Abstract
We sought to investigate the utility of new non-invasive tests of semicircular-canal and otolith function that are usable in the neuro-otology office practice in patients with vestibular schwannoma. Fifty patients with vestibular schwannoma were assessed using a 5-item battery consisting of air-conducted cervical- and bone conducted ocular-vestibular-evoked myogenic potentials (AC cVEMPs and BC oVEMPs) and video head impulse testing (vHIT) in all three canal planes. VEMP asymmetry ratios, latencies, and vHIT gains were used to determine the test sensitivity, relationship with tumour size and the pattern of vestibular nerve involvement. The percentage of abnormalities for each of the five tests for the entire sample ranged between 36.2–61.7 %. In 58.3 % of patients, test abnormalities were referable to both superior and inferior vestibular nerve divisions. Selective inferior nerve dysfunction was identified in 10.4 % and superior nerve dysfunction in 12.5 %. The remaining 18.8 % of patients demonstrated a normal test profile. The sensitivity of the 5-item battery increased with tumour size and all patients with medium to large (>14 mm) schwannoma had at least two abnormal vestibular test result. Our results indicate that dysfunction of the superior and inferior vestibular nerve evolves in parallel for most patients with schwannoma. Unexplained vHIT and VEMP asymmetry should alert otologists and neurologists to undertake imaging in patients presenting with non-specific disequilibrium or vertigo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vestibular schwannoma (VS), a benign tumour of the vestibular nerve, commonly presents with unilateral hearing loss, tinnitus and disequilibrium, which are attributed to a combination of compressional/neurotoxic effects on eighth nerve afferents and/or compromised vascular supply to the inner ear [1–3]. As it enlarges a schwannoma may exert pressure on adjacent nerves and brainstem structures, leading to increased morbidity [4]. These rare, but potentially serious consequences create a need for sensitive tools for screening and monitoring.
Asymmetrical thresholds on pure-tone audiometry are a well recognised “red flag” for the initiation of investigations for a retro-cochlear cause of hearing loss [3, 5]. However, of those who proceed to have MRI, only 1–2 % will be diagnosed with a vestibular schwannoma [5, 6]. Despite its origin from the vestibular nerve, the role of vestibular function testing in VS is poorly defined. Most studies undertaken prior to 2007 were restricted to the use of the caloric test and cervical vestibular evoked myogenic potentials, which assess only horizontal semicircular canal and saccular function. Caloric testing is further renowned for its unpleasant vestibular symptoms and is not easily implemented in the busy office practice.
Vestibular function testing has evolved considerably over the last decade. Semicircular canal function can now be evaluated non-invasively using video head-impulse testing (vHIT) in all canal planes [7, 8]. Ocular and cervical vestibular evoked myogenic potentials (oVEMP and cVEMP) enable testing of the two otolith organs (sacculus and utricle) and their afferents [9–11]. The combined application of these tests provides a more detailed representation of vestibular function, enabling the identification of lesions specific to each end-organ or nerve division.
MRI scanning undeniably represents the gold standard test for excluding vestibular schwannoma in the patient with unilateral tinnitus or asymmetric hearing. The purpose of this study was not to seek an alternative test to magnetic resonance imaging, but to explore the role of vestibular testing as an additional source of information. We further sought the effects of tumour size and patterns of test abnormalities that were indicative of the nerve of origin.
