular reflex (hVOR). HVOR function is essential in daily life to generate visual stabilization during horizontal head movements and walking. Vestibular schwannoma (VS) is a common tumor in the cerebello-pontine angle; since VS originates from the vestibular nerve, it can cause vertigo symptoms by deterioration of hVOR function.
Caloric testing has been the standard examination method for hVOR in unilateral or bilateral vestibular dysfunction for over 100 years. The disadvantages of caloric testing consist in its time-consuming test procedure and its discomfort for patients due to induced vertigo and nausea. Besides this, the thermal stimulus is non-physiologic and the response has a high inter-individual variety with ≤25 % of normal values.
The video head impulse test (vHIT) is a new VOR test method, which is safe, easy to perform and comparable to scleral search-coil measurements [1–3]. It is indicative for a VOR function loss when eye/head velocity gain is low, i.e., compensatory eye movement does not equal head rotation to brief high-acceleration head impulses and is therefore accompanied by corrective catch-up saccades to keep the gaze straight ahead.
Surprisingly, testing hVOR function with caloric test and vHIT can yield conflicting results, e.g., the caloric test indicates a vestibular hypofunction but the vHIT does not. This has been proven in few studies, especially for peripheral vestibular disorders such as Menière’s disease (MD) [4, 5] and vestibular neuritis (VN) [6–8], but not for retrocochlear lesions such as VS in a higher number of patients [9].
Therefore, the aim of this study was to evaluate the diagnostic value of caloric test and vHIT in patients with sporadic unilateral VS, i.e., to investigate whether both tests are sensitive and specific to detect a VS-related hVOR function deficit. Furthermore, vHIT test results were examined in relation to the caloric test and the amount of caloric unilateral weakness (UWCaloric) matching the highest sensitivity and specificity of the vHIT is defined. Since the response of the horizontal semicircular canal to caloric irrigation is equivalent to low-frequency rotatory stimulation (0.002–0.004 Hz) [10] and vHIT tests hVOR in a high-frequency range (5–7 Hz) [2], the results will be discussed in terms of their implications for vestibular frequency dynamics of hVOR function in vestibular schwannoma.
Materials and methods
Subjects
This is a retrospective study of patients with untreated sporadic unilateral VS, based on 1.5 T cranial MRI scanning (Magnetom Avanto, Siemens, Germany; 1 mm axial T2 space and contrast enhanced T1 vibe) in a tertiary referral center. VS was assessed according to the Hanover classification [11]. All patients underwent a detailed clinical history, a standard clinical ENT/neurological examination and vestibular testing (caloric test and vHIT) on the same day. The exclusion criteria were: patients with acute vestibular failure within the 2 weeks immediately preceding the vestibular testing, after microsurgery or radiotherapy for VS or previous mastoid surgery, concomitant diseases influencing the vestibular system, neurofibromatosis type 1/type 2 or the intake of vigilance-affecting drugs. The study subjects comprised 69 patients (39 females and 30 males; mean age 58.1 years; range 27–86 years). In 37 subjects (54 %) the VS was situated on the left side and in 32 (46 %) on the right side. This study was approved by the local ethic committee.
Caloric tests
The caloric tests were performed in a supine position with a 30-degree head elevation with water (30/44 °C, 75 ml, irrigation time 30 s, inter-irrigational interval >7 min). Horizontal eye movements were recorded with a binocular video oculography system (VN 415™, Interacoustics, Denmark). The maximum velocity of the slow phase component of spontaneous nystagmus and bithermal-induced nystagmus during a 30-s culmination phase was analyzed for unilateral weakness (UWCaloric) as determined by Jongkees formula. A pathological UWCaloric was present at >25 % according to normative data in our laboratory. Subjects with a spontaneous nystagmus >4° of slow phase velocity were rejected from the study [12].
