Abstract
The expression and regulation of serum amyloid A (SAA) in patients with chronic rhinosinusitis with nasal polyps (CRSwNP) have not been well documented. This study enrolled 24 CRSwNP patients and 19 controls to evaluate the expression of SAA in polyp tissues in Chinese adult patients and investigate underlying mechanism. The levels of SAA and interleukin (IL)-17A and myeloperoxidase (MPO) in nasal tissues were detected using quantitative reverse transcription polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. In addition, the mRNA expression of SAA was examined in cultured polyp epithelial cells (PECs) in the presence of various cytokines (IFN-γ, IL-4, IL-5 and IL-17A) using qRT-PCR, and the role of extracellular signal-related kinase (ERK) signalling in SAA expression was evaluated by western blot analysis. We found the levels of SAA, IL-17A and MPO were significantly upregulated in polyp tissues compared with the controls (p < 0.05), and significant correlations between SAA and IL-17A mRNA levels, as well as between SAA and MPO protein levels, were observed in polyp tissues (p < 0.05). In the in vitro culture system, IL-17A was found to significantly increase SAA mRNA expression in PECs via ERK signaling pathway, in a time- and dose-dependent manner (p < 0.05). Our results suggested a regulatory mechanism underlying excessive SAA production in polyp tissues, which might gain more insights into the pathophysiology of CRSwNP in Chinese adult patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a common nasal inflammatory disease with region difference and distinct expression of innate immune markers [1]. The contributing factor to polyp formation can be ascribed to infection, allergy, mucociliary impairment, physical obstruction, etc. Currently, the pathogenic mechanisms of CRSwNP remain controversial and need further elucidation.
According to the European position paper on rhinosinusitis and nasal polyps (E3POS, 2007), it is generally recognized that CRSwNP is characterized by extensive infiltrations of eosinophils and skewed Th2 response in Caucasian population [2]. Interestingly, our recent studies [3, 4], together with other report [5], have demonstrated Chinese CRSwNP patients possess a distinct pathogenic phenotype included additional accumulations of neutrophils and mixed Th1/Th2/Th17 response and lower concomitant asthma, etc. However, the determinant factor for the pathogenesis of CRSwNP in Chinese adult patients is uncertain.
Serum amyloid A (SAA) is traditionally regarded as a kind of acute phase protein, which induces chemotaxis of inflammatory cells [6, 7]. Recent study has shown that SAA also possesses cytokine-like activity and can stimulate the production of cytokines included interleukin (IL)-1β, IL-8 and IL-23, which are related to neutrophilic inflammation and increased levels of myeloperoxidase (MPO) [8]. SAA previously was reported to be involved in CRSwNP, but its importance in polyp progression has not been well documented [9]. Since we recently found CRSwNP patients in China had excessive accumulation of neutrophils [4], we therefore hypothesized that enhanced SAA expression might be the characteristics in polyp tissues in Chinese adult patients. To address this issue, we investigated the expression of SAA, IL-17A and MPO in adult Chinese CRSwNP patients, and evaluated the possible modulation of SAA expression in cultured polyp epithelial cells (PECs) in the presence of various cytokines in vitro.
Materials and methods
Subjects
Twenty-four adult patients with extensive polyps were recruited in the study. The diagnosis was established based on history, clinical examination, nasal endoscopy, and computed tomography (CT) scan of the sinuses, accorded with the standard criterion for CRSwNP issued by E3POS guideline [2]. For histologic analysis, polyp specimens were obtained under local anesthesia. And 19 patients undergoing septoplasty because of anatomical variations were considered as controls and the tissues from the inferior turbinate during septal surgery were sampled. None of the subjects used oral or nasal corticosteroids 4 weeks before surgery. All subjects were nonsmokers and free from upper respiratory tract infection for 4 weeks preceding the study. The demographic data from the patients and controls enrolled in this study were listed in Table 1. This study was approved by the local ethical committee and informed consent was obtained from each subjects.
And each specimen was divided into two parts: one was fixed with 4 % paraformaldehyde and then embedded in paraffin for immunohistochemistry (IHC) staining, and the other was stored immediately at −80 °C for quantitative reverse transcription polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA).