Methods
This study was undertaken within the Neurology department of the Royal Prince Alfred Hospital, Sydney Australia from 2012–2014. Fifty patients (25 females and 25 males aged 26–89 years: mean = 57.4 ± 15.5) with confirmed unilateral vestibular schwannoma were recruited from neurology, neurosurgery and otolaryngology clinics. There were 26 left and 24 right vestibular schwannoma identified by brain MRI inclusive of T1 and T2-weighted axial, sagittal and coronal views with fine (0.5 mm) cuts through the internal acoustic meati. For 24 patients, medical attention was initially sought due to auditory/aural symptoms; 13 patients were investigated principally for imbalance or vertigo while a further eight patients were equally concerned about auditory and vestibular symptoms. Five patients had non-vestibular presentations including headache (n = 2), recurrent vomiting (n = 1), facial numbness (n = 1) and blunt head injury secondary to a fall (n = 1), which led to magnetic resonance imaging. All patients underwent a detailed history, clinical examination and audiological assessment. The study received local ethics committee approval and written informed consent was obtained in accordance with the 1964 Declaration of Helsinki and its later amendments.
The five-test battery
A 5-item test battery, designed to represent each individual vestibular end-organ, was used to investigate the pattern of vestibular nerve/end-organ involvement. As indicated by the schematic diagram in Fig. 1, posterior semicircular canal (PSC) vHIT and air-conducted (AC) cVEMPs provided two corroborative tests of inferior nerve function. Conversely, superior nerve function was inferred from the bone-conducted (BC) oVEMP, horizontal and anterior canal (HSC and ASC) vHIT.
Vestibular evoked myogenic potentials (VEMPs)
OVEMPs and cVEMPs were recorded using a Medelec Synergy EMG/EP system (version15). cVEMPs were elicited in response to AC clicks (0.1 ms, 140 dB peak SPL) presented monaurally via TDH 49 head phones. For oVEMPs we used BC “minitaps”. These minitaps were generated in response to a 1.0 ms square-wave pulse (condensation polarity) that was fed through an external amplifier (20 V amplitude = 147 dB force level) and delivered via a hand-held bone-vibrator (Bruel and Kjaer 4810 minishaker) in the midline of the head at Fz. Both AC and BC stimuli were presented at a rate of 5/sec.
All VEMP testing was conducted while the subject lay reclined at an angle approximately 30° above the horizontal, using methods described previously [12]. Briefly, oVEMPs were recorded during maximum upward gaze from infra-orbital electrodes placed beneath the eyes. For cVEMP testing, responses were recorded from the contracted sternocleidomastoid (SCM) muscles during head elevation. The primary outcome measure for both reflexes was the amplitude asymmetry ratio (AR), defined as the percentage difference in peak–peak amplitudes recorded from the tumour (T) and non-tumour (nT) ear: %AR = 100 × [(nT − T)/(nT + T)]. Responses that were absent from one ear were assigned an amplitude of zero, resulting in AR = 100 %. Reflex latencies (first peak of each VEMP response in Fig. 1) provided a secondary outcome measure. In our laboratory, based upon data collected from 77 healthy controls, an abnormal AR (mean + 2SD) is 30.1 % (AC cVEMP) and 38.9 % (BC oVEMP), whereas latencies greater than the mean + 2SD of 13.1 (AC cVEMP) and 10.2 ms (BC oVEMP) are considered significantly prolonged. Three patients had evidence of middle ear pathology and were treated as missing data in the analysis of AC VEMP results.
Video head-impulse-testing (vHIT)
Semicircular canal function was evaluated using a 3-D video head impulse system (GN Otometrics, Taastrup, Denmark). The system consisted of a pair of light-weight goggles containing 3-D gyroscopes to measure head velocity, and a small mounted video camera to record eye position. The video camera was contained within the right eye-frame of the goggles, which were secured firmly to the subject’s head with an adjustable elastic strap.
At the commencement of testing the patient was instructed to fixate on a target positioned approximately 1.1–1.3 metres in front of them. The examiner, while holding the patients head from behind, then made a series of brisk head movements (10–20° amplitude) corresponding to the horizontal, left anterior-right posterior (LARP) and right anterior-left posterior (RALP) canal planes. Eye and head velocities were sampled at 250 Hz and the ratio of eye-to-head velocity (VOR gain) was calculated from the average of at least 20 head impulses performed over a range of velocities (50–300°/s). Results were compared with the mean gain −2SD from a sample of 40 healthy controls (16 male and 24 female) aged 26–89 years (56.4 ± 15.4). Normal limits were calculated separately for left versus right canal-plane head impulses (Table 1) to account for gain asymmetries introduced through use of a monocular recording technique (right eye only) [13, 14]. All vHIT results (controls and patients) were analysed using version 2 of the GN Otometrics software.