Video head impulse testing
The EyeSeeCam™ system was used to record hVOR with vHIT. Subjects were instructed to fixate a dot located on a wall 1.2 m straight ahead. A minimum of 10 head impulses in the horizontal plane (yaw-axis rotation amplitude 15°–20°, duration 150–200 ms, peak velocity 200°/s) were randomly performed manually to both sides, with unpredictable timing and direction. The velocities of eye and head [°/s] were captured at 40, 60 and 80 ms after the head impulse was initiated and were averaged for both. The velocity gain of the hVOR (Mean-GainvHIT) is represented by the ratio of mean eye velocity [°/s] over mean head velocity [°/s]. Gain asymmetry ratio (AR-GainvHIT) is defined by the following equation:
Refixation saccades were sampled within 700 ms after the onset of head impulses and with amplitudes up to 400°/s eye velocity. The vHIT results were classified as abnormal if two conditions were met: abnormal values for either Mean-gainvHIT or AR-GainvHIT and the presence of refixation saccades. Refixation saccades were classified as covert if they occurred before the end of the head impulse and as overt afterwards. Normal hVOR Mean-gainvHIT and AR-GainvHIT are 0.99 ± 0.1 and 3.5 ± 2.5, respectively. For the cut-off value, we defined an abnormal Mean-gainvHIT (<0.79) and AR-GainvHIT (>8.5 %) as the outside of mean ± 2 SD found in 30 normal subjects in our laboratory.
Data analysis
The data of all patients were numerically captured, continuous variables were expressed as mean (SD), and categorical variables were expressed as frequencies and percentages. The significance of any differences between groups was evaluated by the t-test for dependent samples (p < 0.05). Linear regression was used to determine the significance of the correlation between the UWCaloric and the AR-GainvHIT. An analysis of variance (ANOVA) was carried out to assess the influence of predictor variables (sex, age, tumor grade). ROC curve was plotted [sensitivity vs. (1−specificity)] to compare the results of UWCaloric and Mean-GainvHIT/AR-GainvHIT. Statistical analyses were performed using SPSS for Windows 20.0.0.
Results
Evaluation of hVOR in different tumor grades
Abnormal results for Mean-GainvHIT, AR-GainvHIT and UWCaloric were found in 25 (36 %), 30 (44 %) and 50 subjects (72 %), respectively. This corresponds to a test sensitivity of 36–44 % for vHIT and 72 % for caloric test. The side with abnormal caloric test and vHIT values was always in agreement with the side identified as having VS based on MRI scanning.
There was no significant influence of sex or age on tumor grade, caloric or vHIT test results. There were significantly fewer subjects affected at a lower VS tumor grade (T1/T2) in caloric testing compared with subjects at higher VS tumor grades (T3/T4), whereas this dependence on tumor grade was not detected in vHIT testing. AR-GainvHIT revealed an abnormal result in 5/69 subjects (7 %) whereas Mean-GainvHIT detected them as normal. Demographics and details are provided in Table 1. In Table 2, the mean values of UWCaloric, Mean-GainvHIT and AR-GainvHIT are summarized for each tumor grade. Subjects with lower tumor grades exhibited a significant lower UWCaloric compared with higher tumor grades whereas Mean-GainvHIT and AR-GainvHIT values were not significantly different.
Comparison of caloric test and vHIT
There was a significant but modest linear correlation between UWCaloric and AR-GainvHIT values (r = 0.54, p < 0.05) in VS patients (Fig. 1). The intercept of the linear regression line was 32.2. The slope of the line (0.9; p < 0.05) indicates that VS patients have slightly larger asymmetries in their response to vHIT than to caloric. The linear correlation of UWCaloric and GainvHIT (r = 0.48, p < 0.05) was slightly lower than that of the former. Table 3 provides the results of the caloric test and vHIT. An abnormal vHIT result related to a normal caloric test was seen for Mean-GainvHIT in two cases (3 %) and for AR-GainvHIT in 4 cases (6 %), respectively. This was not dependent on tumor grade. A normal vHIT accompanied by a normal caloric test result was found in 17 cases (25 %) for Mean-GainvHIT and in 15 cases (22 %) for AR-GainvHIT, respectively. In two cases (8 %), there was a reduction of the contralateral gain to abnormal values without occurrence of refixation saccades. This was correlated to a gain reduction <0.25 on the VS side and a UWCaloric >90 %.
To enhance test sensitivity for vHIT with reliable specificity, the best cut-off values for Mean-GainvHIT and AR-GainvHIT were calculated from ROC curve analysis and are shown in Table 4. Considering the results of caloric irrigation as the independent variable, the ROC curve, predicting an optimal dichotomous outcome of vHIT (normal or abnormal), is shown in Fig. 2. A cut-off point of 50 % UWCaloric was associated with a vHIT test sensitivity of 0.45 and a specificity of 0.9. The area under the curve was calculated at 0.713 (95 % CI = 0.617/0.810). The positive predictive value of the vHIT was 0.94 and the negative predictive value was 0.42. Subjects with a UWCaloric <50 % exhibited a Mean-GainvHIT and AR-GainvHIT of 0.89 ± 0.23 and 9 ± 13, which were significantly higher (p < 0.05) than in subjects with a UWCalorc ≥50 % exhibiting a Mean-GainvHIT and AR-GainvHIT of 0.67 ± 0.29 and 24 ± 20, respectively.