IHC staining
IHC staining was performed using the avidin–biotin–peroxidase method as we previously reported [4]. Briefly, 5-μm-thick sections were deparaffinized by serial treatment. After blocking the endogenous peroxidase in 3 % hydrogen peroxide and with 1 % bovine serum albumin, the sections were incubated overnight at 4 °C in the presence of a mouse anti-human SAA monoclonal antibody (Abcam, Cambridge, MA, USA) at a dilution of 1:100 according to the manufacturer’s instructions. Each of these sections was incubated with a secondary antibody and then with horse radish peroxidase-labeled streptavidin complex (Zhongshanjinqiao, Beijing, China). Distribution of peroxidase was revealed by incubating the sections in a solution containing 3 % 3,3′-diaminobenzidine tetrahydrochloride before being counterstained with hematoxylin and cover slipped. Negative control studies were performed by omitting the incubation step with the primary antibody.
Sections were inspected by a single “blinded” observer in a coded random order and evaluated by using light-microscopic analysis in high-power fields. The staining intensities of five randomly selected areas (400×) on each section of the subjects were summed and averaged. The staining intensity was semi-quantitatively scored as: 0 absent, 1 sparse and weak immunostaining, 2 moderate, 3 strong, 4 extensive and extremely strong.
qRT-PCR
qRT-PCR was performed as we previously reported [4]. Briefly, total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Reverse transcription (RT) was performed and the cDNA was synthesized from 2 μg of total RNA using an oligo (dT) 18 primer and M-MLV reverse transcriptase (TAKARA, Syuzou, Shiga, Japan) for quantitative PCR. Expression of mRNA was determined using ABI PRISM 7500 Detection System (Applied Biosystems, Foster City, CA, USA) and SYBR Premix Taq™ (TAKARA). The sequences of the primer were as follows: SAA forward: 5′-CTT GGC GAG CCT TTT GAT G-3′; reverse: 5′- TAG TTC CCC CGA GCA TGG-3′; IL-17A forward: 5′- AAT CTC CAC CGC AAT GAG CA-3′; reverse: 5′-ACG TTC CCA TCA GCG TTG A-3′; β-actin forward: 5′-AAG ATG ACC CAG ATC ATG TTT GAG ACC-3′; β-actin reverse 5′-AGC CAG GTC CAG ACG CAG GAT-3′. PRISM samples contained 1 × SYBR Green Master Mix 1.5 μL, 5 μM primers, and 25 ng synthesized cDNA in a 25 μL volume. Reactions were heated to 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 10 s, annealing extension at 60 °C for 60 s. All PCR reactions were performed in duplicate. Melting curve analysis was used to control for amplification specificity. Expression of target gene was expressed as fold increase or decrease relative to the expression of β-actin. The mean value of the replicates for each sample was calculated and expressed as cycle threshold (C t). The amount of gene expression was then calculated as the difference (ΔC t) between the C t value of target gene and the t t value of β-actin. Fold changes in target gene mRNA were determined as 2−ΔCt.
ELISA
ELISA was performed as we previously reported [4]. For nasal tissue, 1 ml of PBS containing a protease-inhibitor cocktail (Keygentec, Nanjing, China) was added for every 100 mg of tissue. The sample was homogenized for 1 min on ice. After homogenization, the suspension was centrifuged at 4,000 rpm for 20 min at 4 °C, and the supernatants were stored at −80 °C for ELISA assay of SAA (A bcam) and MPO (R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The detection limits were as follows: SAA 0.75 ng/mL and MPO 1.56 ng/mL.
Cell culture and stimulation
For in vitro cell culture, primary PECs were collected from four CRSwNP patients by means of enzymatic digestion. Collected PECs were cultured in submersion cultures in BEGM medium (Lonza, Walkersville, MD, USA) until passaged. When 80–90 % confluence was reached, the epithelial cells were washed with PBS (37 °C, pH 7.4), and fresh media without hydrocortisone was added in the presence of different stimulators or PBS (control) for 0–24 h. These stimulators included recombinant cytokines such as human IFN-γ (100 ng/mL), IL-4 (100 ng/mL), IL-5 (100 ng/mL), and IL-17A (100 ng/mL) (all were purchased from R&D systems). To evaluate the role of activated extracellular signal-related kinase (ERK) signalling in SAA expression, PD98059 (10 μM) was added as the specific inhibitor. Then, cell pellets were collected for SAA mRNA examination and western blot analysis. The qRT-PCR analysis followed the protocol mentioned above.