Data analysis
All data were analysed using SPSS version 21. Descriptive statistics are represented in the text and tables as the mean ± SD (continuous data) or as a percentage (categorical data). McNemar’s test was used to compare percentages of test abnormalities for the entire group, whereas rates of abnormalities were compared across groups classified according to maximum tumour diameter (group 1 ≤ 14 mm, group 2 = 15–29 mm and group 3 > 30 mm) using the Chi square test. This method of grouping was based on the recommendation by Stragerup and Caye-Thomasen [15] that 15 mm be used as a cut-off point for schwannoma intervention. As some of the variables were not normally distributed, we used Spearman’s correlation coefficient r s to explore the relationship between the original test results (%AR, vHIT gain and pure-tone asymmetry in dB) and maximum tumour diameter. Unless otherwise stated, significance levels initially set at the 5.0 % level, were adjusted using Holm-Bonferonni corrections.
Results
The maximum tumour diameter ranged from 2.6–60 mm (mean = 19.3 ± 14.6 mm) with 24, 15 and 11 patients in groups 1, 2 and 3 (≤14, 15–29, ≥30 mm). On questioning, auditory symptoms were reported by 92.0 % (tinnitus: 66.0 %, aural fullness: 42.0 %, hearing loss: 76.0 %) and vestibular symptoms by 70.0 % (disequilibrium: 60.0 %, vertigo: 32.0 %) of patients. Additional neurological symptoms included facial numbness (20.0 %), facial weakness (4.0 %) and swallowing difficulties (8.0 %).
Audiological investigations
Three patients with pre-existing severe-profound hearing loss were excluded from the analysis of hearing asymmetries. Using the recommendations of Obolzer et al., asymmetrical hearing loss was defined as a ≥15 dB inter-aural difference at two adjacent frequencies if the average threshold at octave frequencies from 250–8000 Hz was ≤30 dBHL for the better hearing ear. This value was set to ≥20 dB if the average threshold exceeded 30 dBHL [5]. Hearing asymmetry was identified in 37/47 (78.7 %) patients (mean asymmetry at two adjacent frequencies = 33.1 ± 24.9 dB). For 80.9 % of patients, the greatest inter-aural difference was over the high frequency range of hearing (≥2 kHz). Mid-frequency asymmetry (1–2 kHz) was most pronounced in 12.7 % of patients. Three patients (6.4 %) showed greater low frequency (<1 kHz) hearing asymmetry.
VEMPs
cVEMPs were preserved from all healthy (non-tumour) ears. Using our laboratory criteria, which defines the upper limit for normal cVEMP asymmetry as AR = 30.1 %, 23 of 47 patients (48.9 %) had asymmetrical AC cVEMPs localising to the side of the tumour. Most (16/23) had absent responses (AR = 100 %), whereas seven patients demonstrated preserved responses that were reduced in amplitude. BC oVEMP amplitude asymmetry (AR > 38.9 %) localising to the tumour side totalled 25/50 patients (50.0 %). For 16/25 patients, BC oVEMPs were absent; 9/25 patients had a preserved response that was reduced in amplitude. One patient had bilaterally absent BC oVEMPs.