Discussion
Head impulse test and caloric test are both primarily tests of peripheral vestibular function. They have been shown to detect a hVOR deficit since they can be abnormal in vestibular neuritis, Menière`s disease, after intratympanic gentamicin therapy, vestibular nerve deafferentation and even in isolated vestibular nuclear infarction but seldom in vertebrobasilar stroke [4, 5, 7, 8, 15, 24, 29, 30]. Therefore, a retrocochlear lesion like VS can cause a hVOR deficit, which should be detectable with vHIT and caloric test, since VS can either deteriorate peripheral hVOR function due to vestibular nerve compression or diminished blood supply of the inner ear [23].
The results of our study show that caloric testing and vHIT differentiate significantly between the affected and unaffected side of VS according to MRI. However, for the detection of a hVOR deficit, test sensitivities of caloric and vHIT differ, i.e., caloric irrigation reveals an abnormal hVOR function statistically more often than vHIT. Since caloric testing reflects low-frequency hVOR changes and vHIT is able to show high-frequency hVOR deficiencies, the combination of both tests permits the interpretation of hVOR impairment in a frequency-dependent manner. From the results of our study it can be concluded that in VS the whole frequency range of hVOR function can deteriorate, however, the results also clearly show that the low-frequency range is more often affected, whereas the high-frequency range is preserved. To identify both the low- and high-frequency hVOR range, caloric test and vHIT are necessary.
During head motion, vestibular signals have to be encoded in a wide frequency range from static head position to high-acceleration head turns. A frequency-dependent organization of hVOR can be attributed to distinct functional subgroups of vestibular hair cells with different intrinsic properties and response dynamics at each synaptic level. It has been shown, that the sensory–motor transformation occurs in parallel frequency-tuned pathways [31]. In fact, regular vestibular afferents that terminate onto type II hair cells have a low velocity detection threshold and transmit more information about low-frequency head rotations (<4 Hz). In contrast, irregular vestibular afferents which terminate onto type I hair cells make their primary contribution to VOR processing for high-frequency head rotations [32].
Evidence of a frequency-dependent impairment of hVOR function in various peripheral vestibulopathies such as Menière`s disease and vestibular neuritis derives from earlier studies. Perez and Rama-Lopez [13] found the clinical HIT to be a low sensitive but highly specific test when compared with the caloric test. In patients with chronic vestibular neuritis (VN), Mahringer and Rambold [7] have recently demonstrated that a caloric test asymmetry of >25 % was associated with a pathologic vHIT in only 33 %, whereas Schmidt-Priscoveanu et al. [14] found that high-frequency VOR did not recover in a follow-up of VN [7, 13, 14]. The latter is in line with former studies, which have shown that the VOR remains deficient in response to high-frequency, high-acceleration or high-velocity rotations [15–17].
In general, the results of our study and literature data show that in the case of VS and other peripheral vestibulopathies both low (caloric) and high- (vHIT) frequency tests are necessary to reflect the whole hVOR frequency spectrum in case of impairment. The clinical relevance of the caloric test and vHIT is supported by the finding that in only few VS cases a normal caloric test was accompanied by an abnormal vHIT.
Tringali et al. [18] observed a good correlation of caloric weakness and tumor grade in a series of 629 patients. The frequency-dependent diagnostic of VS in our study reveals that there is only a tumor grade dependent influence on caloric test results but not on vHIT results. This can be interpreted as a result of a different susceptibility of regular and irregular vestibular nerve fibers due to tumor pressure, since in semicircular canals regular afferents have smaller axon diameter [19]. Furthermore, it has been shown that even the vertical canals can contribute to hVOR answer thus influencing the caloric test result [20], but in contrast there has been no evidence of vertical canal contribution to hVOR answer in vHIT until now. Since VS is a slow growing tumor, vestibular impairment is a chronic process accompanied by central vestibular compensation. For high-frequency hVOR, there may be proposed a higher ability to recover, which has been demonstrated in a vestibular neuritis follow-up study with vHIT and caloric test [8]. Furthermore, it has been shown that different parts of vestibulo-cerebellum (e.g., nodulus/uvula) are attributed to a different modulation of the slow component hVOR (velocity storage) and VOR gain [21, 22].