Western blot analysis
The cells were collected, and total cellular protein was extracted in 100 μL of RIPA lysis buffer at 4 °C for 30 min. The protein concentration was determined by the Bradford method. Samples containing 10 μg of protein were boiled, subjected to SDS-PAGE in 10 % Tris–glycine gels and transferred electrophoretically to a polyvinylidene fluoride membrane. The membrane was incubated with 5 % fat-free skim milk in Tris-buffered solution (TBS) containing 0.05 % Tween 20 (1 h, room temperature) and then incubated with mouse anti-human p-EKK and ERK (Cell Signalling, Danvers, MA, USA) diluted to 1:2,000 (overnight, 4 °C). The membrane was then incubated with horseradish peroxidase-linked secondary antibody (goat anti-mouse immunoglobulin G, 1:1,000) and finally processed using the ECL chemiluminescence reaction kit (Cell Signalling), followed by exposure on medical film. The relative band density of p-ERK compared with total ERK was quantified with the Bio-Rad Quantity One 1-D Analysis Software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
For tissue examination, data were expressed as the median and interquartile range (IQR), and were analyzed via nonparametric Mann–Whitney U test. The correlation between different mRNA and protein expression levels in CRSwNP patients was assessed using the Pearson correlation test. For in vitro experiment, data were expressed as mean and the standard error of the mean (SEM), and were analyzed with one-way ANOVA and the Student’s t test. A p value of less than 0.05 was considered statistically significant.
Results
SAA immunoreactivity in nasal tissues
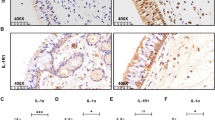
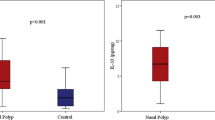
As indicated by IHC staining (Fig. 1a, b), strong immunoreactivity of SAA was mainly observed in the nasal epithelium of polyp tissues. In addition, positive staining was observed in submucosal inflammatory cells and gland in polyp tissues. In contrast, we found the SAA immunoreactivity was quite weak in normal tissues. The semi-quantitative scores of SAA immunoreactivity in polyp and control tissues were 2.20 [1.80, 2.40] and 0.20 [0.20, 0.60], respectively, with significant difference (p < 0.05).
The expression of SAA, IL-17A and MPO in control and polyp tissues. Representative IHC staining SAA is shown in control (a) and polyp tissues (b). Significantly enhanced SAA immunoreactivity is observed in polyp tissues (HPFs). The mRNA levels of SAA and IL-17A in control and polyp tissues (c, d) are shown, as suggested by qRT-PCR. Significantly increased mRNA levels of SAA and IL-17A are observed in polyp tissues. The protein levels of SAA and MPO in control and polyp tissues (e, f) are shown, as suggested by ELISA. Significantly increased protein levels of SAA and MPO are observed in polyp tissues. * p < 0.05
SAA and IL-17A mRNA levels in nasal tissues
As indicated by qRT-PCR analysis, we found SAA and IL-17A mRNA was detectable in all analyzed cases. Both SAA and IL-17A mRNA levels were found to be significantly increased in polyps tissues compared with controls (p < 0.05) (Fig. 1c, d). Significant correlation between SAA and IL-17A mRNA expression was observed in polyps tissues (r = 0.43, p < 0.05).
SAA and MPO protein levels in nasal tissues
As illustrated in Fig. 1e and f, we found both SAA and MPO protein levels were significantly increased in polyps tissues compared with controls (p < 0.05). Similarly, significant correlation between SAA and MPO protein levels was observed in polyps tissues (r = 0.58, p < 0.05).
SAA mRNA expression after cytokine stimulation
In cultured PECs, no significant change in SAA mRNA expression was observed in the presence of IFN-γ, IL-4 and IL-5 (Fig. 2a). However, we found significantly increased SAA mRNA expression in cultured PECs after incubation with IL-17A (100 ng/mL) for 12 h compared with the control (p < 0.05) (Fig. 2a). Moreover, IL-17A was found to increase SAA mRNA expression in cultured PECs in a time- and dose-dependent manner (p < 0.05) (Fig. 2b, c).