On considering reflex latencies, 2/7 patients with reduced AC cVEMP amplitudes and 4/24 patients with normal responses had prolonged p13 latencies. Prolonged BC oVEMP latencies were recorded from 5/9 patients with asymmetrical responses and from three patients with normal oVEMP %AR. This increased the overall percentage of AC cVEMP and BC oVEMP abnormalities (%AR and/or prolonged latencies) to 57.4 and 56.0 %, respectively.
vHIT
Three patients with missing vHIT data in one or more canal planes were excluded from the analysis of vHIT results. Mean VOR gain was significantly lower on the affected side for all three vHIT tests (affected ears: HSC = 0.75 ± 0.24, ASC = 0.74 ± 0.30, PSC = 0.64 ± 0.30; unaffected ears: HSC = 0.90 ± 0.16, ASC = 0.90 ± 0.25, PSC = 0.81 ± 0.26; paired t tests, p ≤ 0.002). On comparing the results of tumour ears with the 95.0 % range of control subjects in Table 1, 61.7, 36.2 and 56.5 % demonstrated reduced VOR gains for HSC, ASC and PSC head impulses. McNemar tests indicated ASC vHIT produced fewer abnormalities than either HSC (p = 0.002) or PSC vHIT (p = 0.031).
Relationship between tumour size and audio-vestibular test results
There were many inter-correlations between the variables and all test parameters were significantly correlated with tumour size (p < 0.01, Table 2). HSC vHIT gain demonstrated the strongest relationship with tumour size (r s = −0.72, p < 0.001), followed by the %AR for the BC oVEMP and AC cVEMP (0.71 and 0.50, p < 0.001). Thus, larger tumours tended to be associated with lower horizontal vHIT gain and higher cVEMP and oVEMP % asymmetry ratios. Significant, but weaker correlations were evident between tumour size and ASC vHIT gain, PSC vHIT gain and hearing asymmetry (r s = −0.48, −0.45, 0.41 p < 0.01).
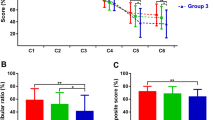
Figure 2 illustrates the percentage of abnormal test results for the three groups classified according to tumour size. Even after adjustment for multiple comparisons (Holm-Bonferroni), Chi square tests confirmed a significant relationship between tumour size and rates of abnormal vHIT gain (all canals), BC oVEMP and AC cVEMP asymmetry i.e., as the size of the tumour increased beyond 14 mm, so did the sensitivity of each vestibular function test. In contrast, the percentage of patients with clinically significant hearing asymmetry was not significantly different across the three groups. Due to the similar percentages of vestibular abnormalities for groups 2 and 3, the data were combined into one group (schwannoma > 14 mm) for the remainder of the analysis. Descriptive statistics for the combined data are provided in Table 3. For schwannoma exceeding a 14 mm diameter, sensitivity was over 90.0 % for both otolith function tests (AC cVEMP and BC oVEMP), and over 59.0 % for all vHIT tests.
Percentage of test abnormalities for patients grouped according to maximum tumour diameter. The relationship between the percentage of abnormal results and tumour size is indicated by the p value for each Chi square test. Hearing asymmetry was based on the average difference (in dB) for the two adjacent pure-tone frequencies demonstrating the largest inter-aural difference: three patients with pre-existing hearing loss were excluded from this part of the analysis; for patients with evidence of middle ear pathology (n = 3), asymmetry was determined from masked bone-conduction thresholds
Vestibular profiles identified using a 5-item vestibular test battery
Over half the patients (58.3 %) demonstrated abnormalities referable to both vestibular nerve divisions. Abnormalities referable to the inferior vestibular nerve were identified in 10.4 % of patients (isolated PSC n = 1; PSC and saccule n = 3; saccule only n = 1), while a further 12.5 % demonstrated a superior pattern of vestibular nerve involvement (HSC only n = 3, HSC and utricle n = 2; combined utricular, ASC and HSC deficits n = 1). Nine of 48 patients (18.8 %), all with schwannoma ≤14 mm (group 1), had no vestibular test abnormalities. The results in Fig. 3, shown separately for schwannoma ≤14 mm (group 1) and >14 mm (groups 2 and 3), indicate that a “mixed pattern” of vestibular nerve involvement was common for larger sized schwannoma.