In our study, linear regression analysis reveals only a moderate correlation of caloric and vHIT test results. Comparable data have been shown in Menière’s disease (MD) before and after gentamicin treatment using UWCaloric and search-coil head thrust test or in VS with motorized head impulse test [4, 5, 23]. The intercepts/slopes of the linear regression equation fitting UWCaloric as a function of AR-GainvHIT of our study is in between that for MD as well as chronic VN and acute VN (54.7/0.26) and near to mixed acute plus chronic VN in former studies (caloric/search-coil measurements) [4, 14]. Therefore, disparity of caloric and vHIT test seems to be dependent more on whether the disease is acute or chronic than on a specific etiology in peripheral vestibular pathologies.
For assessment of high-frequency hVOR recent studies have used besides the occurrence of refixation saccades (overt/covert) either ipsilesional gain or gain asymmetry for quantification of vHIT results. Using the vHIT in different peripheral vestibulopathies it has been demonstrated that both the ipsi- and contralesional gain can be reduced and in rare cases ipsilesional hVOR gain reduction can even occur without refixation saccades [24]. In this study, we therefore compared ipsilesional gain (Mean-GainvHIT) and gain asymmetry ratio (AR-GainvHIT). Combined use of AR-GainvHIT and saccades revealed more abnormal cases in vHIT and provides a better correlation with respect to the UWCaloric than Mean-GainvHIT. But since correlation of UWCaloric and AR-GainvHIT or Mean-GanvHIT in VS patients is significant but only moderate, it cannot conclusively predict that an abnormal caloric test is always associated with an abnormality in vHIT. In ROC analysis, we determined the optimal degree of UWCaloric to separate between normal and abnormal if vHIT test is used to discriminate between these. Our discriminant UWCaloric in VS is close to those which have been reported using caloric and bedside HIT or vHIT in other peripheral vestibulopathies [8, 13]. Therefore, vHIT is more sensitive in the presence of a severe canal paresis [25–27].
Using either Mean-GainvHIT or AR-GainvHIT, we determined vHIT sensitivity and specificity as well as positive and negative predictive test values. Liu et al. [28] found the sensitivity, specificity, positive and negative predictive value of HIT to be of 54.9, 90.6, 86.7, and 64.4 %, respectively, if the results of caloric test were considered as the standard method to evaluate the hVOR. In our study, considering the MRI as standard method to detect a VS, these results could only be reached by changing the cut-off values for Mean-GainvHIT to >0.9 and AR-GainvHIT to <8.
Conclusion
For detection of hVOR impairment within a broad frequency spectrum, both caloric testing and vHIT are necessary in VS. Our findings suggest substantial preservation of high-acceleration canal function. This has to be considered in surgical interventions or radiotherapy for VS, since then they can still cause vertigo symptoms. VS tumor grade has an influence on caloric test results but not on vHIT. AR-GainvHIT should be used in assessment of hVOR function. A frequency dynamic analysis of hVOR function is recommended in acute or chronic peripheral vestibulopathies.
References
Agrawal Y, Schubert MC, Migliaccio AA, Zee DS, Schneider E, Lehnen N, Carey JP (2013) Evaluation of quantitative head impulse testing using search coils versus video-oculography in older individuals. Otol Neurotol 27
Weber KP, MacDougall HG, Halmagyi GM, Curthoys IS (2009) Impulsive testing of semicircular-canal function using videooculography. Ann N Y Acad Sci 1164:486–491
MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS (2009) The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology 73:1134–1141
Park HJ, Migliaccio AA, Della Santina CC, Minor LB, Carey JP (2005) Search-coil head-thrust and caloric tests in Menière`s disease. Acta Otolaryngol 8:852–857
Nguyen KD, Minor LB, Della Santina CC, Carey JP (2009) Vestibular function and vertigo control after intratympanic gentamicin for Menière`s disease. Audiol Neurootol 14:361–372
Zellhuber S, Mahringer A, Rambold HA (2013) Relation of video-head-impulse test and caloric irrigation: a study on the recovery in unilateral vestibular neuritis. Eur Arch Otorhinolaryngol 6
Mahringer A, Rambold HA (2013) Caloric test and video-head-impulse: a study of vertigo/dizziness patients in a community hospital. Eur Arch Otorhinolaryngol 15