The expression of SAA mRNA in PECs after cytokine stimulation in vitro. a IL-17A, but not IFN-γ, IL-4 and IL-5, significantly increased SAA mRNA expression in cultured PECs; b IL-17A (100 ng/mL) significantly increased SAA mRNA expression in cultured PECs in a time- dependent manner; c IL-17A increased SAA mRNA expression in cultured PECs in a dose-dependent manner; d IL-17A significantly increased p-ERK expression in cultured PECs in a time-dependent manner; e IL-17A significantly increased p-ERK expression in cultured PECs in a dose-dependent manner; f Inhibiting activation of p-ERK by PD98059 (10 μM) significantly prevented IL-17A-mediated SAA mRNA expression. The data indicated the means (SEM) of three independent experiments. * p < 0.05
ERK signaling pathway is involved in SAA expression in PECs after IL-17A stimulation
Accordingly, we found IL-17A (100 ng/mL) significantly increased p-ERK expression in cultured PECs in a time- and dose-dependent manner as well (p < 0.05) (Fig. 2d, e). When inhibited activation of p-ERK by adding PD98059 (10 μM), IL-17A-mediated SAA mRNA expression was found to be significantly prevented (p < 0.05) (Fig. 2f).
Discussion
In the present study, we provided the first evidence that SAA expression is significantly upregulated in CRSwNP patients compared with the controls. Given the fact that SAA is able to enhance chemotaxis of neutrophils [7, 8], our finding might be helpful in understanding the pathogenic process for Chinese CRSwNP patients and establishing optimal treatment strategy in the future.
It has been generally accepted that CRSwNP can be differentiated based on the effector T cell pattern, resulting in eosinophilic or neutrophilic accumulation. In Caucasian subjects, CRSNP is considered to be orchestrated by Th2 cells, with IL-5 as major cytokine, resulting in increased eosinophil survival and a predominant eosinophilic inflammation [2]. In contrast, recent findings suggested Chinese CRSwNP patients might possess enhanced Th17 phenotype and increased neutrophil infiltration, as well as Th2 skewed eosinophilic accumulation to a less extent [3–5]. Increased IL-17A has been thought to be the key cytokine resulting in the accumulation and activation of neutrophils [10]. However, the pathogenic mechanism underlying the characteristically neutrophilic phenotype in Chinese CRSwNP patients has not been well understood yet.
SAA is an acute-phase protein which is mainly produced in the liver and plays important role in host defense during various inflammatory conditions [11]. Recombinant SAA was found to exhibit considerable chemoattractant activity for human monocytes, neutrophils, and T lymphocytes in vitro. SAA is responsible for its biological functions through binding to the SAA receptors such as formyl peptide receptor-like 1 genes [12, 13]. Except for liver hepatocyte, SAA has also been localized predominantly to the epithelial components of a variety of tissues, including breast, stomach, prostate, lung, kidney, tonsil, skin epidermis etc. [14]. Recent study has shown that SAA production is up-regulated by proinflammatory mediators, notably the IL-1β and IL-6 and TNF-α [15]. Conversely, SAA has been shown to possess cytokine-like activity and induce the production of cytokines (IL-8, TNF-α, etc.) in monocytes and neutrophils [8]. SAA was revealed to be a significant inducer of IL-23 and IL-1β in synoviocytes of rheumatoid arthritis and potentially activates the IL-23/IL-17A pathway in the synovium of rheumatoid arthritis [16]. However, there is limited literature concerning the importance of SAA in the pathogenesis of NP.
These mentioned findings thus raised the question whether SAA participated in the pathogenesis of CRSwNP in Chinese adult patients characterized by Th17 predominant neutrophilic infiltration. As we expected, SAA mRNA and protein expression were found to be significantly upregulated in polyp tissues compared with the controls, which was in contrast to Lane et al. [9, 17] study which stated that no difference in SAA expression between American CRSwNP patients and normal controls. More interestingly, we provided the evidence that SAA expression is associated with Th17 cell differentiation in polyp tissues in Chinese adult patients, which might subsequently contribute to the enhanced neutrophilia in polyp tissues in Chinese adult patients.