Profiles of vestibular nerve involvement are shown separately for schwannoma ≤14 mm (group 1) and >14 mm (groups 2 and 3). Inferior vestibular nerve involvement was inferred from either a reduced posterior vHIT gain and/or an AC cVEMP asymmetry ratio exceeding normal limits. Classification of superior nerve involvement required either an abnormal asymmetry ratio on BC oVEMP testing and/or a reduced gain for one or both of horizontal and anterior vHIT. The number of patients included in each pie chart (i.e. with sufficient results to reliably infer the pattern of nerve involvement) is indicated
Descriptive statistics based on the number of vestibular abnormalities (1–5) are summarised in Table 4 for small (≤14 mm) and medium to large (>14 mm) schwannoma. All patients with VS > 14 mm had at least two abnormal vestibular tests and ten of 22 (45.5 %) with a complete set of results had abnormalities on all five tests. Thirteen (59.1 %) of 22 patients with VS ≤ 14 mm had at least one vestibular abnormality, yet none of the patients had abnormalities on all 5 tests. Two of 24 patients with VS > 14 mm (8.3 %) and eight of 23 patients with VS ≤ 14 mm (34.8 %) demonstrated symmetrical hearing; four of these eight patients had at least one abnormal vestibular test result. Representative results for a patient with a large schwannoma, showing 5/5 test abnormalities, are compared with the normal vestibular test profile of a patient with a small (12 mm) schwannoma in Fig. 4.
Vestibular test (vHIT and VEMP) profiles and axial MRI results of a small and large schwannoma. The right panel shows the results for a patient with a large 33 mm right VS. The patient demonstrates abnormalities on all five vestibular function tests: VOR gain, calculated as the ratio of eye velocity (left = blue; right = red) to head velocity (black), is reduced in all canal planes and VEMPs are absent from the affected ear. For the patient in the left panel, the tumour measured 12 mm in diameter. Although this patient’s VEMP reflexes appear slightly asymmetrical, the asymmetry ratios fall within normal limits. vHIT results indicate some covert catch-up saccades for horizontal and posterior canal head impulse, yet VOR gains are within the normal range
Discussion
This study represents the first comprehensive evaluation of clinically applicable vestibular tests in patients with vestibular schwannoma. Vestibular test abnormalities were usually referable to both vestibular nerve divisions and all tests demonstrated a significant relationship with tumour size. A normal vestibular test profile was observed only in tumours ≤14 mm while the likelihood of a larger schwannoma increased with the number of test abnormalities.
Sensitivity of new vestibular function tests in VS
The overall prevalence of otolith abnormalities, based on asymmetrical amplitudes, was 48.9 % for the AC cVEMP and 50.0 % for the BC oVEMP. Inclusion of patients with prolonged latencies increased the percentages to 57.4 and 56.0 %, respectively. These results are comparable with those of Kinoshita et al. [16] (AC cVEMP = 64.4 %; BC oVEMP = 68.9 %) who, like us, found no statistically significant difference in the percentage of abnormalities for the two tests. Lower rates of abnormal AC cVEMPs (38.5 %) and BC oVEMPs (15.4 %) were reported by Piras et al. [17] when using 500 Hz tone-bursts. However, these authors investigated only 26 patients with small to medium sized tumours (mean diameter = 10.5 ± 5.2 mm), which probably explains their reduced number of abnormalities and weaker correlations between test results.