Bartolomeo M, Biboulet R, Pierre G, Mondain M, Uziel A, Venail F (2013) Value of the video head impulse test in assessing vestibular deficits following vestibular neuritis. Eur Arch Otorhinolaryngol 29
Machner B, Gottschalk S, Sander T, Helmchen C, Rambold H (2007) Intralabyrinthine schwannoma affecting the low but not high frequency function of the vestibulo-ocular reflex: implications for the clinical diagnosis of chronic peripheral vestibular deficits. J Neurol Neurosurg Psychiatry 78:772–774
Formby C, Robinson DA (2000) Measurement of vestibular ocular reflex (VOR) time constants with a caloric step stimulus. J Vestib Res 10:25–39
Samii M, Matthies C (1997) Management of 1000 Vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery 40:11–23
Sills AW, Baloh RW, Honrubia V (1977) Caloric testing 2: results in normal subjects. Ann Otol Rhinol Laryngol Suppl 86:7–23
Perez N, Rama-Lopez J (2003) Head-impulse and caloric tests in patients with dizziness. Otol Neurotol 24:913–917
Schmid-Priscoveanu A, Bohmer A, Obzina H, Straumann D (2001) Caloric and search-coil head-impulse testing in patients after vestibular neuritis. J Assoc Res Otolaryngol 2:72–78
Halmagyi GM, Curthoys IS, Cremer PD, Henderson CJ, Todd MJ, Staples MJ, D’Cruz DM (1990) The human horizontal vestibulo-ocular reflex in response to high-acceleration stimulation before and after unilateral vestibular neurectomy. Exp Brain Res 81:479–490
Gilchrist DP, Curthoys IS, Cartwright AD, Burgess AM, Topple AN, Halmagyi M (1998) High acceleration impulsive rotations reveal severe long-term deficits of the horizontal vestibulo-ocular reflex in the guinea pig. Exp Brain Res 123:242–254
Lasker DM, Hullar TE, Minor LB (2000) Horizontal vestibulo-ocular reflex evoked by high-acceleration rotations in the squirrel monkey. III. Responses after labyrinthectomy. J Neurophysiol 83:2482–2496
Tringali S, Charpiot A, Ould MB, Dubreuil C, Ferber-Viart C (2010) Characteristics of 629 vestibular schwannomas according to preoperative caloric responses. Otol Neurotol 31:467–472
Baird RA, Desmadryl G, Fernandez C, Goldberg JM (1988) The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60:182–203
Aw ST, Haslwanter T, Fetter M, Heimberger J, Todd MJ (1998) Contribution of the vertical semicircular canals to the caloric nystagmus. Acta Otolaryngol 118(5):618–627
Killian JE, Baker JF (2002) Horizontal vestibulo-ocular reflex (VOR) head velocity estimation in Purkinje cell degeneration (pcd/pcd) mutant mice. J Neurophysiol 87:1159–1164
Cohen H, Cohen B, Raphan T, Waespe W (1992) Habituation and adaptation of the vestibulo-ocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res 90:526–538
Hirvonen M, Aalto H, Petteri Hirvonen T (2008) Motorized head impulse rotator in patients with vestibular schwannoma. Acta Otolaryngol 128:1215–1220
Blödow A, Pannasch S, Walther LE (2013) Detection of isolated covert saccades with the video head impulse test in peripheral vestibular disorders. Auris Nasus Larynx 40:348–351
Beynon GJ, Jani P, Baguley DM (1998) A clinical evaluation of head impulse testing. Clin Otolaryngol 23:117–122
Harvey SA, Wood DJ, Feroah TR (1997) Relationship of the head impulse test and head-shake nystagmus in reference to caloric testing. Am J Otol 18:207–213
Hamid M (2005) More than 50 % canal paresis is needed for the head impulse test to be positive. Otol Neurotol 26:318–319
Liu B, Kong WJ (2011) Evaluation of the vestibular ocular reflex in patients with unilateral peripheral vestibular disorder by the head impulse test. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 46:40–43
Kim HJ, Lee SH, Park JH, Choi JY, Kim JS (2014) Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol 261:121–129
Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE (2009) HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 40:3504–3510
Straka H, Lambert FM, Pfanzelt S, Beraneck M (2009) Vestibulo-ocular signal transformation in frequency-tuned channels. Ann N Y Acad Sci 1164:37–44
Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE (2007) Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci 27:771–781
Conflict of interest
The authors declare no financial interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blödow, A., Blödow, J., Bloching, M.B. et al. Horizontal VOR function shows frequency dynamics in vestibular schwannoma. Eur Arch Otorhinolaryngol 272, 2143–2148 (2015). https://doi.org/10.1007/s00405-014-3042-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-3042-2