Our finding also showed that nasal epithelium of polyps tissues are the major source of SAA in CRSwNP patients, which was consistent with the previous report that SAA was localized predominantly to the epithelial components of a variety of other tissue [14]. Based on the observation that SAA is able to enhance chemotaxis of neutrophils [6, 7], our results thus highlight the central role of airway epithelial cell-derived SAA in modulating the innate and adaptive immunity and in initiating the accumulation of neutrophil in Chinese CRSwNP patients. To further investigate the possible modulation of SAA expression in nasal epithelium, we established an in vitro system of PECs and tested the effects of various cytokines on the expression of SAA mRNA in primary culture PECs. Interestingly, we found IL-17A significantly increased SAA mRNA expression in cultured PECs in a time- and dose-dependent manner. Taken together, our findings expand the understanding on SAA expression and modulation in polyp tissues, suggesting a possible regulatory mechanism underlying IL-17A-mediated neutrophil infiltration in Chinese CRSwNP patients via ERK-mediated SAA production. Therefore, to further investigate the therapeutic strategy against IL-17A/SAA/neutrophil regulatory axis in CRSwNP patients is likely to be of great interest.
Conclusion
Our study showed that SAA expression was significantly upregulated in polyp tissues, and SAA expression was positively correlated with IL-17A production and tissue neutrophilia. Moreover, IL-17A was found to increase SAA production in primarily cultured PECs in vitro. These findings suggested a possible regulatory mechanisms underlying IL-17A-mediated neutrophil infiltration in Chinese CRSwNP patients.
References
Otto BA, Wenzel SE (2008) The role of cytokines in chronic rhinosinusitis with nasal polyps. Curr Opin Otolaryngol Head Neck Surg 16:270–274
Fokkens W, Lund V, Mullol J (2007) European position paper on rhinosinusitis and nasal polyps group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology 20:S1–S136
Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, Wang DY, Desrosiers M, Liu Z (2009) Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol 124:478–484
Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, Luo Q, Zheng J, Wang H, Li Z, Xia J, Jiang H, Liu Z, Shi J, Li H, Xu G (2012) Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol 129:1522–1528
Wang X, Dong Z, Zhu DD, Guan B (2006) Expression profile of immune-associated genes in nasal polyps. Ann Otol Rhinol Laryngol 115:450–456
Badolato R, Wang JM, Murphy WJ, Lloyd AR, Michiel DF, Bausserman LL, Kelvin DJ, Oppenheim JJ (1994) Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med 180:203–209
Xu L, Badolato R, Murphy WJ, Longo DL, Anver M, Hale S, Oppenheim JJ, Wang JM (1995) A novel biologic function of serum amyloid A. Induction of T lymphocyte migration and adhesion. J Immunol 155:1184–1190
Patel H, Fellowes R, Coade S, Woo P (1998) Human serum amyloid A has cytokine-like properties. Scand J Immunol 48:410–418
Lane AP, Truong-Tran QA, Myers A, Bickel C, Schleimer RP (2006) Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol 20:117–123
Hoshino H, Laan M, Sjöstrand M, Lötvall J, Skoogh BE, Linden A (2000) Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol 105:143–149
Urieli-Shoval S, Linke RP, Matzner Y (2000) Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol 7:64–69
He R, Sang H, Ye RD (2003) Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 101:1572–1581
Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, Wang JM (1999) A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med 189:395–402
Urieli-Shoval S, Cohen P, Eisenberg S, Matzner Y (1998) Widespread expression of serum amyloid A in histologically normal human tissues. Predominant localization to the epithelium. J Histochem Cytochem 46:1377–1384
Furlaneto CJ, Campa A (2000) A novel function of serum amyloid A: a potent stimulus for the release of tumor necrosis factor-alpha, interleukin-1beta, and interleukin-8 by human blood neutrophil. Biochem Biophys Res Commun 268:405–408
He R, Shepard LW, Chen J, Pan ZK, Ye RD (2006) Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol 177:4072–4079
Lane AP, Truong-Tran QA, Schleimer RP (2006) Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinol 20:138–144
Acknowledgments
This study was supported by the National Natural Science Fund of China (No. U0832007, 81070771, 81070772, 81271054) and Guangdong Province Natural Science Grant (S2011010004634, S2011020002295) and a grant from the Ministry of Hygiene (No. 201202005) and Program for New Century Excellent Talents in University (No. NCET-10-0851).
Conflict of interest
No conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
H.T. Wang and J. Bai have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Wang, H., Bai, J., Ding, M. et al. Interleukin-17A contributes to the expression of serum amyloid A in chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol 270, 1867–1872 (2013). https://doi.org/10.1007/s00405-012-2295-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-012-2295-x