There have been no comprehensive reports of three dimensional vHIT as a screening tool for VS. Blodow et al. [18], using only horizontal vHIT, reported reduced gain of 0.76 ± 0.28 and sensitivity of 41.0 % for a group of 46 schwannoma patients. Rates of vHIT abnormalities in our study, like the VEMP results, depended on tumour size, ranging from 13.0–34.8 % for schwannoma less than 15 mm to over 70.0 % for schwannoma exceeding a 29 mm diameter. We feel these statistics are probably a conservative estimate of the incidence of canal dysfunction. In addition to VOR gain, which has been validated as an objective outcome measure, another indicator of a semicircular canal deficit is the presence of pathological catch-up saccades [7, 8]. However, the frequency, latency and amplitude characteristics of catch-up saccades have not been fully explored as a means of separating normal from pathological ears. The future identification of other objective parameters may further improve the sensitivity of vHIT testing in VS.
Do test abnormalities identify the nerve of origin?
An advantage of a test battery, combining vHIT with VEMPs, is its potential to identify patterns of nerve/end-organ involvement. For example, Walther and Blodow [19], using vHIT together with AC cVEMP and AC oVEMP, identified four profiles that characterised patients with vestibular neuritis; entire (both nerve divisions), superior, inferior and selective ampullary (canal afferents only). We found little evidence of selective inferior or superior nerve subtypes in our VS patients (Fig. 3). Rather, both nerve divisions tended to be similarly affected. Horizontal vHIT gain, which depends on the superior ampullary nerve afferents, correlated equally well with BC oVEMP and AC cVEMP asymmetry, each reflecting superior and inferior otolith afferent function, respectively. These unexpected inter-correlations and the global deficits observed in larger schwannomas (Fig. 3b) indicate that superior and inferior nerve dysfunction tends to evolve in parallel. This is in accordance with an earlier study by Suzuki et al [20] using only AC cVEMPs and calorics, which showed vestibular abnormalities were largely independent of the nerve of schwannoma origin. For the small percentage (22.9 %) of schwannomas that did produce abnormalities pointing to a single nerve, it is possible that this information could be used in surgical planning.
Do test abnormalities correlate with tumour size?
All vestibular test results demonstrated a relationship with tumour size and the number of abnormalities (0–5) increased significantly for VS greater than 14 mm. However, while we found good correlations between most vestibular test results and tumour size, not all studies have demonstrated a relationship [19, 21]. For some, sample size may have been an issue. Differences in the classification and range of tumour sizes can further be expected to produce different results across laboratories, as can the method of statistical analysis. For vHIT, the technique and experience of the examiner is an additional consideration. Horizontal head impulse testing is relatively easy to perform and not surprisingly this test yielded the strongest correlation with tumour size. Posterior and anterior canal head-impulses are more technically challenging [7] and as our control data suggest, are associated with greater variance in VOR gain measurements. This probably contributed to their weaker association with tumour size and lower rates of abnormalities for anterior canal vHIT.
Clinical implications
Vestibular testing as a potential screening tool for schwannoma
Some schwannoma patients, including 21.4 % in this study, have symmetrical hearing and may seek medical attention for reasons other than auditory symptoms. In a study by Lustig et al. [4], who explored reasons for imaging patients with symmetrical hearing, vertigo and disequilibrium were the most common indicators that led to the discovery of a schwannoma. However, these symptoms are common in the general population [22] and it would be impractical to image all symptomatic patients. Simple laboratory tests such as VEMPs and vHIT could help identify those patients with vertigo or imbalance who merit imaging to exclude medium to large schwannomas.
A limitation of the present study is that we did not include an additional control group with similar presenting symptoms, but with negative MRI results. Therefore the specificity of VEMPs and vHIT in schwannoma remains uncertain. To our knowledge there has been only one study that has examined both the sensitivity and specificity of AC cVEMPs in large series. Ushio et al. [23] reviewed the AC cVEMP results of 803 consecutive patients attending a vertigo clinic, including 78 patients with a schwannoma. They reported a sensitivity of 80.8 % and specificity of 52.7 %, which is similar to specificity statistics reported for hearing asymmetry criteria [5]. Ushio et al. [23] acknowledged that the specificity was not high and suggested that this may have been due in part to selection bias. By investigating patients from a vertigo clinic, vestibular dysfunction is likely to have been over represented.
Despite the uncertain specificity, the prevalence of VEMP and vHIT abnormalities in schwannoma >14 mm is high. All patients in this group had at least two abnormal test results and just under half had abnormalities on all five tests. We therefore recommend that vestibular function, as measured using VEMPs and vHIT, be an additional consideration in the risk assessment for eighth nerve pathology. In our opinion MR imaging should be undertaken in any patient with asymmetric vestibular function not easily explained by another well defined cause such as vestibular neuritis. Conversely, our data suggest a normal vestibular profile (0 abnormalities) virtually excludes schwannoma larger than 14 mm. This finding obviously requires confirmation by other large series studies. Future investigations in patients with both auditory and vestibular symptoms are also needed to confirm the specificity of vHIT and VEMPs in conjunction with existing audiometric criteria.
Schwannoma monitoring
Whereas surgical intervention used to be common practice, most small schwannoma are now managed conservatively using the “watch and rescan” approach [15]. However, there are instances when MRI scanning is precluded due to cardiac pacemakers or metallic foreign bodies, obesity and claustrophobia [24]. Our results, which demonstrate a significant relationship between tumour size and vestibular-test abnormalities, imply a potential role for these tests as adjunctive monitoring tools when MRI is not accessible. Future longitudinal studies involving serial VEMP and vHIT testing, in conjunction with MRI, are needed to clarify the precise relationship between tumour growth and vestibular test abnormalities.
Post-operative planning
Quantitative vestibular testing is not routinely used in the pre- operative/radiation assessment of VS. However, sudden deafferentation and loss of vestibular function can produce disabling vertigo and ongoing balance problems in a proportion of post-ablation VS patients [25–27]. Detailed information regarding residual pre-operative function, as provided through combined VEMP and vHIT testing, may help identify at risk patients and initiate vestibular pre-habilitation.
Conclusions
In patients presenting with non-specific disequilibrium or vertigo, unexplained unilateral VHIT and VEMP asymmetry should alert otologists and neurologists to undertake imaging. The vestibular test profile in most cases does not indicate selective vestibular nerve involvement. In patients with known schwannomas awaiting ablation, these tests will quantify residual vestibular function and help clinicians to plan post-operative management.
References
Merchant S, McKenna M (2010) Neoplastic growth. In: Merchant S, Nadol J Jr (eds) Schuknecht’s pathology of the ear, 3rd edn. People’s Medical Publishing House, USA, pp 492–499
Jackler RK, Schindler RA (1994) A century ago in the American journal of otology. Am J Otol 15:117–120
Selesnick SH, Jackler RK, Pitts LW (1993) The changing clinical presentation of acoustic tumors in the MRI era. Laryngoscope 103:431–436
Lustig L, Rifkin S, Jackler R, Pitts L (1998) Acoustic neuromas presenting with normal or symmetrical hearing: factors associated with diagnosis and outcome. Am J Otolaryngol 19:212–218
Obholzer RJ, Rea PA, Harcourt JP (2004) Magnetic resonance imaging screening for vestibular schwannoma: analysis of published protocols. J Laryngol Otol 118:329–332
Mahrous AK, Kalepu R (2008) Positive findings on MRI in patients with asymmetrical SNHL. Eur Arch Otorhinolaryngol 265:1471–1475
Macdougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP (2013) The video head impulse test (vHIT) detects vertical semicircular canal dysfunction. PLoS One 8:e61488
Weber KP, MacDougall HG, Halmagyi GM, Curthoys IS (2009) Impulsive testing of semicircular-canal function using video-oculography. Ann N Y Acad Sci 1164:486–491
Colebatch JG, Halmagyi GM, Skuse NF (1994) Myogenic potentials generated by a click-voked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 57:190–197
Iwasaki S, McGarvie LA, Halmagyi GM et al (2007) Head taps evoke a crossed vestibulo-ocular reflex. Neurology 68:1227–1229
Todd NP, Rosengren SM, Aw ST, Colebatch JG (2007) Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol 118:381–390
Taylor RL, Wijewardene AA, Gibson WP, Black DA, Halmagyi GM, Welgampola MS (2011) The vestibular evoked-potential profile of Meniere’s disease. Clin Neurophysiol 122:1256–1263
Weber KP, Aw ST, Todd MJ et al (2008) Inter-ocular differences of the horizontal vestibulo-ocular reflex during impulsive testing. Prog Brain Res 171:195–198
Viirre E, Tweed D, Milner K, Vilis T (1986) A reexamination of the gain of the vestibuloocular reflex. J Neurophysiol 56:439–450
Stangerup SE, Caye-Thomasen P (2012) Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am 45:257–268, vii
Kinoshita M, Iwasaki S, Fujimoto C et al (2013) Ocular vestibular evoked myogenic potentials in response to air-conducted sound and bone-conducted vibration in vestibular schwannoma. Otol Neurotol 34:1342–1348
Piras G, Brandolini C, Castellucci A, Modugno GC (2013) Ocular vestibular evoked myogenic potentials in patients with acoustic neuroma. Eur Arch Otorhinolaryngol 270:497–504
Blodow A, Helbig R, Wichmann N, Wenzel A, Walther LE, Bloching MB (2013) Video head impulse test or caloric irrigation? Contemporary diagnostic tests for vestibular schwannoma. HNO 61:781–785
Walther LE, Blodow A (2013) Ocular vestibular evoked myogenic potentials to air conducted sound stimulation and video head impulse test in acute vestibular neuritis. Otol Neurotol 34:1084–1089
Suzuki M, Yamada C, Inoue R, Kashio A, Saito Y, Nakanishi W (2008) Analysis of vestibular testing in patients with vestibular schwannoma based on the nerve of origin, the localization, and the size of the tumor. Otol Neurotol 29:1029–1033
Ushio M, Iwasaki S, Chihara Y, Murofushi T (2008) Abnormal deviation of subjective visual horizontal in patients with vestibular schwannoma. Ann Otol Rhinol Laryngol Suppl 117:641–644
Bisdorff A, Bosser G, Gueguen R, Perrin P (2009) The epidemiology of vertigo, dizziness, and unsteadiness and its links to co-morbidities. Front Neurol 4:29
Ushio M, Iwasaki S, Murofushi T et al (2009) The diagnostic value of vestibular-evoked myogenic potential in patients with vestibular schwannoma. Clin Neurophysiol 120:1149–1153
Fortnum H, O’Neill C, Taylor R et al (2009) The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess 13: iii–iv, ix–xi, 1–154
Parving A, Tos M, Thomsen J, Moller H, Buchwald C (1992) Some aspects of life quality after surgery for acoustic neuroma. Arch Otolaryngol Head Neck Surg 118:1061–1064
Tufarelli D, Meli A, Labini FS et al (2007) Balance impairment after acoustic neuroma surgery. Otol Neurotol 28:814–821
Wiegand DA, Fickel V (1989) Acoustic neuroma—the patient’s perspective: subjective assessment of symptoms, diagnosis, therapy, and outcome in 541 patients. Laryngoscope 99:179–187
Acknowledgments
This research was funded by the Garnett Passe and Rodney Williams Memorial Foundation and the National Health and Medical Research Council of Australia. The authors thank Mr Satendra Pratap, Michael Todd and Leigh McGarvie for technical support, and bio-statisticians Ann-Sophie Veillard and Lucy Davies for their advice on the statistical analysis.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
This study received local ethics committee approval and written informed consent was obtained in accordance with the 1964 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taylor, R.L., Kong, J., Flanagan, S. et al. Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. J Neurol 262, 1228–1237 (2015). https://doi.org/10.1007/s00415-015-7697-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7697-4